Abstract
Ozone (O3) is widely distributed in environments with high levels of air pollution. Since cerebellar morphologic disruptions have been reported with prenatal O3 exposure, O3 may have an effect on some neurotransmitter systems, such as monoamines. In order to test this hypothesis, we used 60 male rats taken from either, mothers exposed to 1 ppm of O3 during the entire pregnancy, or from mothers breathing filtered and clean air during pregnancy. The cerebellum was extracted at 0, 5, and 10 postnatal days. Tissues were processed in order to analyze by HPLC, dopamine (DA) levels, 3,4 dihydroxyphenilacetic acid (DOPAC) and homovanillic acid (HVA), norepinephrine (NA), serotonin, and 5-hydroxy-indole-acetic acid (5-HIAA) contents. Results showed a decrease of DA, NA, DOPAC and HVA mainly in 0 and 5 postnatal days. There were no changes in 5-HT levels, and 5-HIAA showed an increase after 10 postnatal days. DOPAC + HVA/DA ratio showed changes in 0 and 10 postnatal days, while 5-HIAA/5-HT ratio showed a slight decrease in 0 days. The data suggest that prenatal O3 exposure disrupts the cerebellar catecholamine system rather than the indole-amine system. Disruptions in cerebellar NA could lead to ataxic symptoms and also could limit recovery after cortical brain damage in adults. These finding are important given that recovery mechanisms observed in animals are also observed in humans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ozone (O3) is a major pollutant present in photochemical smog that is formed by photochemical reactions in response to intense sunlight. Many studies regarding the health effects of O3 have mainly focused on the respiratory tract. However, there are some papers documenting extra-pulmonary effects, particularly detriments in the central nervous system (CNS) such as decreased paradoxical sleep (PS) [1, 2] effects on kindling development [3], and changes in the metabolism and contents of some neurotransmitters [4, 5], (Gonzalez-Pina et al. 1997). There are a few studies documenting the mechanisms involved O3-induced toxicity of the CNS. For example, it has been demonstrated that paradoxical sleep disruptions caused by O3 exposure are mediated by changes in serotonin (5-HT) activity in the dorsal raphe [6] and also by extracellular acetylcholine in the hypothalamic medial preoptic area [7]. These disruptions could be mediated by secondary products, such as hydrogen peroxide, aldehydes, and free radicals, which are produced by initial reactions between O3 and the respiratory system. Despite the fact that free radicals are highly reactive, these products are thought to reach the brain through the bloodstream [8], because thiobarbituric acid-reactive substances, as an index of lipidic peroxidation produced by oxidative stress, increase in the brain after O3 exposure [9, 10]. The possibility of free radicals reaching other organs and systems implies also that they could pass through the blood–placental barrier. This represents a risk for the offspring when the mother is exposed to O3 during pregnancy. In this context, there are very few reports documenting the effects of prenatal O3 exposure on the developing CNS [11, 12]. Morphological studies have been conducted focusing on the cerebellum of offspring whose mothers were exposed to O3 during pregnancy. It has been described that rat pups show cerebellar necrotic signs at postnatal day 0, diminishment of the molecular layer at day 12 and unusual clumps of chromatin in the nucleus periphery of purkinje cells at postnatal day 60 [13], as well as a reduction in the number of purkinje cells at postnatal day 90 [14]. Due to the important morphological changes produced by prenatal O3 exposure in the cerebellum, it is expected that some neurotransmitter systems could be disrupted by prenatal O3 exposure. Thus, we measured the monoamine contents in cerebellum of pups whose mothers were exposed during pregnancy, as a functional indicator of risk for offspring development derived from prenatal O3 exposure.
Materials and methods
Twelve female wistar rats in estrus, with confirmed vaginal sperm from healthy males, were used to obtain study litters (12 litters). In order to obtain control animals, six pregnant rats were housed individually in acrylic cages (30 cm wide × 30 cm depth × 40 cm height each) and were provided with filtered air airflow as well as hoses for the administration and measurement of gases. The rats were maintained breathing activated coal filtered air (clean-air; 4 l/min) with food and water ad libitum. The other six rats were housed in similar conditions as above breathing clean air containing 1 ppm of O3. Ozone was administered by means of an O3 generator (TRIOZON mod. P-15) coupled to a previously calibrated domestic variant, a device that modifies the domestic line output voltage between 0 and 120 V and varies the UV light contained in the O3 generator. This variant was used to adjust the voltage in order to maintain a constant O3 supply. Concentration was monitored using an UV O3 measuring device (DASIBI mod. 1008-PC). Both, exposure to clean air and O3 were performed for 12 h/day (20:00–8:00 h) during the entire gestation (21 days). At birth, 10 males randomly taken from mothers that breathed clean air and 10 taken from mothers exposed to 1 ppm of O3 during pregnancy, were killed by decapitation. The remaining offspring were distributed to 10 pups per litter, with an equal number of males and females. All animals were nursed in a pollution-free environment, in standard vivarium conditions. Then, 10 males of each group were taken and sacrificed at postnatal day 5 and another 10 pups of each group were taken and sacrificed at postnatal day 10. Thus, we obtained six groups: prenatal exposure to clean-air sacrificed at 0 days after birth (CA + 0), prenatal exposure to 1 ppm of O3 sacrificed at 0 days after birth (O3 + 0), prenatal exposure to clean air sacrificed at 5 days after birth (CA + 5), prenatal exposure to 1 ppm of O3 sacrificed at 5 days after birth (O3 + 5), prenatal exposure to clean-air sacrificed at 10 days after birth (CA + 10) and prenatal exposure to 1 ppm of O3 sacrificed at 10 days after birth (O3 + 10). The brains were immediately extracted and the cerebellum dissected on a cool plate according to the Glowinski and Iversen technique [15]. Tissues were sonicated in 0.4 N perchloric acid with 1% (w/v) sodium metabisulfite, followed by 10 min of centrifugation at 15,000 rpm at 4°C. Supernatants were kept frozen at −70°C until chromatographic analysis. The contents of dopamine (DA), norepinephrine (NE), serotonin (5-HT) and the metabolites 3,4-dihydroxyphenilacetic acid (DOPAC), homovanillic acid (HVA) and 5-hydroxy-indole-acetic acid (5-HIAA) were analyzed by high-performance liquid chromatography with an electrochemical detector as described by Diggory and Buckett [16]. A Perkin–Elmer LC-250 liquid chromatograph coupled to an electrochemical detector (Bioanalytical Systems mod. LC-4C) was used. Calibration curves for monoamines were constructed by known concentrations of standards prepared in a perchloric metabisulfite solution injected into a 20 μl loop of the chromatograph. Peaks were integrated with a Perkin–Elmer LC-1020 program. The monoamine concentrations of samples were obtained by interpolation in their respective standard curves. In order to separate the neurotransmitters, an Alltech adsorbosphere catecholamine analytical column (4.1 × 100 mm) with 3 μm of particle size was used. The mobile phase consisted of an aqueous phosphate buffer solution (0.1 M, pH 3.2) containing 0.2 mM sodium octyl sulphate, 0.1 mM of EDTA and 15% v/v methanol. Flow rate was 1.2 ml/min, and the potential was set at 0.80 V against an Ag/AgCl reference electrode. Monoamines and the metabolite/neurotransmitter ratio found in prenatally O3-exposed rats and prenatally clean-air exposed animals in each age group were statistically compared using a non-paired t-test (P ≤ 0.05).
Results
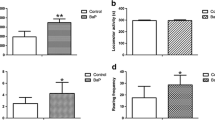
It was found that DA (Fig. 1) is decreased in the cerebellum of O3 + 0 rats when they were compared with the CA + 0 group (P ≤ 0.033). This pattern was equally observed in O3 + 5 rats when they were compared with CA + 5 rats (P ≤ 0.030). However, there were no significant differences between CA + 10 and O3 + 10 groups (P ≤ 0.252). The metabolite DOPAC (Fig. 2) was significantly decreased in O3 + 0 rats when compared with the CA + 0 group (P ≤ 0.045), and it was also observed a marginally significant decrease between CA + 5 and O3 + 5 rats (P ≤ 0.066). There were no observed significant changes between CA + 10 and O3 + 10 groups (P ≤ 0.471). On the other hand, HVA (Fig. 3) showed dramatic changes since lower levels of metabolite were found for all ages when they were compared with their respective controls: 0 days of age (P ≤ 0.010), 5 days of age (P ≤ 0.011), and 10 days of age (P ≤ 0.05). The same pattern of change was found in NE content (Fig. 4) and such decreases were significant for all the ages: 0 days of age (P ≤ 0.037), 5 days of age (P ≤ 0.000), and 10 days of age (P ≤ 0.009). On the other hand, DOPAC + HVA/DA ratio (Fig. 5) was significantly decreased in O3 + 0 rats (P ≤ 0.004) and also in O3 + 5 rats (P ≤ 0.046), while a non-significant decrease was observed in O3 + 10 rats (P ≤ 0.090).
Dopamine (DA) content in the cerebellum of rats after 0, 5, and 10 days of birth. A significant decrease in 0 and 5 postnatal days is observed, while at 10 days the DA levels were found without any difference. Comparisons were performed between prenatal clean-air exposed rats (clear bars) and prenatal O3-exposed rats (dark bars). Non-paired t-test (*P ≤ 0.05)
3,4-di-hydroxyphenilacetic acid (DOPAC) content in the cerebellum of rats after 0, 5, and 10 days of birth. A significant decrease in 0 postnatal day is observed, while a marginally significant (m.s.) decrease was found in 5 days and at 10 days the DOPAC levels were found without any difference. Comparisons were performed between prenatal clean-air exposed rats (clear bars) and prenatal O3-exposed rats (dark bars). Non-paired t-test (*P ≤ 0.05)
Homovanillic acid content in the cerebellum of rats after 0, 5, and 10 days of birth. A significant decrease in 0, 5, and 10 postnatal days is observed. Comparisons were performed between prenatal clean-air exposed rats (clear bars) and prenatal O3-exposed rats (dark bars). Non-paired t-test (*P ≤ 0.05, **P ≤ 0.01)
Norepinephrine content in the cerebellum of rats after 0, 5, and 10 days of birth. A significant decrease in 0, 5, and 10 postnatal days is observed. Comparisons were performed between prenatal clean-air exposed rats (clear bars) and prenatal O3-exposed rats (dark bars). Non-paired t-test (*P ≤ 0.05, **P ≤ 0.01)
DOPAC + HVA/DA ratio in the cerebellum of rats after 0, 5, and 10 days of birth. A significant decrease in 0 and 5 postnatal days is observed, while at 10 days the DOPAC + HVA/DA ratio was found decreased but without a significant difference. Comparisons were performed between prenatal clean-air exposed rats (clear bars) and prenatal O3-exposed rats (dark bars). Non-paired t-test (*P ≤ 0.05)
Cerebellar 5-HT content (Fig. 6) was not observed modified in any of the ages assayed, as depicted in the plot. However, its metabolite 5-HIAA (Fig. 7) was significantly increased in rats of the O3 + 10 group (P ≤ 0.001). In contrast, 5-HIAA/5-HT ratio (Fig. 8) was observed to decrease in O3 + 0 rats (P ≤ 0.042).
5-Hydroxy-indole-acetic acid (5-HIAA) content in the cerebellum of rats after 0, 5, and 10 days of birth. A significant increase in 10 postnatal day rats is observed, while at 0 and 5 days the 5-HIAA levels were found without any difference. Comparisons were performed between prenatal clean-air exposed rats (clear bars) and prenatal O3-exposed rats (dark bars). Non-paired t-test (**P ≤ 0.001)
5-HIAA/5-HT ratio in the cerebellum of rats after 0, 5, and 10 days of birth. A significant decrease in 0 postnatal day rats is observed, while at 5 and 10 days the 5-HIAA/5-HT ratio was found without significant difference. Comparisons were performed between prenatal clean-air exposed rats (clear bars) and prenatal O3-exposed rats (dark bars). Non-paired t-test (*P ≤ 0.05)
Discussion
The negative health effect of air pollution is not a new issue. It is widely known that aged men and newborn children died in December 4–8, 1952 in London and also 500 people in New York in 1963, as a consequence of air pollution [17]. In the case of O3, the benefits of mitigating ozone pollution on global health has been discussed [18], since it has been associated in epidemiological studies with daily premature mortality [19, 20]. Therefore, O3 pollution is a global problem that needs to be studied using diverse perspectives, such as its effects on health and the risks associated with high exposure. In this context, our findings document that prenatal O3 exposure is capable of inducing changes in cerebellar monoamine content in young offspring. It is widely accepted that the cerebellum influences mainly motor behavior, eye movement, and conditioning [21]. Cerebellar projections are also though to influence respiration [22], cognition [23], and prediction of sensory events [24] in addition to being involved in autism [25], and it exerts an influence in recovery after somatosensory and motor cortical brain injury [26, 27, 28]. However, there are no studies that assess the effects of O3 on such behaviors in prenatal animals exposed to O3. However, there are some reports documenting a depression of motor activity in adult animals after acute O3 exposure [29, 30]. Subepithelial sensory nerve stimulation with substance P release has been reported in humans exposed to 0.2 ppm of O3 [31], although such effects could be related to local stimulation.
We found that DA content in the cerebellum was the same in both groups of rats at 10 days of age. We also observed important reductions in the metabolites DOPAC and HVA as well as in the DOPAC + HVA/DA ratio, as an index of neurotransmitter metabolism, suggesting that dopaminergic enzymatic machinery and the release and re-uptake of this neurotransmitter was disrupted. These findings are also supported by the effects observed in NE content, which decreased at all ages studied. NE is a neurotransmitter derived by the β-hydroxylation of DA and is involved in GABAergic response modulation of developing Purkinje cells [32]. It has been also suggested that NE contributes to the wiring of definitive neuronal circuits in the cerebellum [33]. The critical period for the establishment of such functions is at postnatal days 5–9, when NE enhance inhibitory activity [32]. Interestingly, these were the days in which we found disruptions in cerebellar NE content. Thus, it is possible that animals prenatally exposed may show some alterations in Purkinje cell modulation of GABA-mediated inhibition, leading to such symptoms as ataxia. This is consistent with the observation of important morphological alterations in the cerebellum, mainly in Purkinje cells, during development [13] and into adulthood [14] of prenatally exposed animals. On the other hand, some lines of evidence strongly suggest that cerebellar NE is involved in functional recovery after motor brain injury [26, 27, 28]. Since administration of substances that mimic NE enhance recovery in experimental animals and humans, while substances that deplete central NE impair motor recovery [34], then we expect that recovery mechanisms in prenatally O3-exposed animals will present difficulties recovering from motor cortical injury. This represents a potential risk in human health because the mechanisms of recovery observed in animals seem to be similar to those described in humans [35].
Serotonin showed a disruption pattern similar to that found in adult animals after acute O3 exposure. The effects were observed in the metabolite, 5-HIAA as it has been reported in adult animals in other brain regions [4, 5]. Serotonin is involved in the inhibition of glutamate release in the cerebellum [36], suggesting a role in cerebellar glutamatergic modulation. It has been documented that the 5-HT system is highly plastic [37, 38, 39, 40] and the fact that its metabolite is affected by O3 exposure suggest that serotonergic plasticity mechanisms could be mediated in part by mono-amino-oxidase activity. We do not think that another aspect of the metabolism could be involved because we did not observe changes in the 5-HIAA/5-HT ratio. These observations represent an example of resistance of the organism to O3 exposure and open a field of study regarding 5-HT mechanisms as an experimental model of defense from secondary products derived from exposure to such a gas.
It is unlike that O3 causes the observed effects itself, due to its high reactivity. Probably, the formation of free radicals (FR) in the lung [41], which in turn reach the placenta and go beyond via a cascade mechanism [8], could be involved in cerebellar monoamine disruptions observed. There is evidence of extra-pulmonary FR toxicity in the adult brain after O3 exposure since brain malondialdehyde, a product of neuronal membrane peroxidation, increases [9, 10] while antioxidant supplementation diminishes these effects [42]. It is possible that the increase of monoamines reported in adult animals exposed to O3 [4] could be a defensive mechanism against FR since antioxidant properties of monoamines has been documented [43]. In this viewpoint, our results suggest that the antioxidant system could be disrupted at birth in prenatally O3-exposed animals, which could lead to serious cellular malfunctions that are critical in fetal development.
Although we used a high concentration of O3 in order to ensure observable effects in this work, there are reports that describe the effects of O3 exposure on the CNS of adult rats using models that mimic conditions found in the cities with air pollution, such as exposure to concentrations of 0.25 ppm for 4 h that alters locomotor behavior [44], or 0.35 ppm that decreased paradoxical sleep after 2 h breathing O3 [45], and the O3-induced changes in the extracellular 5-HIAA and acetylcholine activity while rats were breathing concentrations ranging from 0.1 to 0.5 ppm [6, 7]. Maximum levels of O3 measured in Mexico City have been 0.49 ppm [46]. These observations suggest that 1 ppm of O3 exposure is valid for assessing environmental risk because emphasize the effects that are also observed using low concentrations. However, there is no direct evidence that atmospheric O3 could affect the CNS of humans, although a study reported fatigue, lethargy, and headache referred by subjects exposed to O3 [47], symptoms that could be a consequence of sleep disturbances, as reported in experimental animals [2]. In order to develop preventive strategies, it is important to design more studies focused evaluate the risk of prenatal O3 exposure, regarding extra-pulmonary effects. The cerebellar monoamine disruptions reported here could lead to poor psychomotor development in children and also affect brain recovery after stroke, making rehabilitation difficult. In addition, while secondary effects are elucidated, care must be advised when medical use of O3 in obstetrics and gynecology [48] is prescribed.
References
Arito H, Uchiyama I, Arakawa H, Yokoyama E (1990) Ozone-induced bradycardia and arrhytmia and their relation to sleep–wakefulness in rats. Toxicol Lett 52:169–178
Huitron-Resendiz S, Custodio-Ramirez V, Escalante-Membrillo C, Gonzalez-Pina R, Paz C (1994) Sleep alterations and brain regional changes of serotonin and its metabolite in rats exposed to ozone. Neurosc Lett 177:119–122
Escalante-Membrillo C, Paz C (1997) Development of an experimental model of epilepsy in rats exposed to ozone. Toxicol Lett 93:103–107
Gonzalez-Pina R, Paz C (1997) Brain monoamine changes in rats after short periods of ozone exposure. Neurochem Res 22:63–66
Cottet-Emard JM, Dalmaz Y, Pequignot J, Peyrin L, Pequignot JM (1997) Long-term exposure to ozone alters peripheral and central catecholamine activity in rats. Pflugers Arch Eur J Physiol 433:744–749
Gonzalez-Pina R, Alfaro-Rodriguez A (2003) Ozone exposure alters 5-hydroxy-indole-acetic acid contents in dialysates from dorsal raphe and medial preoptic area in freely moving rats. Relationships with simultaneous sleep disturbances. Chem Biol Interac 146:147–156
Alfaro-Rodriguez A, Gonzalez-Pina R (2005) Ozone-induced paradoxical sleep decrease is related to diminished acetylcholine levels in the medial preoptic area in rats. Chem Biol Interact 151(3):151–158
Pryor WA, Squadrito GL, Friedman M (1995) The cascade mechanisms to explain ozone toxicity: the role of lipid ozonation products. Free Rad Biol Med 9:935–941
Rahman IU, Massaro GD, Massaro D (1992) Exposure of rats to ozone: evidence of damage to heart and brain. Free Rad Biol Med 12:323–326
Escalante-Membrillo C, Gonzalez-Maciel A, Reynoso-Robles R, Gonzalez-Maciel A, Gonzalez-Pina R (2005) Brain thiobarbituric acid-reactive substances in rats after short periods of ozone exposure. Environ Res 99:68–71
Bignami C, Musi B, Dell’Omo G, Laviola G, Alleva E (1994) Limited effects of ozone exposure during pregnancy on physical and neurobehavioral development of CD-1 mice. Toxicol Appl Pharmacol 129(2):264–271
Santucci D, Sorace A, Francia N, Aloe L, Alleva E (2006) Prolonged prenatal exposure to low-level ozone affects aggressive behavior as well as NGF and BDNF levels in the central nervous system of CD-1 mice. Behav Brain Res 166(1):124–130
Rivas-Manzano P, Paz C (1999) Cerebellar morphological alterations in rats induced by prenatal ozone exposure. Neurosci Lett 276(1):37–40
Romero-Velazquez RM, Alfaro-Rodriguez A, Gonzalez-Pina R, Gonzalez-Maciel A (2002) Effect of ozone prenatal exposure on post-natal development of cerebellum. Proc West Pharmacol Soc 45:65–67
Glowinski J, Iversen LL (1966) Regional studies of catecholamines in the rat brain. Disposition of 3H-norepinephrine, 3H-dopamine and 3H-DOPA in various regions of the brain. J Neurochem 13:655–669
Diggory GL, Buckett WR (1984) An automated method to measure monoamines and metabolites using elevated temperature reversed phase HPLC with electrochemical detection, Application to striatal dopamine and hyppocampal serotonin turnover. J Pharmacol Methods 11:207–217
Maynard RL (1993) Air pollution: should we be concerned about it? J Royal Soc Med 86:63–64
West JJ, Fiore AM, Horowitz LW, Mauzerall DL (2006) Global health benefits of mitigating ozone pollution with methane emission controls. Proc Natl Acad Sci 103(11):3988–3993
Levy JI, Chereynsky SM, Sarnat JA (2005) Ozone exposure and mortality: an empiric bayes metaregression analysis. Epidemiology 16(4):458–468
Schwartz J (2005) How sensitive is the association between ozone and daily deaths to control for temperature? Am J Respir Crit Care Med 171(6):627–631
Saab CY, Willis WD (2003) The cerebellum: organization, functions and its role in nociception. Brain Res Rev 42:85–95
Xu F, Frazier DT (2000) Modulation of respiratory motor output by cerebellar deep nuclei in the rat. J Appl Physiol 89:996–1002
Fiez JA (1996) Cerebellar contributions to cognition. Neuron 16:13–15
Nixon PD, Passingham RE (2001) Predicting sensory events. The role of the cerebellum in motor learning. Exp Brain Res 138:251–257
Nayate A, Bradshaw JL, Rinehart NJ (2005) Autism and Asperger’s disorder: Are they movement disorders involving the cerebellum and/or basal ganglia? Brain Res Bull 67(4):327–334
Boyeson MG, Krobert KA (1992) Cerebellar norepinephrine infusions facilitate recovery after sensorimotor cortex injury. Brain Res Bull 29:435–439
Krobert KA, Sutton RL, Feeney DM (1994) Spontaneous and amphetamine-evoked release of cerebellar noradrenaline after sensorimotor cortex contusion: an in vivo microdyalisis study in the awake rat. J Neurochem 62:2233–2240
Gonzalez-Pina R, Bueno-Nava A, Montes S, Alfaro-Rodriguez A, Gonzalez-Maciel A, Reynoso-Robles R, Ayala-Guerrero F (2006) Pontine and cerebellar norepinephrine content in adult rats recovering from focal cortical injury. Neurochem Res 31(12):1443–1449
Tepper JL, Weiss B, Cox C (1982) Microanalysis of ozone depression of motor activity. Toxicol Appl Pharmacol 64(2):317–326
Dorado-Martinez C, Paredes-Carbajal C, Mascher D, Borgonio-Perez G, Rivas-Arancibia S (2001) Effects of different ozone doses on memory, motor activity and lipid peroxidation levels, in rats. Int J Neurosci 108(3–4):149–161
Krishna MT, Springall D, Meng QH, Withers N, Macleod D, Biscione G, Frew A, Polak J, Holgate S (1997) Effects of ozone on epithelium and sensory nerves in the bronchial mucosa of healthy humans. Am J Respir Crit Care Med 3(Pt 1):943–950
Yeh HH, Woodward DJ (1983) Noradrenergic action in the developing rat cerebellum: interaction between norepinephrine and gamma-aminobutyric acid applied microiontophoretically to immature Purkinje cells. Brain Res 312(1):49–62
Yeh HH, Woodward DJ (1983) Noradrenergic action in the developing rat cerebellum: interaction between norepinephrine and synaptically-evoked responses of immature Purkinje cells. Brain Res 313(2):207–218
Goldstein LB, Coviello A, Miller GD, Davis JN (1991) Norepinephrine depletion impairs motor recovery following sensorimotor cortex injury in the rat. Restor Neurol Neurosci 3:41–47
Goldstein LB (2000) Effects of amphetamines and small related molecules on recovery after stroke in animals and man. Neurophramacology 39:852–859
Maura G, Raiteri M (1996) Serotonin 5-HT1D and 5-HT1A receptors respectively mediate inhibition of glutamate release and inhibition of cyclic GMP production in rat cerebellum in vitro. J Neurochem 66(1):203–209
Gershon MD (2003) Plasticity in serotonin control mechanisms in the gut. Curr Opin Pharmacol 3(6):600–607
Salvinelli F, Casale M, Paparo F, Persico AM, Zini C (2003) Subjective tinnitus, temporomandibular joint dysfunction, and serotonin modulation of neural plasticity: causal or casual triad? Med Hypotheses 61(4):446–448
Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB Jr, Mitchell GS (2001) Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21(14):5381–5388
Kojic L, Dyck RH, Gu Q, Douglas RM, Matsubara J, Cynader MS (2000) Columnar distribution of serotonin-dependent plasticity within kitten striate cortex. Proc Natl Acad Sci 97(4):1841–1844
Kennedy CH, Hatch GE, Slade R, Mason RP (1992) Application of EPR spin-trapping technique to the detection of radicals produced in vivo during inhalation exposure of rats to ozone. Toxicol Appl Pharmacol 114:41–46
Gonzalez-Pina R, Alfaro-Rodriguez A, Castorena-Maldonado A, Morales-Martinez JJ (2002) Acute administration of alpha-tocopherol protects from ozone-induced changes in rat striatal catecholamine levels. Proc West Pharmacol Soc 45:59–61
Sofic E, Denisova N, Youdim K, Vatrenjak-Velagic V, De Filippo C, Mehmedagic A, Causevic A, Cao G, Joseph JA, Prior RL (2001) Antioxidant and pro-oxidant capacity of catecholamines and related compounds. Effects of hydrogen peroxide on glutathione and sphingomyelinase activity in pheochromocytoma PC12 cells: potential relevance to age-related diseases. J Neural Transm 108(5):541–557
Rivas-Arancibia S, Dorado-Martinez C, Colin-Barenque L, Kendrick KM, de la Riva C, Guevara-Guzman R (2003) Effect of acute ozone exposure on locomotor behavior and striatal function. Pharmacol Biochem Behav 74(4):891–900
Paz C, Huitron-Resendiz S (1996) The effects of ozone exposure on the sleep–wake cycle and serotonin contents in the pons of the rat. Neurosci Lett 204(1–2):49–52
Bravo H, Roy-Ocotla G, Sánchez P, Torres R (1991) Contaminación atmosférica por ozono en la zona metropolitana de la ciudad de México: evolución histórica y perspectivas. Rev Coord Gral Estud Posgrad –23:39–48
Hackney JD, Linn WS, Karuza SK, Buckley RD, Pedersen EE, Law DC, Bates DV, Hazucha M, Pengelly LD, Silverman F (1977) Effects of ozone exposure in Canadians and southern Californians. Arch Environ Health 32:110–116
Andikyan VM, Voloshchuk IN, Kovganko PA, Clemente JM (2001) Morphofunctional changes in the placenta after ozone therapy. Bull Exp Biol Med 130: 715–718
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonzalez-Pina, R., Escalante-Membrillo, C., Alfaro-Rodriguez, A. et al. Prenatal Exposure to Ozone Disrupts Cerebellar Monoamine Contents in Newborn Rats. Neurochem Res 33, 912–918 (2008). https://doi.org/10.1007/s11064-007-9534-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9534-3