Abstract
The essential and naturally occurring transition metal manganese (Mn) is present in the soil, water, air, and various foods. Manganese can accumulate in the brain if the Mn intake or exposure is excessive and this can result in neurotoxic effects. Manganese is important for the proper activation of different metabolic and antioxidant enzymes. There are numerous Mn importers and exporters. However, the exact transport mechanism for Mn is not fully understood. On the other hand, iron (Fe) is another well-known essential metal, which has redox activity in addition to chemical characteristics resembling those of Mn. Existing data show that interactions occur between Fe and Mn due to certain similarities regarding their mechanisms of the absorption and the transport. It has been disclosed that Mn-specific transporters, together with Fe transporters, regulate the Mn distribution in the brain and other peripheral tissues. In PC12 cells, a significant increase of transferrin receptor (TfR) mRNA expression was linked to Mn exposure and accompanied by elevated Fe uptake. In both humans and animals, there is a strong relationship between Fe and Mn metabolism. In the present review, special attention is paid to the interaction between Mn and Fe. In particular, Fe and Mn distribution, as well as the potential molecular mechanisms of Mn-induced neurotoxicity in cases of Fe deficiency, are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An amount of about 12–20 mg of the trace element manganese (Mn) in an average human body (70 kg) is essential for the normal metabolism of carbohydrates, lipids, and proteins, since it is a cofactor in some enzymes (Aschner and Aschner 2005; Aschner et al. 1999; Dion et al. 2018; Rodrigues et al. 2018; Zoroddu et al. 2019). The most abundant of these in the human body is glutamine synthetase, which has a key role in brain function (Chen et al. 2018; Zoroddu et al. 2019). Mn also has an important role in physiological and developmental processes such as antioxidant defense, immunity, blood sugar, energy homeostasis, and also growth and reproduction (Avila et al. 2013). Though Mn is crucial for the proper functioning of numerous metabolic and antioxidant enzymes, excessive Mn exposure or intake is accompanied by accumulation in the brain, which can cause neurotoxicity (Crossgrove and Yokel 2004). For non-occupational exposed individuals, the main exposure route of Mn is ingestion (Bjørklund et al. 2017b). Different types of food, legumes, seafood, nuts, tea, spices, and plant-derived beverages are the major dietary sources of Mn. Although overt Mn deficiency diseases seem to be very rare in humans, deficiency states have been associated with symptoms like poor bone formation, weight loss, and reduced fertility (Aschner and Aschner 2005). Symptoms of neurotoxicity attributed to excessive Mn exposure have raised great concern in public health (Crossgrove and Yokel 2004; de Water et al. 2018). The risk of nutritional exposure to excess of Mn is mainly related to certain unhealthy conditions such as people suffering from liver failure, hepatic encephalopathy, or newborns receiving total intravenous parenteral nutrition supplemented with Mn (Peres et al. 2016). In these conditions, the accumulation of Mn is due to the limited excretion through the biliary system or the failure of the gastrointestinal control of Mn absorption, respectively. Moreover, patients with iron deficiency anemia are at risk for increased Mn uptake, since the Fe deficiency increases the expression of the metal importers/transporters that are common between the two metal ions (Chen et al. 2015).
Signs of neurological Mn toxicity include stuttering, progressive bradykinesia, gait disturbance, hallucinations, tremors, and dystonia (Bjørklund et al. 2017b). Chronic Mn encephalopathy (manganism) was originally described among workers involved in grinding of Mn ores. These workers were seen with characteristic symptoms of gait disturbances, cognitive and motor deficiencies, hallucinations, and tremors (Mena et al. 1967). Also, children who were exposed to Mn concentrations above 0.24 mg/L in drinking water showed changed functions in school and reduced results on neurobehavioral tests (Zhang et al. 1995). A clinical sign of manganism is the high levels of Mn in the globus pallidus and striatum neurons, as shown by T1-weighted MRI analysis (Aschner et al. 2015). The build-up of Mn in this area induces a series of effects, in particular, oxidative damage caused by the increased expression of ROS molecules and alterations in metabolism and synthesis of different important neurotransmitters, such as glutamate, GABA, and dopamine (Kim et al. 2015). Genetic factors are also important in Mn toxicity and Parkinson’s disease, as well (Chen et al. 2014). In particular, mutations in Parkin, and ATP13A2 (Park9) genes, that are associated with Mn efflux activity, have been correlated with its toxicity (Gitler et al. 2009; Peres et al. 2016; Remelli et al. 2016). Also, mutations in the Mn transporter gene SLC30A10 have been reported to induce a genetic Mn overload syndrome such as parkinsonism and dystonia. The evidence that the deregulation of this protein is the only genetic factor associated with hereditary Mn-induced parkinsonism highlighted the importance of the Mn efflux mechanism in homeostasis and detoxification of Mn(II). Iron (Fe), another transition element, is essential for most living organisms on the earth, including humans (Fitsanakis et al. 2010; Zoroddu et al. 2019). About 5 g of Fe is found in the average human body, and its role is crucial for the proper functioning of several cellular mechanisms (Zoroddu et al. 2019). This includes DNA synthesis, enzymatic processes, and generation of mitochondrial energy. Iron shares many similar chemical and biochemical properties with Mn. Because of their close similarities, the interaction of Fe and Mn is seen in numerous physiological processes (Roth and Garrick 2003; Ye et al. 2017). Iron and Mn are cofactors for several metalloenzymes that play essential roles in neurochemistry and antioxidant defense in the brain. Due to their similar chemical, biochemical, and structural properties, it is possible if one the metal is in excess, it can impact the other metal’s physiological functions.
The present review aims to give an update on Fe–Mn interactions as regards their shared absorption and distribution. Also, we discuss their toxicological interactions, aiming at highlighting mechanisms involved in Mn-induced neurotoxicity.
Divalent metal transporter DMT1: absorption and distribution of iron and manganese
Innovative studies on DMT-1 (initially known as DCT-1, divalent cation transporter-1 and NRAMP 2, natural resistance-associated macrophage protein 2) in metal transport revealed this protein is actively involved in the transport of a broad spectrum of divalent cations, including Mn(II) (Gunshin et al. 1997). Moreover, it is thought that the affinity of DMT1 to Fe(II) is lower than that of Mn(II) (Garrick and Dolan 2002; Garrick et al. 2006). Experimental study on HEK293T transfected with pMT2–DMT1 expression plasmid revealed competition between Fe and Mn for binding to the transporter and intracellular incorporation (Garrick and Dolan 2002). Correspondingly, a significant decrease of acid-stimulated Fe uptake by DMT1 was observed in 500 µM Mn-treated Caco2 cells (Bannon et al. 2003).
Moreover, 100 µM Mn exposure also resulted in upregulated expression of DMT1 in choroidal epithelia of the blood–cerebrospinal fluid barrier (Wang et al. 2006). The involvement and potential function of DMT1 in the transport of Mn over the blood–brain barrier (BBB) have been discussed (Au et al. 2008). DMT1 expression in the nasal epithelium provides a route for direct absorption of metals into the brain (Fitsanakis et al. 2007). Moreover, DMT1 expression in the brain enhances with age, increasing the susceptibility to metal-induced neuropathology (Ke et al. 2005). Concurrently, an investigation on the A549 cell line revealed that DMT1 in pulmonary cells is not the main Mn transporter (Heilig et al. 2006). Other manganese importers such as ZIP8 (SLC39A8) and ZIP14 (SLC39A14), the latter also being a Fe(II) binder, are important regulators for the Mn absorption through the liver and lung. As in the case of DMT1, the expression of these transporters in the nasal respiratory epithelium allows the inhaled Mn nanoparticles (dust and fumes) to be absorbed directly into the brain (Genter et al. 2009; Zoroddu et al. 2014). The levels of ferroportin (Fpn), a well-known Fe exporter, have been found to be significantly increased in enterocyte basolateral membranes due to Mn exposure. In a metal overload condition, Fpn act as a Mn transporter. A study of Xenopus laevis oocytes showed that the Mn export was mediated and partially inhibited by Fpn in Fe treatments (Madejczyk and Ballatori 2012; Yin et al. 2010). Research on Xenopus oocytes showed that Fpn stimulated Mn efflux (Madejczyk and Ballatori 2012; Mitchell et al. 2013). However, it was not found that hepcidin, which plays a crucial role in Fe homeostasis regulation, inhibited the efflux of Mn (Mitchell et al. 2013). It is noteworthy that Mn treatment had no impact on the gene expression of Fpn in J774 cells (Park and Chung 2008; Park et al. 2013). Many studies have been performed in rodents to determine how DMT1 is related to the tissue transport of Mn. Several studies that used developing rats showed enhanced expression of DMT1 in the brains of newborns due to Mn exposure via milk (Garcia et al. 2006). In Fe-deficient condition, DMT1-mediated uptake of Mn by olfactory epithelium can also be upregulated. DMT1 has been found to be essential for Fe transport, however, not for the transport of Mn in a mouse model having intestinal DMT1 deficiency. In principle, these findings are in accordance with previous indications that Mn transport over the BBB is not only mediated by DMT1 (Pivina et al. 2019).
Consequently, research indicates that DMT1 plays an insignificant role in the interactions in vivo between Fe and Mn (Aisen et al. 1969). The reduced Mn metabolism in mice with Fpn deficiency demonstrated the involvement of Fpn also in manganese transport (Gkouvatsos et al. 2012). Interestingly, this researcher group revealed enhanced absorption of Mn in HFE-deficient mice, whereas a significant difference was not found in the distribution in tissues of Mn after metal installation or injection. Another important investigation of HFE−/− in mice found increased expression of Fpn in mitochondria of the liver, which had a significantly lower concentration of Mn and a higher Fe level compared to wild-type controls (Parkkila et al. 2001). In a rat model with increased DMT1 expression, Mn-induced parkinsonism was linked to Mn exposure and reduced Fpn1 expression in the substantia nigra, which caused Fe overload (DeWitt et al. 2013; Pang et al. 2015; Peres et al. 2019; Sarkar et al. 2019).
Iron and manganese delivery system
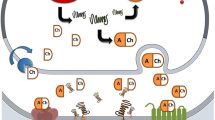
Manganese can form a specific complex with transferrin (Tf), which is crucial in the Fe delivery system (Fig. 1) (Aisen et al. 1969; Gkouvatsos et al. 2012). Later studies established that Mn binds to the Fe-binding sites of Tf molecules and that the oxidation state of Mn determines the metal affinity of Mn to endogenous ligands (Harris and Chen 1994; Vincent and Love 2012). Other research has shown that the Tf mechanism of the Mn3+ transportation to the target cells is similar to Fe3+ transportation, although the transport rate of the Mn–Tf complex was slower than that for other Mn transport mechanisms (Aschner et al. 1999; Gunter et al. 2013). Furthermore, Mn2+ oxidizes to Mn3+ before interacting with Tf (Critchfield and Keen 1992). It is noteworthy that the findings of in vitro research show that ceruloplasmin presumably may participate in the Mn-binding to apo-transferrin (apoTf) through oxidation of the bivalent cation (Fig. 2) (Moshtaghi et al. 1997).
Non-heme iron transport across an intestinal enterocyte. Ferric iron (Fe) is reduced to the ferrous form by a luminal ferrireductase duodenal cytochrome b (DCYTB). Ferrous Fe is then transported into the enterocyte by divalent metal transporter-1 (DMT1), across the apical brush border. Within the enterocyte, Fe is either stored in ferritin or exported out of the cell, in the bloodstream, across the basolateral membrane by ferroportin (Fp). The hepcidin causes ferroportin internalization and degradation, decreasing iron efflux. Ferrous Fe is oxidized to its ferric form by the ferroxidase hephaestin aided by ceruloplasmin. Ferric Fe is then bound by serum transferrin in blood capillaries and transported to various sites in the body
Cellular manganese and iron homeostasis in humans. Manganese (Mn) and iron (Fe) share the same importers, such as divalent metal transporter-1 (DMT1) and zinc transporter 14 (ZIP14). The reduction of Fe3+ to Fe2+ requires the activity of iron reductases such as duodenal cytochrome B (DCYTB), stromal cell-derived receptor 2 (SDR-2), and metalloreductase STEAP2. Secondarily Mn2+ can enter cells via zinc transporter 8 (ZIP8), glutamic acid ionotropic receptor (GLU R), dopamine transporter (DAT), (store-operated calcium channel (SOC Ca2+), voltage-gated Ca+2 channel (VG Ca2+), choline and citrate transporters. Fe3+ and Mn3+ import can occur via endocytosis of the holo-transferrin–transferrin receptor 1 (Tf–TfR1) complex. In the endosome, ferric and manganic ions can be reduced by metalloreductase STEAP3 (STEAP3) and pumped out by DMT1 in ferrous and manganous form. Intracellular Fe in labile Fe pool (LIP) can be stored in ferritin, non-heme Fe enzymes, used for iron–sulfur (Fe–S), and heme protein biosynthesis, energy generation, and regulation of transcription via Fe-responsive element-binding proteins (IRP1/2). Mn2+ can interfere with the homeostasis of Fe2+, through the Fe regulatory protein (IRP) system that regulates Fe metabolism. Both Fe2+ and Mn2+ can generate an excess of reactive oxygen species (ROS). Export of Fe2+ occurs through ferroportin (Fp), often aided by hephaestin (Hp) and/or ceruloplasmin (Cp), and repressed by hepcidin. Mn2+ efflux can occur via Fp and solute carrier family 30 member 10 (SLC30A10), also present in endosomes. Protein as secretory pathway Ca2+-ATPases (SPCA1/2) and probable cation-transporting ATPase 13A2 (ATP13A2) play a role in storage, homeostasis and export of Mn2+
Earlier studies had demonstrated that Mn might act as a substrate for multicopper oxidases (Hellman and Gitlin 2002). Interestingly, significantly increased expression of transferrin receptor (TfR) mRNA in Mn-exposed PC12 cells has been demonstrated, and this effect was accompanied by elevated Fe uptake. However, no similar effect was observed in astrocytes (Zheng and Zhao 2001). These findings match well with the later studies indicating a significant elevation of the cellular concentration of TfR proteins and elevated levels of TfR mRNA in choroidal epithelial cells after Mn exposure (Li et al. 2005). Earlier data indicate that TfR on human neuroblastoma SHSY5Y cells may internalize and bind Mn–Tf complex to the same effect as Fe–Tf complex (Suárez and Eriksson 1993). It is interesting that lactoferrin also complexes Mn, and this lactoferrin-bound Mn was taken up by intestinal brush border membrane vesicles by a receptor-mediated mechanism. However, this complex has a lower affinity for the receptor than that for the Fe–lactoferrin complex (Fe–Lf) (Bo et al. 2019; Davidsson et al. 1989). Also, Mn interferes with the mechanisms of Fe transport, through the system of iron regulatory proteins (IRP) that regulate Fe metabolism (Pantopoulos 2004). Particularly, it has been established in incubated PC12 cells that moderate Mn exposure results in decreased IRP binding activity, while high Mn exposure results in increased IRP binding (Kwik-Uribe et al. 2003). A later study disclosed that dynamics of IRP-1 binding altered in Mn-exposed PC12 cells as well as the abundance of IRP-2 intracellularly (Kwik‐Uribe and Smith 2006). The altered homeostasis of Fe induced by Mn in GABAergic AF5 cells seems to mainly occur due to IRP2 modulation, and in a minor degree, to IRP1 (Crooks et al. 2007). Similar to Fe, Mn has in IRP1 a high affinity to the fourth labile position of the Fe–S cluster (Oshiro et al. 2002). Research has shown that Mn decreases amyloid precursor protein (APP) and heavy-chain ferritin (H-ferritin) protein translation by increasing the binding of IRP1 to the iron-responsive elements (IRE) on the 5′-untranslated regions of their mRNA transcripts (Venkataramani et al. 2018). In younger individuals, APP expression is not associated with amyloidosis. Instead, it acts solely as a neuroprotectant while facilitating cellular ferroportin-dependent iron efflux. Therefore, translational blockage of APP and H-Ferritin results in the accumulation of toxic Fe(II) and the subsequent neurotoxicity due to the generation of ROS.
Iron and manganese interaction in animals and human studies
In an in vitro study of cultured hepatocytes, it was shown that hepcidin levels increased due to Mn treatment. Considering how hepcidin impacts the homeostasis of Fe, the researchers suggested that exposure to Mn, particularly in Fe deficiency, can intensify Fe deficiency (Bartnikas 2012; Chen et al. 2019). The findings from in vivo research, including laboratory animals, at least partially showed under in vitro studies. Several investigations were performed on laboratory rodents to evaluate how DMT1 may impact the transport of Mn in different tissues. In Fe deficiency anemia state, the uptake via olfactory epithelium of Mn was also upregulated through the DMT1 mechanism (Thompson et al. 2007). DMT1 is not essential for Mn or Cu transport but was in a mouse model found essential for Fe transport with intestinal DMT1 deficiency (Chen et al. 2019; Thompson et al. 2006). These findings bear a resemblance to the earlier data that DMT1 does not facilitate the transport of Mn over the BBB (Crossgrove and Yokel 2004).
In Mn transport, Fpn involvement was also established due to impaired metabolism of Mn in mice with Fpn deficiency (Gkouvatsos et al. 2012; Seo et al. 2016; Seo and Wessling-Resnick 2015). Interestingly, it was demonstrated an enhancement of the absorption of Mn in mice with HFE deficiency, while no significant difference in the tissue Mn distribution after metal injection or installation was detected (Kim et al. 2013). However, another in vivo investigation on Hfe−/− mice found that mitochondria of the liver contain significantly lower concentrations of Mn and elevated Fe levels compared to the level of Mn and Fe in wild-type controls (Jouihan et al. 2008). Animal experiments using rodents also showed that Tf works as one of the main Mn carriers in plasma without regard to the route of Mn exposure (Chen et al. 2018; Davidson and Lonnerdal 1989; Erikson and Aschner 2019). Consistently, numerous animal studies, which used hypotransferrinemic mice, demonstrated that normal Tf levels are essential for appropriate targeting of Mn and proper Mn distribution (Dickinson et al. 1996). Besides, in a study on mice, Mn–Tf appeared to be competing with Fe–Tf on the receptor binding sites of lactating mammary gland cells (Moutafchiev et al. 1998).
It has been detected that the rate at which Mn accumulates in the liver increases the sensitivity for toxic Mn effects in rats with dietary Fe deficiency (Amos-Kroohs et al. 2017; Chandra and Shukla 1976). These findings correspond with the results that were achieved by Wessling-Resnick et al., who established that Fe deficiency increases pulmonary absorption of Mn, while excess Fe has the reverse effect (Heilig et al. 2006; Thompson et al. 2006). Interestingly, Fe supplementation by intravenous (IV) or intraperitoneal (IP) injection leads to an increment of Mn and Fe content in the spleen and liver of the experimental rat (Thompson et al. 2006; Vayenas et al. 1998). In one study, to simulate chronic Mn exposure in rats, the effects of 30 days intraperitoneal MnCl2 injections were evaluated. Compared to the controls, the plasma Fe levels in the injected rats decreased with 32%, and their Fe levels in cerebrospinal fluid increased three-fold (Zheng et al. 1999). In turn, chronic exposure to Mn through injection of MnCl2 (30 mg/kg/day) in rats led to a significant increase in the ileum and liver Fe content (Zaloglu et al. 2002). In contrast, a high-dose Fe treatment decreased the absorption of Mn, which was also demonstrated in calves (Hansen et al. 2010).
Human studies have confirmed that interactions between Fe and Mn are tight, particularly in a Fe-deficient state. An investigation of Fe-deficient infants demonstrated a significantly increased Mn level in the blood, while 1–6 months Fe supplementation therapy significantly ameliorated Fe status and declined the Mn concentration (Park et al. 2013). These findings are parallel to the earlier study results in the status of Mn in adults and children suffering from Fe deficiency anemia (Kim et al. 2005; Smith et al. 2013). Korean National Health and Nutritional Examination Survey (KNHANES) in 2008 showed that the groups with low ferritin had significantly higher blood Mn concentrations, in both women and men, compared to groups with normal ferritin (Kim and Lee 2011). In the Nord-Trøndelag Health Study (HUNT 2), similar findings were found. In particular, elevated Mn blood levels were found in the low ferritin group (Meltzer et al. 2010).
Also, serum ferritin has played a vital role and acts as one of the main determinants of the Mn levels in the blood (Meltzer et al. 2010). The findings of these experimental and observational studies match well with a published case of manganism in a 5-year-old girl with contemporaneous Fe deficiency (Brna et al. 2011; Henn et al. 2011). Research has shown that genetic variation in the genes associated with Fe metabolism may significantly modify the status of Mn via their impact on Mn absorption, excretion as well as distribution (Henn et al. 2011).
Supplementary data were obtained on the Mn–Fe interaction in persons exposed to Mn at work. Particularly, in one study, it was shown that elevated levels of Mn in biosamples from welders were strongly related to lower Fe concentrations in erythrocyte and plasma (Bjørklund et al. 2017a; Cowan et al. 2009). An investigation of 241 welders revealed Fe deficiency only in a few persons (Pesch et al. 2012). In this study, there was not a significant association between the serum levels of ferritin and Mn. However, in another investigation, it was found that serum Mn showed an inverse relationship with Tf levels (Lu et al. 2005). Similarly, workers in Mn alloy production had lower levels of serum soluble TfR in comparison with unexposed controls, which indicate that exposure to Mn is strongly associated with higher intracellular levels of Fe (Chen et al. 2019; Ellingsen et al. 2003).
Neurotoxicity of manganese
Manganese effects in human physiological processes depend on the routes of exposure, dose, age, the period of exposure, environmental factors, and nutritional state, and the line between Mn-dependent biology and toxicity is thus blurred (Pfalzer and Bowman 2017). Since Mn plays a key role in brain growth and development, children are more vulnerable than adults in a U-shaped relationship where both deficiency and excessive absorption can cause deleterious outcomes (Lucchini et al. 2017). It is well known that chronic excessive Mn exposure can lead to various psychiatric, motor, and also cognitive disturbances. Early signs are usually of psychiatric nature (Nordberg et al. 2015). These include emotional instability, compulsive behavior, and in some cases, hallucinations. Neurological effects may be observed a few weeks after the initial symptoms. These may include bradykinesia, dystonia, disturbance of gait, and speech difficulties. The neurological presentation resembles Parkinson's disease. On the cellular level, manganism is linked to increased Mn concentrations, especially in the subthalamic nuclei. And in contrast to idiopathic Parkinson's disease, cases with Mn intoxication appear to have preserved nigrostriatal signaling pathway.
Furthermore, Lewy bodies are unusual to find in manganism (Bjørklund et al. 2017b; Bjørklund et al. 2018). Classical manganism seems to only occur in the case of occupational Mn exposure to large amounts of Mn-rich dust (Avila et al. 2013; Flynn and Susi 2010; Zoroddu et al. 2014). Manganese poisoning in humans may probably also be caused by overconsumption when Mn is used as a nutritional supplement, but such overexposure is insufficiently described. However, it is reported in children that ingestion of ≥ 0.241 mg Mn/L drinking water for at least three years can lead to poor school performance as determined by mastery in mathematics and their overall behavior in comparison to non-exposed children. Children who are exposed to Mn score more poorly than the controls on neurobehavioral tests (Menezes-Filho et al. 2011; Zhang et al. 1995). Other researchers have in children reported associations between decreased intelligence quotient (IQ) and Mn exposure from drinking water (Bouchard et al. 2010). In one study, the Mn content in drinking water was measured for 362 children (6–13 years old), who resided in an area where the drinking water gradient of Mn was specified. The model used in the study was adjusted for maternal intelligence, family income, as well as other confounders. A statistically significant link was found between IQ scores and the drinking water levels of Mn. The difference in IQ points was 6.2 between individuals who were exposed to low and high Mn levels in drinking water. The study concluded that elevated Mn in this cohort was closely linked to lower IQ scores and reduced achievement. However, the exposed children in the latter studies might have been exposed already during fetal life. Unfortunately, these reports did not include information on the Fe status of the children or their mothers.
Iron supplementation and manganese toxicity
Early investigations on healthy subjects not occupationally exposed to Mn have provided interesting data. Thus, iron supplementation as NaFe(III)EDTA did not cause significant changes in Mn absorption or urinary excretion in healthy adults (Davidson and Lonnerdal 1989). Correspondingly, modest Fe supplementation in healthy pregnant women did not affect the Mn status (Bjørklund et al. 2019; Flores-Quijano et al. 2019). However, the Mn absorption rate in the healthy young females was highest in the women who had low concentrations of serum ferritin. It has also been found that persons with high levels of ferritin on a diet that is low in Mn have a maximum half-life of Mn. Interestingly, research has shown that non-heme and dietary heme Fe have a different effect on the status of Mn (Finley 1999). Increased intake of non-heme Fe has a particularly negative effect on Mn level in the bioindication substrates, while no similar effect was detected for heme Fe (Chen et al. 2019; Davis and Greger 1992). In one study, 15 mg Mn/day in healthy women did not affect the Fe status. However, excessive Mn intake in a Fe-deficient condition may accelerate not only Fe deficiency but also the toxic potential of Mn (Erikson and Aschner 2019; Erikson et al. 2005).
Concluding remarks
Various types of food, such as legumes, nuts, tea, seafood, and plant-derived beverages, are considered as dietary sources of Mn. Deficiency of Mn can cause symptoms such as poor bone formation, reduced fertility, and weight loss. However, a more frequent condition appears to be precipitated by excessive exposure to Mn, leading to manganism. Multiple regulatory systems manage Mn transport in the body, usually providing adequate adaptive homeostasis and physiological responses. Both Mn- and Fe-specific transporters participate in the regulation of export and import of Mn. Modification of Fe status can induce changed expression of Fe transporters, which subsequently modifies Mn transport and Mn-related neurotoxicity. On the other hand, dietary Fe overload can increase the DMT1 expression, at least in animal studies, thereby inducing increased Mn uptake.
However, high levels of Fe in the diet usually help to reduce bioavailable Mn in duodenum and jejunum. In the future clinical and experimental evaluation of manganism, special attention should be paid to the interaction of Mn overexposure with Fe deficiency.
References
Aisen P, Aasa R, Redfield AG (1969) The chromium, manganese, and cobalt complexes of transferrin. J Biol Chem 244(17):4628–4633
Amos-Kroohs RM, Davenport LL, Atanasova N et al (2017) Developmental manganese neurotoxicity in rats: cognitive deficits in allocentric and egocentric learning and memory. Neurotoxicol Teratol 59:16–26
Aschner JL, Aschner M (2005) Nutritional aspects of manganese homeostasis. Mol Aspects Med 26(4–5):353–362
Aschner M, Vrana K, Zheng W (1999) Manganese uptake and distribution in the central nervous system (CNS). Neurotoxicology 20(2–3):173–180
Aschner JL, Anderson A, Slaughter JC et al (2015) Neuroimaging identifies increased manganese deposition in infants receiving parenteral nutrition. Am J Clin Nutr 102(6):1482–1489. https://doi.org/10.3945/ajcn.115.116285
Au C, Benedetto A, Aschner M (2008) Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology 29(4):569–576
Avila DS, Puntel RL, Aschner M (2013) Manganese in health and disease. Met Ions Life Sci 13:199–227. https://doi.org/10.1007/978-94-007-7500-8_7
Bannon DI, Abounader R, Lees PS, Bressler JP (2003) Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. Am J Physiol Cell Physiol 284(1):C44–C50
Bartnikas TB (2012) Known and potential roles of transferrin in iron biology. Biometals 25(4):677–686
Bjørklund G, Aaseth J, Skalny AV et al (2017a) Interactions of iron with manganese, zinc, chromium, and selenium as related to prophylaxis and treatment of iron deficiency. J Trace Elem Med Biol 41:41–53
Bjørklund G, Chartrand MS, Aaseth J (2017b) Manganese exposure and neurotoxic effects in children. Environ Res 155:380–384
Bjørklund G, Hofer T, Nurchi VM, Aaseth J (2019) Iron and other metals in the pathogenesis of Parkinson's disease: Toxic effects and possible detoxification. J Inorg Biochem 199:110717. https://doi.org/10.1016/j.jinorgbio.2019.110717
Bjørklund G, Stejskal V, Urbina MA, Dadar M, Chirumbolo S, Mutter J (2018) Metals and Parkinson's disease: mechanisms and biochemical processes. Curr Med Chem 25(19):2198–2214
Bo L-Y, Li T-J, Zhao X-H (2019) Effect of Cu/Mn-fortification on in vitro activities of the peptic hydrolysate of bovine lactoferrin against human gastric cancer BGC-823 cells. Molecules 24(7):1195
Bouchard MF, Sauvé S, Barbeau B et al (2010) Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect 119(1):138–143
Brna P, Gordon K, Dooley JM, Price V (2011) Manganese toxicity in a child with iron deficiency and polycythemia. J Child Neurol 26(7):891–894
Chandra SV, Shukla GS (1976) Role of iron deficiency in inducing susceptibility to manganese toxicity. Arch Toxicol 35(4):319–323
Chen P, Parmalee N, Aschner M (2014) Genetic factors and manganese-induced neurotoxicity. Front Genet 5:265. https://doi.org/10.3389/fgene.2014.00265
Chen P, Chakraborty S, Mukhopadhyay S et al (2015) Manganese homeostasis in the nervous system. J Neurochem 134(4):601–610. https://doi.org/10.1111/jnc.13170
Chen P, Bornhorst J, Aschner M (2018) Manganese metabolism in humans. Front Biosci 23:1655–1679
Chen P, Totten M, Zhang Z et al (2019) Iron and manganese-related CNS toxicity: mechanisms, diagnosis and treatment. Expert Rev Neurother 19(3):243–260. https://doi.org/10.1080/14737175.2019.1581608
Cowan DM, Fan Q, Zou Y et al (2009) Manganese exposure among smelting workers: blood manganese–iron ratio as a novel tool for manganese exposure assessment. Biomarkers 14(1):3–16
Critchfield JW, Keen CL (1992) Manganese+ 2 exhibits dynamic binding to multiple ligands in human plasma. Metabolism 41(10):1087–1092
Crooks DR, Ghosh MC, Braun-Sommargren M, Rouault TA, Smith DR (2007) Manganese targets m-aconitase and activates iron regulatory protein 2 in AF5 GABAergic cells. J Neurosci Res 85(8):1797–1809
Crossgrove JS, Yokel RA (2004) Manganese distribution across the blood–brain barrier III: the divalent metal transporter-1 is not the major mechanism mediating brain manganese uptake. Neurotoxicology 25(3):451–460
Davidson LA, Lonnerdal B (1989) Fe-saturation and proteolysis of human lactoferrin: effect on brush-border receptor-mediated uptake of Fe and Mn. Am J Physiol Gastrointest Liver Physiol 257(6):G930–G934
Davidsson L, Lönnerdal B, Sandström B, Kunz C, Keen CL (1989) Identification of transferrin as the major plasma carrier protein for manganese introduced orally or intravenously or after in vitro addition in the rat. J Nutr 119(10):1461–1464
Davis CD, Greger J (1992) Longitudinal changes of manganese-dependent superoxide dismutase and other indexes of manganese and iron status in women. Am J Clin Nutr 55(3):747–752
de Water E, Proal E, Wang V et al (2018) Prenatal manganese exposure and intrinsic functional connectivity of emotional brain areas in children. Neurotoxicology 64:85–93
DeWitt MR, Chen P, Aschner M (2013) Manganese efflux in Parkinsonism: insights from newly characterized SLC30A10 mutations. Biochem Biophys Res Commun 432(1):1–4
Dickinson T, Devenyi A, Connor J (1996) Distribution of injected iron 59 and manganese 54 in hypotransferrinemic mice. J Lab Clin Med 128(3):270–278
Dion L-A, Saint-Amour D, Sauvé S, Barbeau B, Mergler D, Bouchard MF (2018) Changes in water manganese levels and longitudinal assessment of intellectual function in children exposed through drinking water. Neurotoxicology 64:118–125
Ellingsen DG, Haug E, Ulvik RJ, Thomassen Y (2003) Iron status in manganese alloy production workers. J Appl Toxicol Int J 23(4):239–247
Erikson KM, Aschner M (2019) Manganese: Its Role in Disease and Health. Essent Metals Med Ther Use Tox Metal Ions Clin 19:253
Erikson KM, Syversen T, Aschner JL, Aschner M (2005) Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ Toxicol Pharmacol 19(3):415–421
Finley JW (1999) Manganese absorption and retention by young women is associated with serum ferritin concentration. Am J Clin Nutr 70(1):37–43
Fitsanakis VA, Piccola G, Marreilha dos Santos AP, Aschner JL, Aschner M (2007) Putative proteins involved in manganese transport across the blood-brain barrier. Hum Exp Toxicol 26(4):295–302. https://doi.org/10.1177/0960327107070496
Fitsanakis VA, Zhang N, Garcia S, Aschner M (2010) Manganese (Mn) and iron (Fe): interdependency of transport and regulation. Neurotox Res 18(2):124–131
Flores-Quijano ME, Vega-Sánchez R, Tolentino-Dolores MC et al (2019) Obesity is associated with changes in iron nutrition status and its homeostatic regulation in pregnancy. Nutrients 11(3):693
Flynn MR, Susi P (2010) Manganese, iron, and total particulate exposures to welders. J Occup Environ Hyg 7(2):115–126. https://doi.org/10.1080/15459620903454600
Garcia SJ, Gellein K, Syversen T, Aschner M (2006) A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicol Sci 92(2):516–525
Garrick MD, Dolan KG (2002) An expression system for a transporter of iron and other metals. Methods Mol Biol 196:147–154. https://doi.org/10.1385/1-59259-274-0:147
Garrick MD, Singleton ST, Vargas F et al (2006) DMT1: which metals does it transport? Biol Res 39(1):79–85
Genter MB, Kendig EL, Knutson MD (2009) Uptake of materials from the nasal cavity into the blood and brain. Ann NY Acad Sci 1170(1):623–628. https://doi.org/10.1111/j.1749-6632.2009.03877.x
Gitler AD, Chesi A, Geddie ML et al (2009) Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet 41(3):308–315. https://doi.org/10.1038/ng.300
Gkouvatsos K, Papanikolaou G (1820) Pantopoulos K (2012) Regulation of iron transport and the role of transferrin. Biochim Biophys Acta 3:188–202
Gunshin H, Mackenzie B, Berger UV et al (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388(6641):482
Gunter TE, Gerstner B, Gunter KK et al (2013) Manganese transport via the transferrin mechanism. Neurotoxicology 34:118–127
Hansen S, Ashwell M, Moeser A, Fry R, Knutson M, Spears J (2010) High dietary iron reduces transporters involved in iron and manganese metabolism and increases intestinal permeability in calves. J Dairy Sci 93(2):656–665
Harris WR, Chen Y (1994) Electron paramagnetic resonance and difference ultraviolet studies of Mn2+ binding to serum transferrin. J Inorg Biochem 54(1):1–19
Heilig EA, Thompson KJ, Molina RM, Ivanov AR, Brain JD, Wessling-Resnick M (2006) Manganese and iron transport across pulmonary epithelium. Am J Physiol Lung Cell Mol Physiol 290(6):L1247–L1259
Hellman NE, Gitlin JD (2002) Ceruloplasmin metabolism and function. Annu Rev Nutr 22(1):439–458
Henn BC, Kim J, Wessling-Resnick M et al (2011) Associations of iron metabolism genes with blood manganese levels: a population-based study with validation data from animal models. Environ Health 10(1):97
Jouihan HA, Cobine PA, Cooksey RC et al (2008) Iron-mediated inhibition of mitochondrial manganese uptake mediates mitochondrial dysfunction in a mouse model of hemochromatosis. Mol Med 14(3–4):98
Ke Y, Chang YZ, Duan XL et al (2005) Age-dependent and iron-independent expression of two mRNA isoforms of divalent metal transporter 1 in rat brain. Neurobiol Aging 26(5):739–748. https://doi.org/10.1016/j.neurobiolaging.2004.06.002
Kim Y, Lee B-K (2011) Iron deficiency increases blood manganese level in the Korean general population according to KNHANES 2008. Neurotoxicology 32(2):247–254
Kim Y, Park JK, Choi Y et al (2005) Blood manganese concentration is elevated in iron deficiency anemia patients, whereas globus pallidus signal intensity is minimally affected. Neurotoxicology 26(1):107–111
Kim J, Buckett PD, Wessling-Resnick M (2013) Absorption of manganese and iron in a mouse model of hemochromatosis. PLoS ONE 8(5):e64944
Kim G, Lee HS, Seok Bang J, Kim B, Ko D, Yang M (2015) A current review for biological monitoring of manganese with exposure, susceptibility, and response biomarkers. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 33(2):229–254. https://doi.org/10.1080/10590501.2015.1030530
Kwik-Uribe C, Smith DR (2006) Temporal responses in the disruption of iron regulation by manganese. J Neurosci Res 83(8):1601–1610
Kwik-Uribe CL, Reaney S, Zhu Z, Smith D (2003) Alterations in cellular IRP-dependent iron regulation by in vitro manganese exposure in undifferentiated PC12 cells. Brain Res 973(1):1–15
Li GJ, Zhao Q, Zheng W (2005) Alteration at translational but not transcriptional level of transferrin receptor expression following manganese exposure at the blood–CSF barrier in vitro. Toxicol Appl Pharmacol 205(2):188–200
Lu L, Zhang L-l, Li GJ, Guo W, Liang W, Zheng W (2005) Alteration of serum concentrations of manganese, iron, ferritin, and transferrin receptor following exposure to welding fumes among career welders. Neurotoxicology 26(2):257–265
Lucchini R, Placidi D, Cagna G et al (2017) Manganese and Developmental Neurotoxicity Adv Neurobiol 18:13–34. https://doi.org/10.1007/978-3-319-60189-2_2
Madejczyk MS (1818) Ballatori N (2012) The iron transporter ferroportin can also function as a manganese exporter. Biochim Biophys Acta 3:651–657
Meltzer HM, Brantsæter AL, Borch-Iohnsen B et al (2010) Low iron stores are related to higher blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT 2 study. Environ Res 110(5):497–504
Mena I, Marin O, Fuenzalida S, Cotzias GC (1967) Chronic manganese poisoning. Neurology 17(2):128–136
Menezes-Filho JA, Novaes Cde O, Moreira JC, Sarcinelli PN, Mergler D (2011) Elevated manganese and cognitive performance in school-aged children and their mothers. Environ Res 111(1):156–163. https://doi.org/10.1016/j.envres.2010.09.006
Mitchell CJ, Shawki A, Ganz T, Nemeth E, Mackenzie B (2013) Functional properties of human ferroportin, a cellular iron exporter reactive also with cobalt and zinc. Am J Physiol Cell Physiol 306(5):C450–C459
Moshtaghi AA, Badiei A, Hasanzadeh T (1997) Role of ceruloplasmin and ethanolamine in manganese binding to human serum apo-transferrin. Iran J Sci Technol Trans B- Eng 21(2):157–168
Moutafchiev D, Sirakov L, Bontchev P (1998) The competition between transferrins labeled with59Fe, 65Zn, and54Mn for the binding sites on lactating mouse mammary gland cells. Biol Trace Elem Res 61(2):181–191
Nordberg GF, Fowler BA, Nordberg M (2015) Handbook on the toxicology of metals, 4th edn. Elsevier, Academic Press, Amsterdam
Oshiro S, Nozawa K, Hori M et al (2002) Modulation of iron regulatory protein-1 by various metals. Biochem Biophys Res Commun 290(1):213–218
Pang L, Wang J, Huang W, Guo S (2015) A study of divalent metal transporter 1 and ferroportin 1 in brain of rats with manganese-induced parkinsonism. Chin J Ind Hyg Occup Dis (Zhonghua lao dong wei sheng zhi ye bing za zhi=Zhonghua laodong weisheng zhiyebing zazhi) 33(4):250–254
Pantopoulos K (2004) Iron metabolism and the IRE/IRP regulatory system: an update. Ann NY Acad Sci 1012(1):1–13
Park B-Y, Chung J (2008) Effects of various metal ions on the gene expression of iron exporter ferroportin-1 in J774 macrophages. Nutr Res Pract 2(4):317–321
Park S, Sim C-S, Lee H, Kim Y (2013) Blood manganese concentration is elevated in infants with iron deficiency. Biol Trace Elem Res 155(2):184–189
Parkkila S, Niemelä O, Britton RS et al (2001) Molecular aspects of iron absorption and HFE expression. Gastroenterology 121(6):1489–1496
Peres TV, Schettinger MR, Chen P et al (2016) Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol Toxicol 17(1):57. https://doi.org/10.1186/s40360-016-0099-0
Peres TV, Horning KJ, Bornhorst J, Schwerdtle T, Bowman AB, Aschner M (2019) Small molecule modifiers of in vitro manganese transport alter toxicity in vivo. Biol Trace Elem Res 188(1):127–134
Pesch B, Weiss T, Kendzia B et al (2012) Levels and predictors of airborne and internal exposure to manganese and iron among welders. J Eposure Sci Environ Epidemiol 22(3):291
Pfalzer AC, Bowman AB (2017) Relationships between essential manganese biology and manganese toxicity in neurological disease. Curr Environ Health Rep 4(2):223–228. https://doi.org/10.1007/s40572-017-0136-1
Pivina L, Semenova Y, Doşa MD, Dauletyarova M, Bjørklund G (2019) Iron deficiency, cognitive functions, and neurobehavioral disorders in children. J Mol Neurosci 68(1):1–10. https://doi.org/10.1007/s12031-019-01276-1
Remelli M, Peana M, Medici S, Ostrowska M, Gumienna-Kontecka E, Zoroddu MA (2016) Manganism and Parkinson's disease: Mn(II) and Zn(II) interaction with a 30-amino acid fragment. Dalton Trans 45(12):5151–5161. https://doi.org/10.1039/c6dt00184j
Rodrigues JL, Araújo CF, dos Santos NR et al (2018) Airborne manganese exposure and neurobehavior in school-aged children living near a ferro-manganese alloy plant. Environ Res 167:66–77
Roth JA, Garrick MD (2003) Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem Pharmacol 66(1):1–13
Sarkar S, Malovic E, Jin H, Kanthasamy A, Kanthasamy AG (2019) The role of manganese in neuroinflammation. Role Inflamm Environ Neurotox 3:121
Seo YA, Wessling-Resnick M (2015) Ferroportin deficiency impairs manganese metabolism in flatiron mice. FASEB J 29(7):2726–2733
Seo YA, Elkhader JA, Wessling-Resnick M (2016) Distribution of manganese and other biometals in flatiron mice. Biometals 29(1):147–155
Smith EA, Newland P, Bestwick KG, Ahmed N (2013) Increased whole blood manganese concentrations observed in children with iron deficiency anaemia. J Trace Elem Med Biol 27(1):65–69
Suárez N, Eriksson H (1993) Receptor-mediated endocytosis of a manganese complex of transferrin into neuroblastoma (SHSY5Y) cells in culture. J Neurochem 61(1):127–131
Thompson K, Molina R, Donaghey T, Brain JD, Wessling-Resnick M (2006) The influence of high iron diet on rat lung manganese absorption. Toxicol Appl Pharmacol 210(1–2):17–23
Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M (2007) Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J 21(1):223–230
Vayenas D, Repanti M, Vassilopoulos A, Papanastasiou D (1998) Influence of iron overload on manganese, zinc, and copper concentration in rat tissues in vivo: study of liver, spleen, and brain. Int J Clin Lab Res 28(3):183–186
Venkataramani V, Doeppner TR, Willkommen D et al (2018) Manganese causes neurotoxic iron accumulation via translational repression of amyloid precursor protein and H-Ferritin. J Neurochem 147(6):831–848. https://doi.org/10.1111/jnc.14580
Vincent JB (1820) Love S (2012) The binding and transport of alternative metals by transferrin. Biochim Biophys Acta 3:362–378
Wang X, Li GJ, Zheng W (2006) Upregulation of DMT1 expression in choroidal epithelia of the blood–CSF barrier following manganese exposure in vitro. Brain Res 1097(1):1–10
Ye Q, Park JE, Gugnani K, Betharia S, Pino-Figueroa A, Kim J (2017) Influence of iron metabolism on manganese transport and toxicity. Metallomics 9(8):1028–1046
Yin Z, Jiang H, Lee ESY et al (2010) Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J Neurochem 112(5):1190–1198
Zaloglu N, Yildirim G, Bastug M, Koc E, Ficicilar H, Sayal A (2002) High dosage of manganese chloride application and iron zinc copper status in rats. Trace Elem Electrolytes 19(3):138–142
Zhang G, Liu D, He P (1995) Effects of manganese on learning abilities in school children. Zhonghua yu fang yi xue za zhi [Chin J Prev Med] 29(3):156–158
Zheng W, Zhao Q (2001) Iron overload following manganese exposure in cultured neuronal, but not neuroglial cells. Brain Res 897(1–2):175–179
Zheng W, Zhao Q, Slavkovich V, Aschner M, Graziano JH (1999) Alteration of iron homeostasis following chronic exposure to manganese in rats1. Brain Res 833(1):125–132
Zoroddu MA, Medici S, Ledda A, Nurchi VM, Lachowicz JI, Peana M (2014) Toxicity of nanoparticles. Curr Med Chem 21(33):3837–3853. https://doi.org/10.2174/0929867321666140601162314
Zoroddu MA, Aaseth J, Crisponi G, Medici S, Peana M, Nurchi VM (2019) The essential metals for humans: a brief overview. J Inorg Biochem 195:120–129. https://doi.org/10.1016/j.jinorgbio.2019.03.013
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bjørklund, G., Dadar, M., Peana, M. et al. Interactions between iron and manganese in neurotoxicity. Arch Toxicol 94, 725–734 (2020). https://doi.org/10.1007/s00204-020-02652-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-020-02652-2