Abstract
Biosurfactants offer numerous advantages over the chemical surfactants, especially in energy and environment-related applications. Microbial enhanced oil recovery (MEOR) is a technique to recover oil from reservoirs by using microbes and their metabolites. In present study, total sixteen morphologically distinct bacterial strains isolated from different salty areas of the district Khairpur Mir’s, Pakistan, were investigated for their MEOR potential. Screening assays for thermotolerance and halotolerance declared 7 out of 16 (43.75%) bacterial isolates as thermotolerant (capable of growing in the temperature range 60–70 °C) and halotolerant (tolerating NaCl concentrations up to 17%, w/v). Moreover, five of them were screened as biosurfactant producers. Among, the lowest surface tension reduction was achieved with biosurfactants produced by the strains KJ2MO (27.8 mN/m) and KJ2SK (29.3 mN/m). The biosurfactant activity was found stable at temperature (100–121 °C, 1 h) and pH (4–10). Moreover, maximum oil recovery was obtained with biosurfactant of bacterial strain KJ2MO (54.7%, 51.25%) followed by KJ2SK (44.7%, 40.5%), KJ1WB (37%, 35.5%) and KJ2MD (37.8%, 31.9%) by using either techniques, i.e., soil washing and sand-packed column, respectively. Moreover, the potent species were identified as Pseudomonas pseudoalcaligenes KJ1WB, Bacillus aerius KJ2MD, Bacillus licheniformis KJ2SK, and Bacillus subtilis KJ2MO using 16S rRNA ribo-typing. The investigated species were found to be promising biosurfactants producers having potential for enhanced oil recovery and could be used in other environmental applications like bioremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pakistan is one of the top ten countries having highest shale oil reserves comprising almost 227 billion barrels oil, but only 9.1 billion barrels oil has been reported as recoverable with currently available technology (USA EIA Report, 2013) [1]. The microbial enhanced oil recovery (MEOR) offers an environment-friendly, cost-effective and easy alternative to conventional technology such as chemical flooding [2]. Among proposed MEOR applications, the use of biosurfactants has received much interest of the scientists as reflected from the increased review publications and patents worldwide [3,4,5,6,7,8].
Although MEOR has been acknowledged and applied around the world, at the same time, it is criticized for its failure on fields due to challenges posed by extreme conditions of the oil reservoirs, e.g., decreased permeability and porosity, elevated temperature as well as salinity. Such extreme conditions, however, are currently a limitation for MEOR applicability, but at the same time, it opens new vistas of research to explore appropriate solutions to such problems.
Microorganisms, especially bacteria, can grow under extreme conditions and produce enormous amounts of valuable metabolites by growing on cheap substrates [9, 10]. The development of biotechnological research has resulted in the oil industry to be more open for microbial evaluation to enhance oil production. On the basis of microbial adaptability to the reservoir, both indigenous as well as injected microorganisms can be used [11, 12]. Both, microorganisms and biosurfactants, are sensitive to among other things, the salinity and temperature in the reservoir, which may cause some limitations to the use of biosurfactants in enhanced oil recovery. For example, the sodium chloride content in the reservoirs is a very important factor for MEOR. Sodium chloride makes up over 90% of the total amount of dissolved solids in a reservoir; it is therefore important that the selected bacteria are tolerant of sodium chloride. The existing halophilic bacteria can grow in a saturated solution of sodium chloride [12]. Another challenge for the bacteria in an oil reservoir is the osmotic pressure, caused by the high level of sodium chloride. Under normal conditions, the bacteria will be shrinking because of the high level of sodium chloride that drives the water out of the cell membrane [13].
Likewise, the temperature in the oil reservoir is also a very crucial factor regarding the growth of the bacteria. The temperature increases with the increasing depth of the reservoir and can reach up to 200 °C. The temperature in most oil reservoirs lies below 150 °C. Bacteria can be split into groups depending on their optimum temperature: Thermophilic bacteria, with minimum temperature requirement of 65 °C, are called hyperthermophiles. Since the temperature is an extremely important factor for the growth of microorganisms, it is essential to choose bacteria that have adaptability for high-temperature ranges. Likewise, the bacteria also need to be selected according to the specific conditions of the oil reservoir. If the temperature is too high or low for the selected bacteria, there will be no bacterial growth [14]. Therefore, one of the possible options is to use either halotolerant and thermotolerant microbes or their metabolic products such as biosurfactants, which show stability at a wide range of temperature and salinity. The increasing temperature in the reservoir cause a decrease in critical micelles concentration (CMC) of the biosurfactants produced by bacteria, making biosurfactants more effective for decreasing the surface tension at high temperatures [15], for which the salinity will be an advantage. It is also important to mention that the characteristics of biosurfactant like their efficacy and solubility might be affected by pH and high salt concentrations [16, 17].
The extreme environments of Pakistan such as deserts, hot-springs, salt-mines and hyper-saline environments have been recently reviewed and were considered as treasure chest for novel halophilic/halotolerant and thermophilic/thermotolerant microorganisms [18]. Exploring such environments could also lead to the discovery of some high-potential microbial candidates for biotechnological applications. Therefore, having such aim, it is the first study from Sindh, Pakistan, to explore halo-thermotolerant bacteria from unexplored hyper-saline environments having great potential for biosurfactants production and application in MEOR.
Materials and Methods
Isolation of Halo-thermotolerant Bacteria
Different samples of muddy soil, salt, and saline water were collected from different sites of hyper-saline areas near Kolab Jeal, and Naroo Dhoro towns of district Khairpur, Sindh, Pakistan, for the isolation of halo-thermotolerant bacteria. All the collected samples were processed for bacterial isolation using a tenfold serial dilution method. Briefly, the mud, water, and salt samples (1 g or 1 mL for solid and liquid samples, respectively) were suspended into sterile distilled water (9 mL) and successive dilutions were made by transferring 1 mL from each test tube to the next up to 10–4 dilutions (v/v). Subsequently, an aliquot of 0.1 mL of each dilution was taken and spread evenly over the surface of nutrient agar medium (Oxoid, UK) plates followed by incubation (37 °C for 24 h) [19]. After incubation, the isolates were characterized based on their cultural, morphological and biochemical characteristics.
Screening for Halotolerant and Thermotolerance
The pure bacterial isolates were screened for their growth at different salt concentration and elevated temperature. For this purpose, the bacterial isolates were inoculated on the medium (Nutrient agar) containing different NaCl concentrations ranging from 1 to 17% and at temperatures ranging from 45 to 70 °C for different time intervals, for the growth of potential bacteria.
Screening of Biosurfactants Production
Luria bertani (LB) medium (Bactotrptone 10 g, NaCl 10 g, yeast extract 5 g/L) and minimal salt medium (MSM) were used for screening of biosurfactants as described by Najafi et al. [20] MSM medium containing (g/L); (NH4)2SO4 1, MgSO4 0.25, NaCl 50, K2HPO4 13.7, KH2PO4 2.7, and 1% of the trace elements MnSO4⋅H2O 3, FeSO4⋅7H2O 0.1,CaCl2⋅2H2O 0.1, ZnSO4⋅7H2O 0.1, CuSO4⋅5H2O 0.01, H3BO3 0.01 and 1% Kerosene oil (v/v) as hydrocarbon source. The pH of the medium was adjusted to 7.0 using hydrochloric acid (HCl) and 1 N (NaOH). A single colony of each isolate was taken from the plate and transferred into 100 mL of LB and MSM liquid media. The culture broths were incubated at 50 °C in the rotatory shaker (Innova 4900, Germany) 150 rpm for 4 days. Growth and biosurfactant production were examined under aerobic conditions. Samples (10 mL) were taken at different time points during the fermentation to determine biomass concentration and biosurfactant production. Bacterial growth was determined by measuring the optical density using a spectrophotometer (Jenway 6300) at 600 nm. Afterward, the samples were centrifuged (1792×g for 15 min) and cell-free supernatants (CFS) were collected and used to measure the different assays such as oil displacement activity by standard and modified method (glass slide), emulsifying activity and surface tension measurement.
Oil Displaced Activity (ODA) (Modified Method)
This method of oil displaced technique was done by an oily glass slide. The 10 µL of CFS was placed on the surface of oily glass slide and compared with control which contained a drop of distilled water, and the zone of displacement was measured.
Oil Spreading Technique (Standard Method)
Oil spreading technique (OST) was carried out according to the method as described by Youssef et al. [21]. Fifty milliliters of distilled water was added in a Petri dish followed by addition of 100 µL of crude oil (Bonny Light) to the surface of the water. Then 10 µL of the supernatant were dropped on the crude oil surface. The diameter of clear zone on the oil surface was measured using a meter rule and the time taken to achieve the spread was also noted, the distilled water was used as control in this method.
Emulsification Assay
The emulsification potential of the biosurfactants was determined using method of Cooper et al. [22]. In this method, 1 mL kerosene oil was taken in a tube and 1 mL of cell-free supernatant was added and the mixture was vortexed at high speed for 2 min. After leaving the mixture to stand for 24 h, height of the stable emulsion layer was measured in terms of emulsification index (EI24). This was calculated as the ratio of the height of the emulsion layer and the total height of the liquid.
Surface Tension Measurement
The surface tension was measured by using Tensiometer (K20 Easy Dyne tensiometer, Kruss, Germany) according to the instructions of the manufacturer. The platinum plate was dipped just below the surface of 15 mL of the liquid. Subsequently, the force to move this plate from the liquid phase to the air phase was determined. The values were taken as the mean of 20 measurements were calculated.
Biosurfactant Stability
The stability of biosurfactants at different temperature ranges viz. 80 to 100 °C, at 10 °C, and 121 °C was carried out by incubating the biosurfactant solution for 30 min for 15 min, and then cooled to room temperature.
Effect of Sodium Chloride
The effect of sodium chloride on biosurfactants produced by test bacterial isolates was determined by adding concentrations ranging from (5 to 20% w/v) to the biosurfactant solution and allowed to stand for 30 min. The emulsification indexes of each treatment were determined at the end of each experiment.
pH Stability
Stability studies were carried out using 0.1% (w/v) biosurfactant solution in 0.1 M-phosphate buffer, pH 7.0. The effect of pH on the biosurfactant activity was performed by introducing the biosurfactant solution into test tubes and the pH adjusted to various values (4-6-7-8-10) using HCl and NaOH solution and kept at room temperature.
Soil Washing Technique
The recovery of oil using soil washing technique was carried out according to Urum and Pekdemir [23]. In this method, the oil recovery was evaluated using soil (loamy soil collected from garden) artificially contaminated with used engine oil (UEO). The soil samples (10 g) were dried in hot air oven (initially at 200 °C for 2 h and then at 50 °C for 24 h) and mixed with 20 mL used motor oil in 100 ml Erlenmeyer flasks by shaking (100 rpm) for 24 h. On the next day, oil effluent was determined and flasks were washed with brine solution (5% NaCl) 4–6 time, oil recovered with brine was calculated. Afterward, the cell-free broth containing biosurfactants was loaded and allowed for 24 h. After this, the soil was washed twice with CFS and final recovery of oil and its volume was measured gravimetrically. The control experiments were performed using distilled water under condition, whereas this experiment was performed in triplicate.
Sand-Packed Column
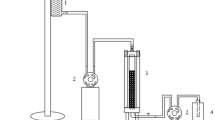
This experiment was carried according to Pathak and Keharia [24] with minor modifications in sand packed column preparation. A 100-g dried sand was packed in an empty glass column and wetted with brine solution (5% NaCl w/v) and PV (pore volume) was determined. Column was completely saturated with 3 × PVs brine solution, then completely saturated with UEO. Once the UEO entered in the column, the brine solution was released out of the matrix of sand and it was collected and calculated as initial oil saturation (Soi). This oil-saturated glass column was then washed with 4–6 PVs of brine solution until no more oil was discharged in the effluent. The oil retained such as residual oil saturation (Sor) after the brine solution wash was calculated on the bases of oil loaded and oil discharged in the effluent from the column. Finally, CFS of 4-day-old bacterial culture containing biosurfactants was then loaded into the oil-saturated column and allowed to stand for one day. The amount of additional oil recovered after 24 h was calculated. This experiment was repeated three times to estimate the efficiency of biosurfactant-producing bacterial isolates for EOR using sand-pack column the percentage of oil recovery was also calculated as described by Pathak and Keharia [24].
Molecular Identification and Phylogenetic Analysis
The bacterial isolates having maximum halo-thermotolerance and potential biosurfactant producers were selected for molecular characterization using 16S rRNA sequence homology as described previously [25]. The bacterial isolates in pure glycerol stocks were sent for commercial amplification and sequencing of the 16S rRNA gene to Macrogen Inc., Seoul, Korea. The amplified nucleotide sequences were obtained and interpreted for similarity index or sequence homology at NCBI GenBank database using the Basic Local Alignment Search Tool (BLAST) against available reference nucleotide library. The phylogenetic distance trees with closely related bacteria were re-constructed using Molecular Evolutionary and Genetic Analysis-X (MEGA-X) software version 10.0 using the Maximum Likelihood method and bootstrap tests [26]. All the 16S rRNA sequences were then submitted in the GenBank database and respective GenBank accession numbers were obtained.
Statistical Analysis
All the experiments were run in triplicate under specified laboratory conditions. The data of each triplicate experiment were managed and statistically analyzed for mean, standard deviation, and analysis of variance (ANOVA) followed by Least significant difference (LSD) estimation at an alpha 0.5, i.e., 95% probability (P < 0.05) using MS-Excel (v2010). Furthermore, all the figures and tables were prepared in MS-Excel (v2010), unless mentioned otherwise.
Results
Sixteen (16) morphologically distinct bacterial colonies were isolated from the seven collected samples. Among them, only 1 bacterium was found Gram-negative bacillus, 2 g-positive cocci and the other 13 were Gram + bacilli. Furthermore, all the bacterial isolates were studied for their cultural and biochemical characteristics (Supplementary file Table S2). Thereafter, the bacterial isolates were preliminarily screened for thermotolerance and halotolerance based on their growth under different temperature and salt concentrations, respectively. The bacterial isolates KJ1MC, KJ2MD, KJ2WE, KJ2MF, KJ2SK, KJ2SL, and KJ2MO were found to grow at 60 to 70 °C (Supplementary file Table S3). On the other hand, KJ1MC, KJ2SK, KJ2SL, and KJ2MO isolates were found to grow up to 17% of salt concentration (Supplementary file Table S4).
Screening for Biosurfactant Production
All sixteen bacterial isolates were screened for their biosurfactants production capability using LB and MSM media (NaCl conc. 5%, 50 °C, 150 rpm for 4 days). Among them, 8 isolates viz. KJ1WA, ND1I, KJ1WM, KJ1WN, KJ1WP, NDG, NDH, and ND2J exhibited no or slow growth, while the remaining 8 isolates viz. KJ1WB, KJ1MC, KJ2MD, KJ2WE, KJ2MF, KJ2SK, KJ2SL, and KJ2MO were found to exhibit best growth absorbance (Fig. 1). Afterwards, the collected CFS was used to screen the biosurfactant production potential of the selected isolates using ODA, OST, emulsification and SFT measuring methods. For OST, Fig. 2a illustrates that among all bacterial isolates, nine isolates (i.e., KJ1WB, KJ1MC, KJ2MD, KJ2WE, KJ2MF, ND2J, KJ2SK, KJ2SL, and KJ2MO) revealed positive results ranging from 5 to 19 mm of a clear zone of oil displacement, while others found to be negative. The CFS containing biosurfactants would displace oil and form a clear zone which indicates the potential of bacterial isolate for biosurfactants production. Likewise, the modified ODA method was also validated to screen and confirm the biosurfactant-producing bacterial isolates. This method is easy to perform as it requires small sample volume (10 µL) and performed on the surface of an oily glass slide as shown in the supplementary file Fig. S1. Among, only 4 bacterial isolates displayed positive result, whereas 12 were found negative for oil displacement activity by a modified method. Therefore, it was considered as to be more robust method in terms screening best microbial isolates having biosurfactant production potential.
Growth absorbance (OD at 600 nm) of the bacterial isolates in LB broth medium for 96 h. The clustered column represents average values of triplicate experiments, while the error bars indicate standard deviation among the replicates. The symbol ‘*’ indicates significantly high biomass-yielding isolates
Bacterial screening for biosurfactant production using three different screening methods. Oil displacement activity (a), emulsification index (b), and surface tension measurement (c). The clustered column represents average values of triplicate experiments, whereas the error bars display standard deviation (SD) among the replicates. The data were statistically analyzed for analysis of variance (ANOVA) followed by Least significant difference (LSD) estimation at an alpha 0.5. The symbols ‘*’ and ‘**’ above column bars indicate most significant results in increasing order of significance at 95% probability (P < 0.05)
Moreover, the kerosene-emulsifying potential of the biosurfactants produced by the bacterial isolates was checked using emulsification assay as described earlier. Out of 16 bacterial isolates, only 7 isolates viz. KJ1WB, KJ1MC, KJ2MD, KJ2MF, ND2J, KJ2SK, and KJ2MO showed positive results (Fig. 2b). The best biosurfactants production was exhibited by KJ1WB, KJ1MC KJ2MD, KJ2SK, and KJ2MO isolates, which were then selected for secondary screening experiments, i.e., SFT measurement, for the confirmation of biosurfactant production. The results of SFT reduction revealed that the lowest SFT values were recorded by the isolate KJ2MO (27.8 mN/m) followed by KJ2SK (29.3 mN/m). However, the SFT reduction values for the isolates KJ2MD, KJ1WB, KJ1MC were found 35, 41, and 42 mN/m, respectively, as shown in Fig. 2c.
Stability Study
The applicability of biosurfactants in several fields depends on their stability at different temperatures and pH values. Therefore, the biosurfactants stability was evaluated for selected bacterial isolates only, i.e., KJ1WB, KJ1MC, KJ2MD, KJ2SK, and KJ2MO. Interestingly, the biosurfactants produced by the isolates were stable over a wide range of temperatures and pH. The stability in terms of ODA of the biosurfactants produced by the isolates KJ1WB, KJ1MC and KJ2SK was achieved at 121 °C, whereas for the isolates KJ2MD and KJ2MO, it displayed maximum ODA at 100 °C (Fig. 3a). Contrarily, the emulsification was maximum for all the biosurfactants at 121 °C as compare to 80–100 °C. It was thus found that the biosurfactants displayed excellent activity at elevated temperature ranges, i.e., 100 °C or above. In addition, the maximum stability of biosurfactants produced by KJ1WB, KJ1MC and KJ2MD isolates was achieved at pH 6–8, pH 10, and pH 8, respectively. Unlike others, the biosurfactants produced by the isolate KJ2SK displayed best functional stability at pH 4; however, the isolate KJ2MO indicates maximum stability at pH 8 (in terms of ODA) and at pH 10 (in terms of emulsifying activity) as shown in Fig. 3c, d.
Functional stability of extracellular biosurfactants produced by selected bacteria over a wide range of temperature and pH. The biosurfactants’ stability at extreme temperature (80 °C, 100 °C and 121 °C) in terms of final activity in ODA (a, c) and emulsification index (b, d) assays is shown using clustered column charts (a, b), while the stability at varying pH (4–10) is shown using stacked column charts (c, d)
Enhance Oil Recovery
The results of enhanced oil recovery (EOR) experiments carried out by two different methods, viz. soil washing and sand packed column, are represented in Fig. 4. For soil washing technique, the percentage additional oil recovery (%AOR) was achieved up to 54.7% (i.e., ≈ 30% more than control), when the CFS of bacterial isolate KJ2MO was used. Similarly, the CFS of the bacterial isolate KJ2SK containing biosurfactants resulted in %AOR of 44.7% from oil-contaminated soil. Additionally, the CFS of the other two bacterial isolates viz. KJ1WB and KJ2MD displayed %AOR of 37% and 37.8%, respectively, as compared to control, i.e., 24% after 24 h of incubation.
Enhanced oil recovery by selected bacterial strains using soil washing technique and sand-packed column methods. The clustered column represents average values of triplicate experiments, whereas the error bars display standard deviation (SD) among the replicates. The data were statistically analyzed for analysis of variance (ANOVA) followed by Least significant difference (LSD) estimation at an alpha 0.5. The symbols ‘*’ and ‘**’ above column bars indicate most significant results in increasing order of significance at 95% probability (P < 0.05)
Similarly, the enhanced oil recovery was also evaluated using sand-packed column under two different experimental settings, i.e., with and without CFS of the bacterial isolates. The results of %AOR through sand-packed column are given in Fig. 4. It was observed that the maximum oil recovery of 51.25% was attained with CFS of the isolate KJ2MO followed by the bacterial isolates KJ2SK (40.5%), KJ1WB (35.5%) and KJ2MD (31.9%), when compared with control (i.e., 19%).
Molecular Identification of Selected Bacterial Isolates
After preliminary identification and screening experiments, the bacterial isolates were further selected for molecular characterization using 16S rRNA ribo-typing. The 16S rRNA gene sequence homology results revealed a 99% similarity index for all the (four) bacterial species, with aligned sequences using the BLAST search tool of NCBI. In general, the isolate KJ1WB shared a close resemblance with Pseudomonas pseudoalcaligenes M10. Contrarily, the other three isolates viz. KJ2MD, KJ2SK and KJ2MO shared the closest similarity with different species of genus Bacillus, i.e., Bacillus aerius RGS230, Bacillus licheniformis BAB-1826, and Bacillus subtilis VKK-30L, respectively, as shown in Neighbor-Joining (NJ) tree Fig. 5. The nucleotide sequences of all the four isolates, i.e., KJ1WB, KJ2MD, KJ2SK and KJ2MO were then submitted to NCBI GenBank under accession numbers MF470189, MF470190, MF470191 and MF470192, respectively.
Neighbor-Joining (NJ) tree of bacterial isolates showing evolutionary relationship with closely related taxa. The evolutionary history was inferred using the Neighbor-Joining method [42]. The optimal tree with the sum of branch length = 0.28681359 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches [43]. The evolutionary distances were computed using the Maximum Composite Likelihood method [25] and are in the units of the number of base substitutions per site. This analysis involved 28 nucleotide sequences. Codon positions included were 1st + 2nd + 3rd + Noncoding. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There were a total of 1650 positions in the final dataset. Evolutionary analyses were conducted in MEGA X [26].
Discussion
In the present study, halo-thermotolerant bacteria were isolated from high salinity areas of district Khairpur, in order to use them for enhanced oil recovery applications through soil washing and sand packed column methods. Microbial isolation and preliminary characterization results revealed very high abundance (94%, n = 15) of Gram-positive bacteria than Gram-negative. Such dominance of Gram-positive bacteria over Gram-negative could be attributed to their strong survival strategies such as endospore formation and thicker cell wall, which help them to tolerate harsh conditions like high salinity and elevated temperatures [27]. The most dominant Gram-positive bacterial genera found to inhibit saline environments include Bacillus and Micrococcus. Similar results were observed by Roohi and co-workers, who also reported more number of Gram-positive isolates from hyper-salinity areas [28]. Thus, our findings were also congruent with previous studies [27,28,29,30,31].
Subsequently, 5–9 bacterial strains displayed efficient biosurfactant production in media containing 5% NaCl, as detected by different screening methods. According to the previous studies, contradictory statements were found for the use of NaCl ranging from 0 to 8% concentration for the production and activity of biosurfactants [32, 33]. Therefore, the NaCl concentration was maintained at 5% (w/v) in the medium used for biosurfactant production throughout this study. Although different assays related variation in number of biosurfactant-producing isolates, the biomass determination was used to rule out possible false-positive results. It can be clearly noticed that the isolates KJ2WE and KJ2WM showed significant biomass values Fig. 1, but at the same time, could not display encouraging results in biosurfactant screening assays. Therefore, on the basis of biosurfactant screening results, finally four bacterial isolates viz. KJ1WB, KJ2MD, KJ2SK and KJ2MO, were selected for further studies. The molecular identification on the basis of the 16S rRNA sequence confirmed the isolates to be species of genera Pseudomonas and Bacillus. Congruently, the biosurfactant producing Pseudomonas and Bacillus species have been reported previously [17, 34,35,36,37,38]. Moreover, all the four selected bacterial isolates were subjected to biosurfactant production in MSM containing kerosene (1% v/v) as a major carbon source for achieving the CFS containing biosurfactants. These CFS samples were used for MEOR experiments to recover UEO from contaminated soil/sand using soil washing and sand-packed column techniques.
In general, the sand packed column method has been appreciated worldwide as a simulated environment to the oil reservoir as compared to the soil washing technique. The later was mostly performed as a screening test, where only ex-situ bioremediation has been targeted [23]. In this connection, many scientists have reported oil recovery through a sand packed column either by CFS containing biosurfactants [24] or whole live microbial cells through the bioaugmentation approach, [10, 38,39,40]. In the present study, maximum oil recovery was achieved with CFS of Bacillus subtilis KJ2MO (50–55%) followed by Bacillus licheniformis KJ2SK isolate (40–45%), while the CFS of P. pseudoalcaligenes KJ1WB isolates displayed oil recovery ranging between (35–38%) by either of two methods Fig. 4. It was observed that the recovery of oil was slightly higher up to 55% in the soil washing technique when compared with sand packed column (51%). This difference could be attributed to the frequent availability of oil as well as agitation speed. However, the results of oil recovery by CFS of B. subtilis KJ2MO through sand packed columns were also significantly better as compared to the previous reports, i.e., 11.7% higher AOR than the B. subtilis K1 and 25.6% higher than B. mojavensis JF2 [24, 41]. The biosurfactants-producing halo-thermotolerant bacterial isolates of this study offer great potential for MEOR applications and could be used in variety of other environmental applications such as bioremediation of oil-contaminated sites.
Conclusion
The biosurfactants-producing halo-thermotolerant bacterial strains of present study have shown promising growth and functional stability over a wide range of salinity and temperature. The biosurfactants produced by the isolates also maintained their functional stabilities at extreme temperature (80–121 °C) and pH (4–10) ranges. Significant EOR outcomes with CFS of the isolates KJ2MO (54.7 and 51.25%) followed by KJ2SK (44.7 and 40.5%), KJ1WB (37 and 35.5%) and KJ2MD (37.8 and 31.9%) as compared to control (i.e., 24 and 19%) using both methods, i.e., soil washing and sand-packed column, respectively, ascertain the future potential of these biosurfactants-producing halo-thermotolerant isolates for variety of environmental applications including enhanced oil recovery, biodegradation, cosmetics, pharmacological, and agricultural fields.
References
Oil TRS (2013) Shale gas resources: an assessment of 137 shale formations in 41 countries outside the United States. Independent Statistics & Analysis and US Department of Energy, Washington
Zhan Y, Wang Q, Chen C, Kim JB, Zhang H, Yoza BA, Li QX (2017) Potential of wheat bran to promote indigenous microbial enhanced oil recovery. J Ind Microbiol Biot 44(6):845–855
Banat IM (1995) Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: a review. Bioresource Technol 51(1):1–12
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol R 61(1):47–64
Cameotra S, Makkar R (1998) Synthesis of biosurfactants in extreme conditions. Appl Microbiol Biot 50(5):520–529
Banat IM, Makkar RS, Cameotra S (2000) Potential commercial applications of microbial surfactants. Appl Microbiol Biot 53(5):495–508
Makkar R, Cameotra S (2002) An update on the use of unconventional substrates for biosurfactant production and their new applications. Appl Microbiol Biot 58(4):428–434
Singh A, Singh B, Ward O (2012) Potential applications of bioprocess technology in petroleum industry. Biodegradation 23(6):865–880
Kowalewski E, Rueslåtten I, Steen K, Bødtker G, Torsæter O (2006) Microbial improved oil recovery—bacterial induced wettability and interfacial tension effects on oil production. J Petrol Sci Eng 52(1):275–286
Sen R (2008) Biotechnology in petroleum recovery: the microbial EOR. Prog Energy Combust 34(6):714–724
Nielsen SM, Shapiro AA, Michelsen ML, Stenby EH (2010) 1D simulations for microbial enhanced oil recovery with metabolite partitioning. Transp Porous Med 85(3):785–802
Haq B, Liu J, Liu K, Al Shehri D (2019) The role of biodegradable surfactant in microbial enhanced oil recovery. J Petrol Sci Eng 189:106688
Henney JE, Taylor CL, Boon CS, Intake IoMCoStRS (2010) Preservation and physical property roles of sodium in foods. In: Strategies to reduce sodium intake in the United States. National Academies Press (US),
Madigan M, Martinko J, Stahl D, Clark D (2012) Methods in microbial ecology. In: Brock biology of microorganisms. Pearson Education, pp 670–696
Cosgrove T (2010) Colloid science: principles, methods and applications. Wiley, Hoboken
Amani H, Sarrafzadeh MH, Haghighi M, Mehrnia MR (2010) Comparative study of biosurfactant producing bacteria in MEOR applications. J Petrol Sci Eng 75(1):209–214
Al-Bahry S, Al-Wahaibi Y, Elshafie A, Al-Bemani A, Joshi S, Al-Makhmari H, Al-Sulaimani H (2013) Biosurfactant production by Bacillus subtilis B20 using date molasses and its possible application in enhanced oil recovery. Int Biodeter Biodegr 81:141–146
Khan R, Khan MI, Zeb A, Roy N, Yasir M, Khan I, Qazi JI, Ahmad S, Ullah R, Bhutto Z (2018) Prokaryotic diversity from extreme environments of Pakistan and its potential applications at regional levels. bioRxiv:342949
González-Rocha G, Muñoz-Cartes G, Canales-Aguirre CB, Lima CA, Domínguez-Yévenes M, Bello-Toledo H, Hernández CE (2017) Diversity structure of culturable bacteria isolated from the Fildes Peninsula (King George Island, Antarctica): a phylogenetic analysis perspective. PLoS ONE 12(6):e0179390
Najafi A, Rahimpour M, Jahanmiri A, Roostaazad R, Arabian D, Soleimani M, Jamshidnejad Z (2011) Interactive optimization of biosurfactant production by Paenibacillus alvei ARN63 isolated from an Iranian oil well. Colloid Surf B 82(1):33–39
Youssef NH, Duncan KE, Nagle DP, Savage KN, Knapp RM, McInerney MJ (2004) Comparison of methods to detect biosurfactant production by diverse microorganisms. J Microbiol Meth 56(3):339–347
Cooper DG, Goldenberg BG (1987) Surface-active agents from two Bacillus species. Appl Environ Microb 53(2):224–229
Urum K, Pekdemir T (2004) Evaluation of biosurfactants for crude oil contaminated soil washing. Chemosphere 57(9):1139–1150
Pathak KV, Keharia H (2014) Application of extracellular lipopeptide biosurfactant produced by endophytic Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) in microbially enhanced oil recovery (MEOR). Biotechnology 4(1):41–48
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci 101(30):11030–11035. https://doi.org/10.1073/pnas.0404206101
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549
de Almeida Couto CR, Alvarez VM, Marques JM, de Azevedo JD, Seldin L (2015) Exploiting the aerobic endospore-forming bacterial diversity in saline and hypersaline environments for biosurfactant production. BMC Microbiol 15(1):1
Roohi A, Ahmed I, Khalid N, Iqbal M, Jamil M (2014) Isolation and phylogenetic identification of halotolerant/halophilic bacteria from the salt mines of Karak, Pakistan. Int J Agric Biol 16:564–570
Pakpitcharoen A, Potivejkul K, Kanjanavas P, Areekit S, Chansiri K (2008) Biodiversity of thermotolerant Bacillus sp. producing biosurfactants, biocatalysts, and antimicrobial agents. Sci Asia 34:424–431
Safary A, Moniri R, Mirhashemi SM, Nikzad H, Khiavi MA (2012) Phylogenetic and biochemical characterization of a new halo-thermotolerant, biofilm-forming Bacillus from Saline Lake of Iran. Pol J Microbiol 62(4):419–425
Zargari S, Ramezani A, Ostvar S, Rezaei R, Niazi A, Ayatollahi S (2014) Isolation and characterization of gram-positive biosurfactant-producing halothermophilic bacilli from Iranian petroleum reservoirs. Jundishapur J Microb 7(8):4228–4241
Freitas F, Alves VD, Carvalheira M, Costa N, Oliveira R, Reis MA (2009) Emulsifying behaviour and rheological properties of the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carbohyd Polym 78(3):549–556
Shavandi M, Mohebali G, Haddadi A, Shakarami H, Nuhi A (2011) Emulsification potential of a newly isolated biosurfactant-producing bacterium, Rhodococcus sp strain TA6. Colloid Surf B 82(2):477–482
Dastgheib S, Amoozegar M, Elahi E, Asad S, Banat I (2008) Bioemulsifier production by a halothermophilic Bacillus strain with potential applications in microbially enhanced oil recovery. Biotechnol Lett 30(2):263–270
Xia W-J, Dong H-P, Yu L, Yu D-F (2011) Comparative study of biosurfactant produced by microorganisms isolated from formation water of petroleum reservoir. Colloid Surf A 392(1):124–130
Ghojavand H, Vahabzadeh F, Shahraki AK (2012) Enhanced oil recovery from low permeability dolomite cores using biosurfactant produced by a Bacillus mojavensis (PTCC 1696) isolated from Masjed-I Soleyman field. J Petrol Sci Eng 81:24–30
Gudiña EJ, Pereira JF, Rodrigues LR, Coutinho JA, Teixeira JA (2012) Isolation and study of microorganisms from oil samples for application in microbial enhanced oil recovery. Int Biodeter Biodegr 68:56–64
Youssef N, Simpson DR, McInerney MJ, Duncan KE (2013) In-situ lipopeptide biosurfactant production by Bacillus strains correlates with improved oil recovery in two oil wells approaching their economic limit of production. Int Biodeter Biodegr 81:127–132
Cunningham AB, Sharp RR, Caccavo F, Gerlach R (2007) Effects of starvation on bacterial transport through porous media. Adv Water Resour 30(6):1583–1592
Arora P, Kshirsagar P, Rana DP, Dhakephalkar P (2019) Hyperthermophilic Clostridium sp. N-4 produced a glycoprotein biosurfactant that enhanced recovery of residual oil at 96°C in lab studies. Colloids Surf B 182:110372
Suthar H, Hingurao K, Desai A, Nerurkar A (2008) Evaluation of bioemulsifier mediated microbial enhanced oil recovery using sand pack column. J Microbiol Meth 75(2):225–230
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Felsenstein J (1985) Confidence-limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Acknowledgements
The authors are extremely thankful for the support of the Higher Education Commission (HEC), Government of Pakistan for funding this research under Start-up Research Grant program to the principle investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Phulpoto, I.A., Jakhrani, B.A., Phulpoto, A.H. et al. Enhanced Oil Recovery by Potential Biosurfactant-Producing Halo-thermotolerant Bacteria Using Soil Washing and Sand-Packed Glass Column Techniques. Curr Microbiol 77, 3300–3309 (2020). https://doi.org/10.1007/s00284-020-02172-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02172-3