Abstract
Highly hydrophobic compounds like petroleum and their byproducts, once released into the environment, can persist indefinitely by virtue of their ability to resist microbial degradation, ultimately paving the path to severe environmental pollution. Likewise, the accumulation of toxic heavy metals like lead, cadmium, chromium, etc., in the surroundings poses an alarming threat to various living organisms. To remediate the matter in question, the applicability of a biosurfactant produced from the mangrove bacterium Bacillus pumilus NITDID1 (Accession No. KY678446.1) is reported here. The structural characterization of the produced biosurfactant revealed it to be a lipopeptide and has been identified as pumilacidin through FTIR, NMR, and MALDI-TOF MS. The critical micelle concentration of pumilacidin was 120 mg/L, and it showed a wide range of stability in surface tension reduction experiments under various environmental conditions and exhibited a high emulsification index of as much as 90%. In a simulated setup of engine oil-contaminated sand, considerable oil recovery (39.78%) by this biosurfactant was observed, and upon being added to a microbial consortium, there was an appreciable enhancement in the degradation of the used engine oil. As far as the heavy metal removal potential of biosurfactant is concerned, as much as 100% and 82% removal was observed for lead and cadmium, respectively. Thus, in a nutshell, the pumilacidin produced from Bacillus pumilus NITDID1 holds promise for multifaceted applications in the field of environmental remediation.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine oil spills are a planetary concern as they can dramatically hamper the soil and aquatic ecology and seriously threaten human health (Patowary et al. 2017; Zhou et al. 2023). Annually a million gallon of waste oil are released untreated into the environment from various workshops and industrial sectors, especially the automobile industries (Sharma and Pandey 2020). Such oil contains several toxic and carcinogenic petroleum hydrocarbons (Muthukamalam et al. 2017; Zargar et al. 2022). Therefore, the remediation of oil pollution is the need of the hour to combat and prevent the consequential environmental hazards associated with oil spills. Another major environmental peril that comes into the picture is pollution due to inorganic heavy metals. With the rapid increase in industrialization, the unchecked release of heavy metals like arsenic, lead, mercury, cadmium, chromium, and others into soil and water bodies presents a significant health risk for humans and animals (Das et al. 2009; Mishra et al. 2021). In order to mitigate these environmental problems, commercially available traditional surfactants such as linear alkyl benzene sulfonates (LAS) are currently in use. However, since these conventional chemical treatments are efficient only at high concentrations, their application and reliability are questionable from the viewpoint of ecological toxicity. Such compounds cause respiratory problems and reduce the resistance of aquatic organisms to environmental stress (Badmus et al. 2021; Collivignarelli et al. 2019). Keeping this in mind, the scientific community has focused its interest on environmental remediation via safer means. In this context, biological surfactants play a crucial role in various remediation purposes as they are easily degraded in the surroundings and are considered eco-friendly as compared to chemical surfactants(Joy et al. 2017).
Biosurfactants are bioactive metabolites produced by microorganisms like yeast, fungi, and bacteria (Zhang et al. 2016). They are amphipathic molecules that can accumulate at the water–oil or air–water interface, thus reducing interfacial or surface tensions (Khopade et al. 2012). Biosurfactants are grouped into glycolipids, lipopeptides, phospholipids, neutral lipids,and fatty acids. Bacillus strains can produce a broad range of cyclic lipopeptides, including surfactins, fengycins, and iturin (Jemil et al. 2017). Biosurfactants have a wide range of applications in the field of bioremediation of pollutants. They improve the solubility and biodegradability of petroleum hydrocarbons, especially polycyclic aromatic hydrocarbons (Bezza and Chirwa 2016). A very recent research work of Zhang et al. (2022) revolves around oil degradation by biosurfactant obtained from Bacillus siamensis HoB-1. In another work, Sharma et al.(2019) have studied crude oil degradation by biosurfactant produced from Agrobacterium fabrum SLAJ731, isolated from the oil field regions of Assam. Similarly, biosurfactants from Bacillus subtilis BR-15 and Bacillus amyloliquefaciens SAS-1 showed efficient supplementation of engine oil degradation with microbial consortia in the experimental study by Sharma et al. (2018). Nayak et al. (2020) investigated engine oil biodegradation by the biosurfactant derived from Bacillus licheniformis LRK1. Biosurfactants can also chelate heavy metal ions; hence, they may be a promising alternative for the remediation of heavy metals in contaminated areas (Gomaa and El-Meihy 2019). Sun et al. (2021) have studied the remediation of lead, copper and cadmium by the action of biosurfactant produced by Pseudomonas sp. CQ 2, a bacterium isolated from Chonqing coalfield of China. Chen et al. (2021) described the remediation of mercury and lead by employing a rhamnolipid biosurfactant isolated from the bacterium Pseudomonas aeruginosa ASW-4.
It may be noted here that an extensive, in-depth study of the available reports on this subject indicated that all of the research works in the area of pumilacidin biosurfactants center around the biomedical potential of the same. The environmental remediation potential of pumilacidin has not been explored yet. Thus, this research gap has been exploited to tap the unrealized potential of pumilacidin in environmental applications like remediation of heavy metals, enhanced oil recovery, and facilitation of bioremediation of used engine oil. The present study delves into the characterization and environmental application of pumilacidin lipopeptide biosurfactant, produced by Bacillus pumilus NITDID1, isolated from mangrove soil of Jambudwip, Sundarban (West Bengal, India). The produced biosurfactant has been characterized by different spectroscopy techniques and studied further for critical micelle concentration (CMC) determination.
Materials and methods
Chemicals and reagent
Used engine oil was procured from a nearby automobile garage. The crude glycerol was obtained from Emami Agrotech Ltd., Haldia, West Bengal, India.
Sample collection, isolation, and screening of biosurfactant-producing bacteria
The soil sample was collected from a depth of 15 cm of the Jambudwip mangrove forest area (21.5811°N, 88.1828°E), West Bengal, India. The soil sample was immediately sieved through a 2 mm mesh, and the sieved fraction was sealed in a sterile container and transported to the laboratory. After that, 10 g of homogenized soil sample was suspended in 90 ml of distilled water to obtain a suspension which was incubated at 120 rpm in a shaker incubator at 30ºC for 10 min at room temperature, followed by serial dilution up to 10–6 and spread on nutrient agar (HiMedia, Mumbai). All the plates were incubated for 48 h at 30 °C. At the end of the incubation period, all morphologically distinct bacteria were sub-cultured and stored at 4 °C for further analysis.
All the isolates were screened for biosurfactant production by inoculating them in a minimal salt medium (MSM) containing 4% (v/v) crude glycerol and incubated under shaking conditions for 72 h at 30 °C and 120 rpm. After incubation, cells were removed from the broth by centrifugation at 10,000 rpm for 30 min, and the supernatant was used for high throughput screening process, which included Oil spreading assay (Sharma et al. 2022), Para film M test (Yalçın et al. 2018), and Emulsification index determination (Cooper and Goldenberg 1987). The oil spreading method is an important screening test for biosurfactant production in which the production of the biosurfactant is determined by the presence of a central clear zone formed after the addition of biosurfactant to an oil drop (Hassanshahian 2014). The diameter of the clear zone formed is proportional to the effectiveness of the biosurfactant. The emulsification test is a simple quantitative method for investigating biosurfactant-producing microorganisms. An emulsification index (E24) greater than 50% was used as a selection criterion for biosurfactant-producing isolates (Oliveira et al. 2021). The detailed protocol of the above-mentioned screening methods has been provided in the supplementary file. For all the methods, sodium dodecyl sulfate (SDS) and uninoculated MSM were the respective positive and negative controls.
Identification of potent biosurfactant-producing bacteria
The potent isolate showing significant biosurfactant activity was further identified by morphological and molecular characterization techniques. Morphological characterization was done by gram staining (HiMedia, India) and scanning electron microscopy (SEM) according to the method described previously by Sharma et al. (2022). 16S rRNA sequencing was done according to method described by Sharma and Singh (2005) and Fatty Acid Methyl Ester (FAME) analysis were done according to the method developed by Sasser (2001). The isolate was routinely grown in Nutrient Agar (NA) medium and preserved as glycerol stock (20%) at −80 °C (Tekinay et al. 2015).
Biosurfactant production and recovery
The bacterium Bacillus pumilus NITDID1 was grown overnight in Nutrient Broth (NB) media at 30 ºC. 1% of cell suspension from this overnight culture was then inoculated in mineral salt medium (MSM) supplemented with crude glycerol 4% (v/v) and incubated at 30 ºC for 96 h with an agitation of 120 rpm (Bezza and Chirwa 2015). After incubation, the pH was adjusted to 7, and the cell-free supernatant was obtained by centrifugation (8000 rpm at 4 ºC for 20 min). The crude biosurfactant was precipitated from the cell-free supernatant by adjusting the pH to 2.0 using 6N HCl and allowing overnight incubation at 4 ºC. The acid precipitate was then recovered by centrifugation (8000 rpm at 4 ºC for 20 min) and washed with alkaline water to accomplish final pH of 7.0. The acid precipitate was then extracted by chloroform:methanol (2:1). The solvent from this suspension was evaporated, and the residue was dissolved in methanol and filtered through a 0.22 µm Whatman syringe filter. The crude biosurfactant extract was purified through a Silica gel column (silica gel 60) (Bezza and Chirwa 2015). The sample was loaded in a column and eluted with chloroform first to get rid of the yellow pigment and impurities of the hydrocarbons. Following the elution of these undesired components, the first fraction was eluted with a mixture of chloroform: methanol 80:20 (v/v). After that, the second fraction was eluted with chloroform: methanol 35:65. Both eluted fractions were collected, and the solvents were evaporated from the same. The fraction showing biosurfactant activity in drop collapse assay, was selected for further characterization.
Structural characterization of the biosurfactant
The functional groups of the purified biosurfactant were determined using FTIR and 1HNMR. For FTIR spectroscopy, a translucent pellet was formed by mixing and pressing the biosurfactant sample with potassium bromide (KBr). The resultant pellet was used for infrared scanning from 400–4000 cm−1in FTIR (Perkin-Elmer) (Shibulal et al. 2013). For NMR evaluation, the biosurfactant was dissolved in deuterated chloroform (CDCl3), and the 1HNMR spectra were recorded at 25 °C using a Bruker Avance 400 spectrometer. The chemical shifts were expressed in parts per million (ppm) downfield from internal standard tetramethylsilane (TMS) (Mani et al. 2016). For determination of molecular weight of the purified biosurfactant MALDI-TOF mass spectrometry (MS) was employed ) (Bruker Daltonics flex Analysis). For this purpose, an equal amount of biosurfactant and matrix solution were mixed together. The matrix solution contained 0.1% α cyano-4-hydroxycinnamic acid in acetonitrile and TFA; mixed in the ratio 50:50:0.01, v/v/v (Mani et al. 2016). The mixture solution was then spotted in the sample plate, dried, and analyzed in the mass range of 1000 Da to 1500 Da.
Thermogravimetric (TG) analysis
In order to study the effect of high temperatures on the structural integrity of this biosurfactant, the weight loss of the biosurfactant with respect to temperature was analyzed in a thermogravimetric (TG) analyzer (Perkin Elmer thermal analysis system). The platinum pan loaded with 4.5 mg of biosurfactant was heated under a nitrogen stream atmosphere with a gradual increase of 20 °C/min. The weight (%) was plotted against temperatures from 30 to 800 °C to obtain a TG thermogram.
Determination of critical micelle concentration (CMC)
CMC is described as the biosurfactant concentration at which micelles are formed, and no additional consequence can be observed on the surface activity (Hentati et al. 2019). The purified biosurfactant was dissolved at different concentrations (0 to 200 mg/L) in sterilized distilled water at 25 °C by the Du-Nouy ring method using a LAUDA digital surface tensiometer (Lauda-Koenigshofen, Germany). The CMC value of the biosurfactant produced from NITDID1 was determined according to Khopade et al. (2012).
Stability studies of the biosurfactant
The biosurfactant stability was determined under a broad range of temperatures, pH, and salt concentrations. Unless specified, the experiments were conducted with a biosurfactant concentration of 1X CMC at 30 °C, pH 7.0, and without NaCl supplementation. The thermal stability test was conducted by incubating the biosurfactant for 2 h at a temperature ranging from 15 to 90 °C, and the surface tension was measured after the biosurfactant solution was cooled down to 30 °C. To conduct the salinity test, different concentrations of NaCl solution ranging from 2.5% to 10% (w/v) were added to the biosurfactant solution. The biosurfactant’s stability was also examined against a broad range of pH. The pH of the biosurfactant suspension was adjusted to different values ranging from 3 to 11, and surface tension was measured to determine stability.
Emulsification (E24) Index
In a clean glass tube, 3 ml of the biosurfactant solution (1 X CMC) was mixed with 3 ml of used engine oil. Then the mixtures were vortexed and incubated for 24 h. The E24 index was calculated using Cooper and Goldenberg's formula (Cooper and Goldenberg 1987).
Environmental application of the biosurfactant
Application of biosurfactant in used engine oil removal from sand
Soil washing
For this experiment, 100 g of sand was added to 10 g of used engine oil in a conical flask and aged for 24 h under shaking condition which caused the oil to gain entry inside the sand particles. 10 g of this used engine oil-impregnated sand was supplemented with 10 ml of different concentrations of biosurfactant (0.5, 1, and 2 g/L), and sterile water was used as a control. The samples were incubated overnight and then centrifuged to determine the phase separation of the sand particles and biosurfactant emulsified oil (Gudiña et al. 2015). The quantity of oil recovered after the biosurfactant was determined gravimetrically, which is the actual amount of oil extracted from sand by hexane (Liu et al. 2014).
Sand pack column
The sand pack column experiment was performed according to the method described by Das and Kumar (2018) to investigate the oil recovery ability of pumilacidin from the sand matrix. Briefly, 100 g of acid-washed sand was packed into a glass column (40 × 2.5 cm). Subsequently, brine solution (5% NaCl (w/v)) was passed through the sand pack column, and the pore volume (PV) was calculated by determining the amount of brine necessary to wet the sand matrix. Three PVs of brine solution were run through the sand-pack column to ensure complete saturation. Under constant pressure, the used engine oil was injected into the column, replacing the brine solution from the sand matrix. The amount of brine released from the column was collected and quantified to determine the initial oil saturation. The oil-saturated column was washed with brine solution until no more oil was released in the effluent. The amount of oil retained in the sand matrix after the brine wash was determined on the basis of the amount of oil injected and released in the effluent. The pumilacidin was then used to enhance the recovery of the oil residing in the sand matrix even after the brine wash. 0.6 PV of biosurfactant (1 g/L) was injected into the column and allowed to stand for 24 h. After incubation, the column was washed with the brine solution, and the eluent was collected. The residual oil was extracted from the eluent with n-hexane and evaporated to quantify the amount of residual oil. The oil recovery percentage was calculated using the formulas described by Suthar et al. (2008).
Biosurfactant enhanced biodegradation of used engine oil
Oil-contaminated soil was collected from the Andal diesel shed, Andal, West Bengal, India (23.5815° N, 87.1910° E). The enrichment culture technique isolated the oil-degrading bacterial consortium (Bezza and Chirwa 2015). The bacterial consortium was grown in Bushnell Hass Medium (BHM) supplemented with used engine oil (2% (v/v) as a carbon source in a flask and incubated at 30 °C for 7 days under shaking conditions at 120 rpm. The culture broth was centrifuged after incubation, and the pellets were resuspended in BHM to make a uniform bacterial suspension of OD600nm 0.5. To examine the role of biosurfactants in improving the biodegradation of used engine oil, the following three different experimental setups were created:
-
(i)
Bacterial suspension (2% v/v) + used engine oil (2% v/v) + BHM;
-
(ii)
Bacterial suspension (2% v/v) + used engine oil (2% v/v) + BHM + biosurfactant (1 X CMC)
-
(iii)
Used engine oil (2%, v/v) + BHM (acts as Uninoculated control)
The conical flasks containing the above-mentioned samples were incubated for 21 days in an orbital shaker at 30 °C. After the incubation period, the experimental samples were centrifuged to separate the biomass. The organic fractions were extracted by adding an equal volume of hexane and further diluted before analysis. The samples were analyzed with Gas Chromatography (GC) (Agilent, USA) equipped with a flame ionization detector (FID) (Ben Ayed et al. 2015). The percentage of degradation was calculated using the following equation
Metal chelating property of the biosurfactant
For this experiment, 20 ml of biosurfactant of 0.5 g/L concentration was incubated with different concentrations of lead and cadmium (100, 500, and 1000 ppm) for 24 h in three respective centrifuge tubes. After incubation, the solution was centrifuged at 10,000 rpm for 30 min to separate the metal-biosurfactant co-precipitate (Ravindran et al. 2020). The supernatant was analyzed through atomic absorption spectroscopic (AAS) to determine the residual quantities of the heavy metals. Heavy metal removal efficiencies (R) were calculated using the following equation
Phytotoxicity assay of biosurfactant
Different concentrations of the biosurfactant (0.5X, 1X, 2X, and 5X CMC) were prepared with sterile distilled water, which was also used as a negative control. SDS at the concentration of 2 mg/ml (amount equal to CMC) was used as a positive control. 5 ml of every concentration of biosurfactant solutions of varying concentrations, positive control, and negative control were inoculated in glass petri dishes containing 10 seeds of cabbage (Brassica sp.) and covered with filter paper (Whatman no. 1). After 5 days of incubation, seed germination, root elongation, and the germination index were recorded (Araújo et al. 2019).
Statistical analysis
All the experiments except structural characterization were performed done in triplicate, and biodegradation of used engine oil was performed in duplicate. The value represents the mean ± standard deviation (SD). The t-test was used to determine significant differences.
Results
Isolation and screening of biosurfactant-producing bacteria
Twenty seven morphologically distinct bacterial colonies have been isolated from a mangrove soil sample of one of the archipelagoes of Sundarban, named Jambudwip. All the isolates have been analyzed for high throughput screening of biosurfactant production which includes tests like oil spreading assay, parafilm M test, and emulsification index determination (Table. S1). Isolate NITDID1 showed the highest diameter of a clear zone (76 ± 0.5 mm) on the oil surface in the oil spreading assay result (Fig. S1), and for the emulsification index test, the E24 value of NITDID1 was 78 ± 0.2% (Fig. S1). So, based on the screening methods, NITDID1 was selected for further characterization.
Identification of biosurfactant-producing bacterium
The morphological analysis by gram staining revealed that the isolate NITDID1 was gram-positive with a rod-like appearance (Fig. S2a). The SEM image showed the length of these bacterial cells to be over 1 μm (Fig. S2b). 1.2% Agarose gel showed a single 1500 bp of 16S rDNA amplicon, and the obtained 16S rRNA gene sequence was deposited in GenBank (accession number KY678446.1). BLAST analysis and phylogenetic tree construction (Fig.S3) for NITDID1 showed maximum sequence similarity (> 99%) to members of the Bacillus pumilus. The cellular fatty acid composition was determined by FAME analysis. The major cellular fatty acids in the strain Bacillus pumilus NITDID1 were 15:0 iso (52.26%) and 15:0 anteiso (30.04%). Other fatty acids present were 17:0 anteiso (3.27%), 17:0 iso (3.12%), 16:0 iso (2.65%), etc. (Table. S2).
Characterization of the biosurfactant
The biosurfactant was subjected to structural characterization, TG analysis, determination of CMC, emulsification index, and stability studies.
Structural characterization
Figure 1a indicates the FT-IR spectra of the biosurfactant produced by strain NITDID1. The peaks between 3400 to 3200 cm−1 were due to the stretching of N–H and O–H bonds, which indicated the presence of amide and hydroxyl groups. The peaks between 2956 and 2853 cm−1 were due to the stretching of C-H bond, indicating the presence of an aliphatic chain (Li et al. 2016). The peak at 1648.40 cm−1 showed the presence of the CO–N bond, which indicated the existence of peptide moieties, and a peak at 1542.90 cm−1 revealed the stretching of the C-N bond (Abdeshahian et al. 2016). The characteristic peaks between 1450.4 cm−1and 1401.10 cm−1 suggested the presence of C = O (Chittepu 2019).
Figure 1b indicated the 1H NMR spectra of the biosurfactant produced by strain NITDID1. Signals of 0.8–2.0 ppm suggested the presence of hydrocarbon chains (Saggese et al. 2018).Signals around 7.0 ppm suggest N–H protons, which, together with signals at about 4.0 ppm, indicated a peptide backbone (Sharma et al. 2019). Therefore, from the above findings, it can be concluded that the produced biosurfactant from strain NITDID1 is a lipopeptide by nature.
MALDI TOF spectroscopy analysis
The MALDI-TOF spectra of the lipopeptide biosurfactants produced by strain NITDID1are depicted in Fig. 2. The range indicated the presence of five prominent sodium adduct peaks at m/z 1044.69, 1058.70, 1072.72, 1086.73 Da, and 1100.75 Da. Each peak is assigned to different isoforms of pumilacidin, indicating multiple isoforms of pumilacidin produced from B. pumilus NITDID1. Peaks at m/z of 1044.66 Da can be assigned to pumilacidin with fatty acid chain (F.A.) length of 13 or 14 carbon atoms;1058.70 Da for F.A. length of 14 or 15 carbon atoms; 1072.72 Da for F.A. length of 15 or 16 carbon atoms; 1086.73 Da for F.A. length of 16 or 17 carbon atoms; and 1100.75 Da for F.A. length of 17 or 18 carbon atoms (Jemil et al. 2017; Saggese et al. 2018).
MALDI-TOF MS/MS analysis was employed to detect the sequence of amino acids from the peptide fraction of the lipopeptide. The three abundant signals 1058.70, 1072.72, and 1086.72 Da of the mass spectrum were selected for MS/MS analysis. Figure 3a displays the MS/MS spectrum of the ion at m/z1058.70 Da. The fragmentation pattern of the peak 1058.70 Da depicted the presence of product ions at m/z 959.66 and 846.55 Da relative to the successive losses of Val (99 Da) and Val-Leu (212 Da) residues, respectively. Other peaks were observed at m/z 707.44, 594.34, and 481.23 Da, corresponding to the loss of C15 β-hydroxy fatty acid chain-Glu (351 Da), C15 β-hydroxy fatty acid side chain-Glu-Leu (464 Da) and C15 β-hydroxy fatty acid chain-Glu-Leu-Leu (577 Da) (Saggese et al. 2018). The obtained inference indicated that the peak at m/z 1058.70 Da could be assigned to pumilacidin, a cyclic lipopeptide with a fatty acid chain of 15 carbons, and Val at position 7 (Fig. 3b).MS/MS spectrum acquired from the precursor ion at m/z 1072.72 and 1086.70 Da are shown in Figs. 4 and 5, respectively. The peak at m/z 1072.72 and m/z 1086.7 Da indicated a pumilacidin, with a cyclic lipopeptide fatty acid chain of 15 and 16 carbons, respectively, and Leu or Ile amino acid residues at the 7th position (Hentati et al. 2019).
TG analysis
TG analysis was performed to study the thermostable nature of pumilacidin, as shown in Fig. 6a. It was found that pumilacidin degradation occurred in multiple well-differentiated steps. A 5% reduction in the weight was found when the temperature was increased from 30 °C to 130 °C. A drastic weight loss of pumilacidin followed this, approximately by 45% between 130 °C and 300 °C, followed by another weight loss of roughly 25% between 300 °C and 600 °C. The final part was decomposed by around 20% over the temperature range from 600 °C to 800 °C. All the degradation processes involved the loss of moisture and pyrolysis of the pumilacidin during heating (Chandankere et al. 2014). The results suggest that the biosurfactant from B. pumilus NITDID1 is thermostable and can be used in various industrial applications.
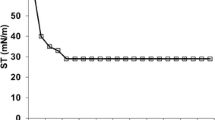
Determination of CMC
CMC is the minimum concentration of the biosurfactant at which the micelle formation is initiated in the solution. With an increase in the concentration of pumilacidin in water, the surface tension of the latter reduced from 69.30 ± 0.6 mN/m to 41.30 ± 0.9 mN/m. Any further increase beyond this point shows no significant decrease in the surface tension. As shown in Fig. 6b, the CMC of the pumilacidin was 120 mg/L.
Stability studies of Pumilacidin
The stability studies of pumilacidin were monitored in a temperature range of 15 °C to 90 °C, pH range of 3 to 11, and salt concentration of 2.5% to 10% (w/v) (Fig.S4). The pumilacidin was found to be thermostable, showing only a mild change (P > 0.05) in its activity even after being exposed to a high temperature of 90 °C for 2 h. Further, the surface tension reduction activity of the pumilacidin was found to be enhanced under alkaline conditions than acidic ones (P ≤ 0.05). Likewise, the surface tension reduction activities of the pumilacidin were found to be better in highly saline conditions (7.5–10%) (P ≤ 0.01).
Emulsification index
The emulsification index of pumilacidin produced by NITDID1 was 90% for used engine oil, suggesting its potential application in bioremediation. The emulsion formed was stable for up to 4 weeks.
Application of the pumilacidin
Application of pumilacidin in used engine oil removal from sand
Soil washing method
Pumilacidin (1 g/L) from Bacillus pumilus NITDID1 recovered 32.94% and 2.47% used engine oil from oil-impregnated sand and control (Fig.S5). However, the increase in pumilacidin concentration from 1 g/L to 2 g/L showed no significant change in oil recovery, suggesting that higher pumilacidin concentrations didn't affect the oil recovery.
Sand pack column method
A sand pack column method was employed as another laboratory scale approach for assessing the potency of pumilacidin produced by Bacillus pumilus NITDID1 in the oil recovery process. After flooding the column with brine, 55.91 ± 3.66% of the oil in the oil-saturated column was recovered, while the remaining 44.09 ± 3.66% was entrapped in the sand matrix. To extract the entrapped oil, pumilacidin (0.6 PV) was injected into the column. The oil recovered from the sand matrix was then eluted with brine flooding after incubation, and the percentage of additional oil recovery (AOR) was measured. Employing pumilacidin produced by NITDID1 resulted in 39.78 ± 1.48% AOR. (Table S3).
Pumilacidin—enhanced bioremediation of used engine oil
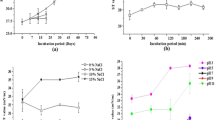
The biodegradation study of used engine oil was conducted to check the role of pumilacidin in the biodegradation of hydrocarbons after 21 days of incubation. The GC-FID results showed more reduction in the peak areas for bacterial consortium samples aided with pumilacidin as compared to ones where the latter was absent. The decrease in the peak areas indicates the degradation of used engine oil components. Supplementation of the pumilacidin increased the degradation of different alkanes in used engine oil (Fig. 7).
Metal chelating property of the pumilacidin
Figure 8 shows that the percentage removal of the heavy metals by 0.5 g/L biosurfactant for Pb was 100%, 91.98%, and 67.67% for 100, 500, and 1000 ppm of Pb, respectively. For cadmium, the removal percentage was 81.98%, 53.83%, and 34.61% for 100, 500, and 1000 ppm of Cd, respectively.
Phytotoxicity assay of pumilacidin
In this study, the germination index (G.I.) has been used to analyze the toxicity of the pumilacidin on Brassica sp. seeds, and the results were represented in Fig.S6. The G.I. values for pumilacidin solutions of 0.5X, 1X, 2X, and 5X CMC were 113%, 105%, 103%, and 91% respectively. However, the germination index declined when seeds were treated with SDS (19%).
Discussion
Biosurfactants have emerged as potential substitutes to their chemical counterparts. They are biodegradable and efficient even under harsh environmental conditions. Biosurfactant-producing microbes can be found in different ecosystems. Here, in this work, mangrove soil was chosen because a mangrove area is one which is diverse in ecology. Jambudwip is one of the remote islands of Sundarban located near Bakkhali, West Bengal, India. From here, a soil sample was collected to isolate and screen for biosurfactant-producing bacteria. In all, 27 pure colonies were isolated from the obtained mangrove soil sample and screened for their biosurfactant production capacity using an array of techniques. Based on the various screening methods observed, isolate NITDID1 was selected further for molecular characterization, whereby NITDID1 was identified as Bacillus pumilus based on 16S rRNA sequencing. The major cellular fatty acids in the strain Bacillus pumilus NITDID1 were found to be 15:0 iso (52.26%) and 15:0 anteiso (30.04%). The branched-chain fatty acids, iso-structure, or anteiso-structure (CH3 group on 2nd or 3rd carbon atom respectively from the end) which accounted for more than 70% of the total cell membrane fatty acid was a characteristic observed in other Bacillus sp. as well (De Faria et al. 2011).
The data obtained from FTIR and 1H NMR analysis showed that B. pumilus NITDID1 produced a mixture of lipopeptide biosurfactants similar to other B. pumilus strains (Sharma et al. 2015). The MALDI TOF MS data additionally confirmed that the biosurfactants produced by B. pumilus NITDID1 were found to be isoforms of pumilacidin with a fatty acid (F.A.) chain length between C13 and C18 (Saggese et al. 2018). MALDI-TOF MS/MS spectra indicated that the biosurfactant strain NITDID1 contains a mixture of pumilacidin isoforms with structures similar to the pumilacidin which have been already reported in the literature (Saggese et al. 2018). TG analysis suggested that this pumilacidin possessed good thermal stability and can be applied in various industrial applications.
A potent biosurfactant is determined by its CMC value and its efficacy in the reduction of surface tension. The CMC of the pumilacidin in this work was as low as 120 mg/L, which is industrially relevant. The CMC value of the pumilacidin was similar to that of chemical surfactants, such as Tween 20,Tween 80, and Triton X-100 (16–110 mg/l), and was considerably lower than tetradecyl trimethyl ammonium bromide (TTAB), and sodium dodecyl sulfate (SDS) (1000–2300 mg/l) (Datta et al. 2018; Hentati et al. 2019). The CMC values of biosurfactant produced from Bacillus sp. I-15 was 200 ± 12 mg/L (Ismail et al. 2013), 200 mg/L from Bacillus pumilus (Oliveira and Garcia-Cruz 2013), 300 mg/L from Acinetobacter junii B6 (Ohadi et al. 2018), 157.5 mg/L from Bacillus licheniformis DS1 (Purwasena et al. 2019), and 185 ± 10 mg/L from Bacillus subtilis, CN2 (Bezza et al. 2020). Interestingly, the aforesaid reported values are much higher than that of the pumilacidin in the present study which is of relevance since biosurfactants having lower CMC values are industrially significant as only a small amount of biosurfactant would be sufficient for micelle formation. The application of a biosurfactant in different industrial sectors hugely relies on its stability at different pH, salinity, and temperature. Biosurfactant produced by B. pumilus NITDID1 was stable at extremely harsh temperatures, alkaline pH, and high salinity, which is in agreement with previous reports (Ju et al. 2016; Liu et al. 2014). The thermostability of biosurfactants makes them suitable for application in food, cosmetic and pharmaceutical industries where sterilization at high temperatures is of utmost importance (Sharma et al. 2018). Thus, the aspect of resistance to elevated levels of temperature is an extremely significant characteristic from the aforesaid viewpoint. Now, the improved stability of this biosurfactant in an alkaline environment can be attributed to the increased solubility of biosurfactant micelles (Bezza and Chirwa 2015). Along with this, the stability of this biosurfactant in high salt conditions makes it ideal for bioremediation of contaminated marine environmental niches (Hentati et al. 2019).
The pumilacidin produced by NITDID1 exhibited high emulsification capability (90%) for used engine oil. In fact, pumilacidin produced by NITDID1 showed a higher emulsification index in comparison to previously reported biosurfactants produced by B. subtilis CN2(76 ± 2.5%) (Bezza and Chirwa 2015), Bacillus cereusNK1 (80%)(Sriram et al. 2011), and Bacillus cereus DRDU1(20%) (Borah and Yadav 2017) for engine/motor oil. The significant emulsification of a hydrophobic compound, which is engine oil in this context, suggests that the obtained biosurfactant can be implemented for the cleanup of oil spill-contaminated sites (Hassanshahian 2014) and thereby is also capable of playing an essential role in the enhancement of oil recovery (Long et al. 2017).
The inaccessible oil that stays entrapped in the reservoir rocks even after secondary restoration can be effectively mobilized by biosurfactants through the reduction of surface tension between the oil and rock, thereby reducing capillary forces that impede oil transport through rock pores (Sarubbo et al. 2022). This property of biosurfactant is called microbial-enhanced oil recovery (MEOR) (Das and Kumar 2019) and is widely utilized by petroleum industries. In the soil washing experiments, pumilacidin resulted in 32.94% recovery of residual oil. Previous reports on this subject suggest that biosurfactant produced from Bacillus methylotrophicus UCP 1616 recovered 25% of motor oil from sandy soil (Chaprão et al. 2018). Similarly, biosurfactants from B. subtilis (strain 309, 311, 573) at the concentration of 1 g/L recovered between 19 and 22% of Arabian light oil (Campinas et al. 2015). Likewise, the recovery rate of crude oil by biosurfactant produced from B. velezensis BSA1 was 21.6% (Yin et al. 2023). Thus, the biosurfactant produced from NITDID1 has shown better results than the ones reported previously. The sand-pack column method was also used along with soil washing experiment to investigate MEOR employing pumilacidin for its environmental application. A sand-pack column is a simple and cost-effective laboratory procedure for evaluating biosurfactants having oil recovery potential (Pandey et al. 2022). From the sand pack column experiment, the pumilacidin produced by NITDID1 resulted in 39.78 ± 1.48% recovery of residual oil. The achieved AOR by this pumilacidin is much better from other Bacillus strains previously reported, such as B. aerius KJ2MD (31.9%) (Phulpoto et al. 2020), B. licheniformis (16.6%) (El-Sheshtawy et al. 2015), B. licheniformis TT42 (34.6%), B. mojavensis JF2 (29.4%) (Suthar et al. 2008), and B. subtilis 20B (30.2%) (Joshi et al. 2008) and comparable with B. licheniformis KJ2SK (40.5%) (Phulpoto et al. 2020), B. licheniformis K125 (43%) (Suthar et al. 2008), and B. subtilis K1 (43.3%) (Pathak and Keharia 2014). This shows that Bacillus pumilus NITID1-derived biosurfactant holds promise in enhancing oil recovery for the petroleum industry.
The emulsification of used engine oil in water is proof of concept that if pumilacidin is brought into play, the oil degradation by microbial consortia in oil-contaminated sites will increase by 14.20%. This can be attributed to the fact that the addition of biosurfactant improves the bioavailability of the oil by breaking down large oil molecules into small micelles and thus increases the rate of action by oil-scavenging bacterial species. The findings from this experiment are in line with those reported by previous authors. For example, biosurfactants from Pseudozyma sp. along with P.putida have shown a better reduction of alkanes in crude oil (Sajna et al. 2015). Sharma et al. have also reported that microbial consortium and biosurfactants produced from B. amyloliquefaciens SAS-1 and B. subtilis BR-15 significantly increased the bioremediation of engine oil (Sharma et al. 2018). An enhancement in microbial consortium-mediated biodegradation of diesel was observed with the addition of SPB1 lipopeptide (Mnif et al. 2017).
In this work, addition of the pumilacidin biosurfactant augmented the biodegradation of C14 (57.32%), C19 (17.64%), C16 (15.66%), C22 (10.78%), and C13 (9.72%) fatty acid chains. The effect of the biosurfactant is much more pronounced on C14 fatty acids because the rate of degradation of these fatty acids, with mixed consortia solely, is much lower than the rest. This is because the available percentage of C14 fatty acids in the experimental setup at a given time is higher than the long-chain hydrocarbons. A plausible explanation of this phenomenon is that the long-chain hydrocarbons first degrade partially into chains of intermediate length (Sharma et al. 2018). Thus, adding biosurfactant markedly enhances the degradation of C14 fatty acids. Empirically, it was seen that hydrocarbon-degrading bacterial consortium could interact synergistically with pumilacidin from NITDID1. The biosurfactant emulsifies and solubilizes the used engine oil, thereby increasing its bioavailability of hydrocarbon-degrading microorganisms. This proves its application in bioremediation in the oil-contaminated site.
Apart from oil emulsification ability of this biosurfactant, the heavy metal chelating capacity was also observed in evaluation of environmental remediation potential of the product. So far, traditional techniques to separate heavy metal contaminants from industrial wastewater include treating them with water, acids, chemical surfactants, and metal-chelating agents such as EDTA. However, these methods failed to remove toxic metal ions adequately (Luna et al. 2016). In this context, biosurfactants come to the rescue as metal-chelating agents that have been reported to be effective in the remediation of heavy metal-contaminated ecosystems (Das et al. 2009). The AAS analysis showed that in biosurfactant-treated artificially prepared solutions of lead and cadmium, the concentration of the heavy metals was drastically reduced with time as compared to the control sets. The chemistry that comes into play is that the metal gets chelated with the biosurfactant and forms a coordination complex, which results in the precipitation of suspended metal ions. Therefore, the metal-pumilacidin complex was seen as a white precipitate when the pumilacidin was allowed to incubate with the metal solution. The precipitation was due to charge neutralization between the two components (Gonçalves et al. 2015). This potential of the biosurfactant can be exploited to design strategies to purify heavy metal-contaminated waste water since the metal-pumilacidin precipitate complex, being heavier than water, can be easily separated, to leave behind safe metal-free water.
For any compound to stand out as an eco-friendly alternative, its EHS (environment, health, and safety) impact is as important as its functionality. In this context, phytotoxicity tests allowed for the assessment of the toxicity of various substances through plant seeds (Mendes et al. 2021). Hence, researchers worldwide resort to phyto-toxicological assays to evaluate the hazard posed by a particular compound on seed germination. Considering that the 80% germination index value was used as a bio-indicator of the disappearance of phytotoxicity (Araújo et al. 2019), the results indicated that the pumilacidin did not show inhibitory effects on the seed germination and root elongation of Brassica sp. It was therefore concluded that for the same CMC concentration, pumilacidin was found to be non-toxic in comparison to the chemical surfactant SDS. Similar results have been reported previously, where it was found that the biosurfactant does not pose any inhibitory effect on seed germination and root elongation (Das and Kumar 2019; Silva et al. 2010). So far, this is the first report demonstrating the detailed characterization and environmental application of a pumilacidin from Bacillus pumilus NITID1.
Conclusion
Although the potential of pumilacidin has been vastly explored in the field of biomedicine, its application in the realm of environmental remediation was identified as a major research gap and has been touched upon for the very first time in this particular research work. In the present study, microbial surfactant from mangrove origin Bacillus pumilus NITDID1 opens fascinating avenues for the environmental remediation of oil and heavy metal-contaminated sites. The biosurfactant exhibited a lipopeptide nature, as confirmed by the FTIR and NMR spectra, and the MALDI-TOF MS results identified it to be a pumilacidin. The pumilacidin was stable under extreme environmental conditions (temperature of 90 ºC, pH 11, 10% NaCl) and showed excellent emulsification efficiency (90%) against used engine oil, thereby increasing the bioavailability for its degradation. Therefore, it shows an interesting potential application in MEOR (39.78% additional oil recovery) and is capable of chelating toxic heavy metals (100% and 82% for lead and cadmium, respectively). This report demonstrates that this pumilacidin biosurfactant has remarkable unharnessed potential in the field of bioremediation and, thus, is a prospective alternative to harmful synthetic surfactants for the same.
Data availability
The authors confirm that the data supporting the study’s finding are included in the article and its supplementary materials. 16S rRNA gene sequence are available in NCBI database.
References
Abdeshahian P, Fooladi T, Hamid AA et al (2016) Characterization, production and optimization of lipopeptide biosurfactant by new strain Bacillus pumilus 2IR isolated from an Iranian oil field. J Pet Sci Eng 145:510–519. https://doi.org/10.1016/j.petrol.2016.06.015
Araújo HWC, Andrade RFS, Montero-Rodríguez D et al (2019) Sustainable biosurfactant produced by Serratia marcescens UCP 1549 and its suitability for agricultural and marine bioremediation applications. Microb Cell Fact 18:1–13. https://doi.org/10.1186/s12934-018-1046-0
Badmus SO, Amusa HK, Oyehan TA, Saleh TA (2021) Environmental risks and toxicity of surfactants: overview of analysis, assessment, and remediation techniques. Environ Sci Pollut Res 28:62085–62104. https://doi.org/10.1007/s11356-021-16483-w
Ben Ayed H, Jemil N, Maalej H et al (2015) Enhancement of solubilization and biodegradation of diesel oil by biosurfactant from Bacillus amyloliquefaciens An6. Int Biodeterior Biodegrad 99:8–14. https://doi.org/10.1016/j.ibiod.2014.12.009
Bezza FA, Chirwa EMN (2015) Production and applications of lipopeptide biosurfactant for bioremediation and oil recovery by Bacillus subtilis CN2. Biochem Eng J 101:168–178. https://doi.org/10.1016/j.bej.2015.05.007
Bezza FA, Chirwa EMN (2016) Biosurfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarbons (PAHs) in creosote contaminated soil. Chemosphere 144:635–644. https://doi.org/10.1016/j.chemosphere.2015.08.027
Bezza FA, Tichapondwa SM, Chirwa EMN (2020) Synthesis of biosurfactant stabilized silver nanoparticles, characterization and their potential application for bactericidal purposes. J Hazard Mater 393:122319. https://doi.org/10.1016/j.jhazmat.2020.122319
Borah D, Yadav RNS (2017) Bioremediation of petroleum based contaminants with biosurfactant produced by a newly isolated petroleum oil degrading bacterial strain. Egypt J Pet 26:181–188. https://doi.org/10.1016/j.ejpe.2016.02.005
Campinas UEDE, Gurjar J, Sengupta B et al (2015) Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel 111:259–268. https://doi.org/10.1016/j.biortech.2015.04.013
Chandankere R, Yao J, Cai M et al (2014) Properties and characterization of biosurfactant in crude oil biodegradation by bacterium Bacillus methylotrophicus USTBa. Fuel 122:140–148. https://doi.org/10.1016/j.fuel.2014.01.023
Chaprão MJ, da Silva R, de CFS, Rufino RD, et al (2018) Production of a biosurfactant from Bacillus methylotrophicus UCP1616 for use in the bioremediation of oil-contaminated environments. Ecotoxicology 27:1310–1322. https://doi.org/10.1007/s10646-018-1982-9
Chen Q, Li Y, Liu M et al (2021) Removal of Pb and Hg from marine intertidal sediment by using rhamnolipid biosurfactant produced by a Pseudomonas aeruginosa strain. Environ Technol Innov 22:101456. https://doi.org/10.1016/j.eti.2021.101456
Chittepu OR (2019) Isolation and characterization of biosurfactant producing bacteria from groundnut oil cake dumping site for the control of foodborne pathogens. Grain Oil Sci Technol 2:15–20. https://doi.org/10.1016/j.gaost.2019.04.004
Collivignarelli MC, Carnevale Miino M, Baldi M et al (2019) Removal of non-ionic and anionic surfactants from real laundry wastewater by means of a full-scale treatment system. Process Saf Environ Prot 132:105–115. https://doi.org/10.1016/j.psep.2019.10.022
Cooper DG, Goldenberg BG (1987) Surface-active agents from two Bacillus species. Appl Environ Microbiol 53:224–229. https://doi.org/10.1128/aem.53.2.224-229.1987
Das AJ, Kumar R (2018) Utilization of agro-industrial waste for biosurfactant production under submerged fermentation and its application in oil recovery from sand matrix. Bioresour Technol 260:233–240. https://doi.org/10.1016/j.biortech.2018.03.093
Das AJ, Kumar R (2019) Production of biosurfactant from agro-industrial waste by Bacillus safensis J2 and exploring its oil recovery efficiency and role in restoration of diesel contaminated soil. Environ Technol Innov 16:100450. https://doi.org/10.1016/j.eti.2019.100450
Das P, Mukherjee S, Sen R (2009) Biosurfactant of marine origin exhibiting heavy metal remediation properties. Bioresour Technol 100:4887–4890. https://doi.org/10.1016/j.biortech.2009.05.028
Datta P, Tiwari P, Pandey LM (2018) Isolation and characterization of biosurfactant producing and oil degrading Bacillus subtilis MG495086 from formation water of Assam oil reservoir and its suitability for enhanced oil recovery. Resour Technol. https://doi.org/10.1016/j.biortech.2018.09.047
De Faria AF, Teodoro-Martinez DS, De Oliveira Barbosa GN et al (2011) Production and structural characterization of surfactin (C 14/Leu7) produced by Bacillus subtilis isolate LSFM-05 grown on raw glycerol from the biodiesel industry. Process Biochem. https://doi.org/10.1016/j.procbio.2011.07.001
De Oliveira EM, Sales VHG, Andrade MS et al (2021) Isolation and characterization of biosurfactant-producing bacteria from amapaense amazon soils. Int J Microbiol. https://doi.org/10.1155/2021/9959550
de Oliveira JG, Garcia-Cruz CH (2013) Properties of a biosurfactant produced by Bacillus pumilus using vinasse and waste frying oil as alternative carbon sources. Brazilian Arch Biol Technol 56:155–160. https://doi.org/10.1590/S1516-89132013000100020
El-Sheshtawy HS, Aiad I, Osman ME et al (2015) Production of biosurfactant from Bacillus licheniformis for microbial enhanced oil recovery and inhibition the growth of sulfate reducing bacteria. Egypt J Pet 24:155–162. https://doi.org/10.1016/j.ejpe.2015.05.005
Gomaa EZ, El-Meihy RM (2019) Bacterial biosurfactant from Citrobacter freundii MG812314.1 as a bioremoval tool of heavy metals from wastewater. Bull Natl Res Cent. https://doi.org/10.1186/s42269-019-0088-8
Gonçalves LRB, de Santana HB, Melo VMM et al (2015) Production of a biosurfactant by Bacillus subtilis ICA56 aiming bioremediation of impacted soils. Catal Today 255:10–15. https://doi.org/10.1016/j.cattod.2015.01.046
Gudiña EJ, Fernandes EC, Rodrigues AI et al (2015) Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Front Microbiol 6:1–7. https://doi.org/10.3389/fmicb.2015.00059
Hassanshahian M (2014) Isolation and characterization of biosurfactant producing bacteria from Persian Gulf (Bushehr provenance). Mar Pollut Bull 86:361–366. https://doi.org/10.1016/j.marpolbul.2014.06.043
Hentati D, Chebbi A, Hadrich F et al (2019) Production, characterization and biotechnological potential of lipopeptide biosurfactants from a novel marine Bacillus stratosphericus strain FLU5. Ecotoxicol Environ Saf 167:441–449. https://doi.org/10.1016/j.ecoenv.2018.10.036
Ismail W, Al-Rowaihi IS, Al-Humam AA et al (2013) Characterization of a lipopeptide biosurfactant produced by a crude-oil-emulsifying Bacillus sp. I-15. Int Biodeterior Biodegrad 84:168–178. https://doi.org/10.1016/j.ibiod.2012.04.017
Jemil N, Manresa A, Rabanal F et al (2017) Structural characterization and identification of cyclic lipopeptides produced by Bacillus methylotrophicus DCS1 strain. J Chromatogr B Anal Technol Biomed Life Sci 1060:374–386. https://doi.org/10.1016/j.jchromb.2017.06.013
Joshi S, Bharucha C, Desai AJ (2008) Production of biosurfactant and antifungal compound by fermented food isolate Bacillus subtilis 20B. Bioresour Technol 99:4603–4608. https://doi.org/10.1016/j.biortech.2007.07.030
Joy S, Rahman PKSM, Sharma S (2017) Biosurfactant production and concomitant hydrocarbon degradation potentials of bacteria isolated from extreme and hydrocarbon contaminated environments. Chem Eng J 317:232–241. https://doi.org/10.1016/j.cej.2017.02.054
Ju M, Li X, Yu Q et al (2016) Purification and characterization of biosurfactant produced by Bacillus licheniformis Y-1 and its application in remediation of petroleum contaminated soil. Mar Pollut Bull 107:46–51. https://doi.org/10.1016/j.marpolbul.2016.04.025
Khopade A, Ren B, Liu XY et al (2012) Production and characterization of biosurfactant from marine Streptomyces species B3. J Colloid Interface Sci 367:311–318. https://doi.org/10.1016/j.jcis.2011.11.009
Li J, Deng M, Wang Y, Chen W (2016) Production and characteristics of biosurfactant produced by Bacillus pseudomycoides BS6 utilizing soybean oil waste. Int Biodeterior Biodegrad 112:72–79. https://doi.org/10.1016/j.ibiod.2016.05.002
Liu Q, Lin J, Wang W et al (2014) Production of surfactin isoforms by Bacillus subtilis BS-37 and its applicability to enhanced oil recovery under laboratory conditions. Biochem Eng J 93:31–37. https://doi.org/10.1016/j.bej.2014.08.023
Long X, He N, He Y et al (2017) Biosurfactant surfactin with pH-regulated emulsification activity for efficient oil separation when used as emulsifier. Bioresour Technol 241:200–206. https://doi.org/10.1016/j.biortech.2017.05.120
Luna JM, Rufino RD, Sarubbo LA (2016) Biosurfactant from Candida sphaerica UCP0995 exhibiting heavy metal remediation properties. Process Saf Environ Prot 102:558–566. https://doi.org/10.1016/j.psep.2016.05.010
Mani P, Sivakumar P, Balan SS (2016) Economic production and oil recovery efficiency of a lipopeptide biosurfactant from a Novel marine bacterium Bacillus simplex. Achiev Life Sci 10:102–110. https://doi.org/10.1016/j.als.2016.05.010
Mendes PM, Ribeiro JA, Martins GA et al (2021) Phytotoxicity test in check: Proposition of methodology for comparison of different method adaptations usually used worldwide. J Environ Manage. https://doi.org/10.1016/j.jenvman.2021.112698
Mishra S, Lin Z, Pang S et al (2021) Biosurfactant is a powerful tool for the bioremediation of heavy metals from contaminated soils. J Hazard Mater 418:126253. https://doi.org/10.1016/j.jhazmat.2021.126253
Mnif I, Sahnoun R, Ellouz-Chaabouni S, Ghribi D (2017) Application of bacterial biosurfactants for enhanced removal and biodegradation of diesel oil in soil using a newly isolated consortium. Process Saf Environ Prot 109:72–81. https://doi.org/10.1016/j.psep.2017.02.002
Muthukamalam S, Sivagangavathi S, Dhrishya D, Sudha Rani S (2017) Characterization of dioxygenases and biosurfactants produced by crude oil degrading soil bacteria. Brazilian J Microbiol 48:637–647. https://doi.org/10.1016/j.bjm.2017.02.007
Nayak NS, Purohit MS, Tipre DR, Dave SR (2020) Biosurfactant production and engine oil degradation by marine halotolerant Bacillus licheniformis LRK1. Biocatal Agric Biotechnol 29:101808. https://doi.org/10.1016/j.bcab.2020.101808
Ohadi M, Dehghannoudeh G, Forootanfar H et al (2018) Investigation of the structural, physicochemical properties, and aggregation behavior of lipopeptide biosurfactant produced by Acinetobacter junii B6. Int J Biol Macromol 112:712–719. https://doi.org/10.1016/j.ijbiomac.2018.01.209
Pandey R, Krishnamurthy B, Singh HP, Batish DR (2022) Evaluation of a glycolipopepetide biosurfactant from Aeromonas hydrophila RP1 for bioremediation and enhanced oil recovery. J Clean Prod 345:131098. https://doi.org/10.1016/j.jclepro.2022.131098
Pathak KV, Keharia H (2014) Application of extracellular lipopeptide biosurfactant produced by endophytic Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) in microbially enhanced oil recovery (MEOR). 3 Biotech 4:41–48. https://doi.org/10.1007/s13205-013-0119-3
Patowary K, Patowary R, Kalita MC, Deka S (2017) Characterization of biosurfactant produced during degradation of hydrocarbons using crude oil as sole source of carbon. Front Microbiol 8:1–14. https://doi.org/10.3389/fmicb.2017.00279
Phulpoto IA, Jakhrani BA, Phulpoto AH et al (2020) Enhanced oil recovery by potential biosurfactant-producing halo-thermotolerant bacteria using soil washing and sand-packed glass column techniques. Curr Microbiol 77:3300–3309. https://doi.org/10.1007/s00284-020-02172-3
Purwasena IA, Astuti DI, Syukron M et al (2019) Stability test of biosurfactant produced by Bacillus licheniformis DS1 using experimental design and its application for MEOR. J Pet Sci Eng 183:106383. https://doi.org/10.1016/j.petrol.2019.106383
Ravindran A, Sajayan A, Priyadharshini GB et al (2020) Revealing the efficacy of thermostable biosurfactant in heavy metal bioremediation and surface treatment in vegetables. Front Microbiol 11:1–11. https://doi.org/10.3389/fmicb.2020.00222
Saggese A, Culurciello R, Casillo A et al (2018) A marine isolate of Bacillus pumilus secretes a pumilacidin active against staphylococcus aureus. Mar Drugs 16:1–13. https://doi.org/10.3390/md16060180
Sajna KV, Sukumaran RK, Gottumukkala LD, Pandey A (2015) Crude oil biodegradation aided by biosurfactants from Pseudozyma sp. NII 08165 or its culture broth. Bioresour Technol 191:133–139. https://doi.org/10.1016/j.biortech.2015.04.126
Sarubbo LA, da Silva MGC, Durval IJB et al (2022) Biosurfactants: production, properties, applications, trends, and general perspectives. Biochem Eng J. https://doi.org/10.1016/j.bej.2022.108377
Sasser M (2001) Identification of bacteria by gas chromatography of cellular fatty acids. Tech Note 101:1–6
Sharma AD, Singh J (2005) A nonenzymatic method to isolate genomic DNA from bacteria and actinomycete. Anal Biochem 337:354–356. https://doi.org/10.1016/j.ab.2004.11.029
Sharma S, Pandey LM (2020) Production of biosurfactant by Bacillus subtilis RSL-2 isolated from sludge and biosurfactant mediated degradation of oil. Bioresour Technol 307:123261. https://doi.org/10.1016/j.biortech.2020.123261
Sharma D, Pruthi V, Al Ghamdi A et al (2015) Structural characterization and antimicrobial activity of a biosurfactant obtained from Bacillus pumilus DSVP18 grown on potato peels. Jundishapur J Microbiol. https://doi.org/10.5812/jjm.21257
Sharma R, Singh J, Verma N (2018) Production, characterization and environmental applications of biosurfactants from Bacillus amyloliquefaciens and Bacillus subtilis. Biocatal Agric Biotechnol 16:132–139. https://doi.org/10.1016/j.bcab.2018.07.028
Sharma S, Verma R, Pandey LM (2019) Crude oil degradation and biosurfactant production abilities of isolated Agrobacterium fabrum SLAJ731. Biocatal Agric Biotechnol 21:101322. https://doi.org/10.1016/j.bcab.2019.101322
Sharma J, Kapley A, Sundar D, Srivastava P (2022) Characterization of a potent biosurfactant produced from Franconibacter sp. IITDAS19 and its application in enhanced oil recovery. Colloids Surfaces B Biointerfaces 214:112453. https://doi.org/10.1016/j.colsurfb.2022.112453
Shibulal B, Joshi S, Al-Bemani A et al (2013) Biosurfactant production by Bacillus subtilis B30 and its application in enhancing oil recovery. Colloids Surf B Biointerfaces 114:324–333. https://doi.org/10.1016/j.colsurfb.2013.09.022
Silva SNRL, Farias CBB, Rufino RD et al (2010) Glycerol as substrate for the production of biosurfactant by Pseudomonas aeruginosa UCP0992. Colloids Surf B Biointerfaces 79:174–183. https://doi.org/10.1016/j.colsurfb.2010.03.050
Sriram MI, Kalishwaralal K, Deepak V et al (2011) Biofilm inhibition and antimicrobial action of lipopeptide biosurfactant produced by heavy metal tolerant strain Bacillus cereus NK1. Colloids Surf B Biointerfaces 85:174–181. https://doi.org/10.1016/j.colsurfb.2011.02.026
Sun W, Zhu B, Yang F et al (2021) Optimization of biosurfactant production from Pseudomonas sp. CQ2 and its application for remediation of heavy metal contaminated soil. Chemosphere 265:129090. https://doi.org/10.1016/j.chemosphere.2020.129090
Suthar H, Hingurao K, Desai A, Nerurkar A (2008) Evaluation of bioemulsifier mediated microbial enhanced oil recovery using sand pack column. J Microbiol Methods 75:225–230. https://doi.org/10.1016/j.mimet.2008.06.007
Tekinay T, Angun P, Han D et al (2015) Production and structural characterization of biosurfactant produced by newly isolated Staphylococcus xylosus STF1 from petroleum contaminated soil. J Pet Sci Eng 133:689–694. https://doi.org/10.1016/j.petrol.2015.07.011
Yalçın HT, Ergin-Tepebaşı G, Uyar E (2018) Isolation and molecular characterization of biosurfactant producing yeasts from the soil samples contaminated with petroleum derivatives. J Basic Microbiol 58:782–792. https://doi.org/10.1002/jobm.201800126
Yin J, Wei X, Hu F et al (2023) Halotolerant Bacillus velezensis sustainably enhanced oil recovery of low permeability oil reservoirs by producing biosurfactant and modulating the oil microbiome. Chem Eng J. https://doi.org/10.1016/j.cej.2022.139912
Zargar AN, Lymperatou A, Skiadas I et al (2022) Structural and functional characterization of a novel biosurfactant from Bacillus sp IITD106. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2021.127201
Zhang J, Xue Q, Gao H et al (2016) Production of lipopeptide biosurfactants by Bacillus atrophaeus 5–2a and their potential use in microbial enhanced oil recovery. Microb Cell Fact 15:1–11. https://doi.org/10.1186/s12934-016-0574-8
Zhang J, Feng W, Xue Q (2022) Biosurfactant production and oil degradation by Bacillus siamensis and its potential applications in enhanced heavy oil recovery. Int Biodeterior Biodegrad 169:105388. https://doi.org/10.1016/j.ibiod.2022.105388
Zhou H, Liu Q, Jiang L et al (2023) Enhanced remediation of oil-contaminated intertidal sediment by bacterial consortium of petroleum degraders and biosurfactant producers. Chemosphere 330:138763. https://doi.org/10.1016/j.chemosphere.2023.138763
Acknowledgements
Authors thank NIT Durgapur for AAS and TG analysis, Xcelris Labs Ltd, Ahmadabad for microbial identification, Royal Life Sciences Pvt. Ltd., Secunderabad for GC-FAME analysis, Bose Institute, Kolkata for MALDI-TOF and FTIR analysis, IIT Kharagpur for using tensiometer instrument, TCG life sciences Pvt Ltd, Kolkata for 1H NMR analysis and IISER Kolkata for GC analysis. We also thank Dr. Sudipa Mondal for helping us with the analysis of characterization data.
Funding
The author declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
AD performed all the experiments, initial data analysis and prepared the initial draft of the manuscript. SS carried out major data analysis, figure and graph preparation and revised the draft manuscript. PG performed sub-experiment under Phyto-toxicity assay, revised and edited the final version of the manuscript. ID carried out isolation and identification of bacteria. DD and SC supervised the work and carried out review and editing of final draft. All authors read and consented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dasgupta, A., Saha, S., Ganguli, P. et al. Characterization of pumilacidin, a lipopeptide biosurfactant produced from Bacillus pumilus NITDID1 and its prospect in bioremediation of hazardous pollutants. Arch Microbiol 205, 274 (2023). https://doi.org/10.1007/s00203-023-03619-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03619-4