Abstract
Here, the taxonomical composition and seasonal dynamics of airborne microbial communities were described in the urban city of Lanzhou, Northwest China. Year-long samples were studied in two filter membranes (Quartz and PTFE). Higher microbial loads were reported in the PTFE than in the quartz filter. Onefold decrease was reported in bacterial loads in spring and summer than winter and autumn for both filters. The fungal loadings were lowest during winter and highest during autumn, followed by summer. The microbial communities included Actinobacteria and Proteobacteria, Ascomycota, and Basidiomycota as major components. Maximum abundance of the members from Gammaproteobacteria, Coriobacteria and Clostridia were studied in all seasons on PTFE membrane, followed by, Erysipelotrichia, Negativicutes and Fusobacteria. Members of Actinobacteria and Bacilli showed higher abundance in spring and winter, with a small proportion during autumn. Members of Clostridia, Gammaproteobacteria, Bacilli, and Actinobacteria showed maximum abundance on the quartz filter in all the seasons. Similarly, on the PTFE, fungi including Dothideomycetes and Agaricomycetes were dominant, followed by Saccharomycetes during summer and winter. The result showed that PM2.5, SO42−, NO2−, Na+, EC, and OC are important environmental parameters influencing the seasonal microbial community. However, the relation of the microbiome with the environment cannot be confidently defined because the environmental factors are changeable and yet interrelated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air pollution is acknowledged as a global risk factor affecting human health and the environment. Studies have shown a strong relationship between poor air quality and human diseases, including emerging infectious diseases (Hodges and Tomcej 2016; Li et al. 2019; Sharma Ghimire et al. 2020; Ghimire et al. 2022). Besides infections, human exposure to bioaerosols is linked with several acute and chronic health issues, including asthma, allergies, sinusitis, rhinitis and bronchitis, and the dispersal of pathogens and health effects from occupational exposure (Eduard et al. 2012; Heederik and Von Mutius 2012). Airborne particles of biological origin (bacteria, viruses, fungi, toxins, pollen, etc.) are suspended in the atmosphere with thousands to millions of cells per cubic meter of air (Bowers et al. 2013; Madhwal et al. 2020; Sharma Ghimire et al. 2020). Atmosphere plays a significant role in the survival, exchange, and transport of microbes in the air, water, soil, plants, animals and humans (Šantl-Temkiv et al. 2018; Sajjad et al. 2020). Such airborne microbes can affect human health by causing several diseases such as cardiovascular diseases, respiratory diseases, infections, allergies, acute toxic effects, and even cancers (Sharma Ghimire et al. 2019; Li et al. 2019).
The atmosphere primarily assists as a transport medium for nutrients (Sajjad et al. 2022) and microorganisms rather than to serve as a reservoir for growth. Hence, the microbial composition in the air is greatly influenced by the geography and characteristics of the given environment, the nature of microorganisms and the transmission and transformation processes (Lighthart and Stetzenbach 1994a, b; Haas et al. 2013; Sharma Ghimire et al. 2019). The microbial concentration and community are governed mainly by the sources of microbial emissions, which could be either natural or anthropogenic, with meteorological influences such as season, temperature, relative humidity (RH) and time of day (Troutt and Levetin 2001; Haas et al. 2013). Previous studies supported that meteorological parameters, temperature, RH, and wind speed are highly influenced by season and significantly impact the microbial community (Mouli et al. 2005; Kaarakainen et al. 2008). However, other factors such as solar radiation, ozone and other chemical co-pollutants are equally supposed to stimulate microbial cell viability (Kaarakainen et al. 2008; Haas et al. 2013). Furthermore, the air pollution due to increasing urbanization and industrialization processes has supplemented more stress on the survival and transport of microbes in the air by adding chemical pollutants such as greenhouse gases, carbon monoxide, hydrocarbons, nitrogen oxides, sulfur dioxide and other trace elements in the atmosphere (Kim 1994; Haas et al. 2013).
Studies have shown the influence of high concentrations of particulate matter and chemical pollutants suspended in the air (derivative from biomass burning, vehicle exhaust and fuel combustion that induces haze events, especially during winter) (Tomasi et al. 2017). It is further found to be proportionally correlated with the relative abundance of total and pathogenic bacteria and fungi in the air (Zhong et al. 2019) because particulate matter and chemical components can act as energy sources, carriers and refugees (Zhai et al. 2018; Wei et al. 2019). Furthermore, it has been estimated that the chemical composition in aerosol particles mainly includes elements, major ions (SO42−), NO3− and NH4+, K+, Cl−, Mg2+, Ca2+ and Na+) and carbonaceous species (e.g., organic carbon, elemental carbon), represents approximately 55% of the variance in microbial species-environment correlations (Innocente et al. 2017; Ruiz-Gil et al. 2020a, b). Hence, studying physicochemical composition and its influence on bioaerosol dispersion processes is relevant to understanding the biological and physical link present in ambient air.
However, the relationship between aerial microbial community composition and meteorology is still not well characterized (Gunthe et al. 2016). The seasonal variability of microbial communities is an essential factor that proposes perception into atmospheric biodiversity and biogeography because bioaerosols play an important role in atmospheric processes. Several studies have reported the association of bioaerosols composition with different meteorological parameters. In northern Colorado, US, the RH and wind speed control airborne bacterial composition (mostly Actinobacteridae and the Pseudomonadales) during the early summer season (Bowers et al. 2013). In southwest Greenland, bacterial communities are positively correlated with air temperature and negatively correlated with RH during mid-summer, showing higher activity for Rubrobacteridae and Clostridiales and a lower activity for Proteobacteria (Šantl-Temkiv et al. 2018). Similarly, some microorganisms are predominant for reproductive reasons during certain times of the year; some co-exist in the air throughout the atmospheric cycle, while others may disperse over long distances (Barberán et al. 2015). Past study on RH done at an urban site of Tokyo revealed that the temporal variation of phylum Proteobacteria (51.4%) was the most common, followed by Firmicutes (13.6%), Cyanobacteria (7.9%), Actinobacteria (7.7%), Bacteroidetes (5.2%), and Acidobacteria (3.0%). Similarly, only Parcubacteria (2.6%) displayed seasonal changes among all phyla, highest during August and September trailed by Sphingomonas (3.5%), Chroococcidiopsis (3.3%), and Bacillus (3.2%) (Uetake et al. 2019). Hence, studying seasonal and temporal differences is challenging yet crucial in understanding how various factors shape the composition of microorganisms in different environments. Additionally, it is reported that microorganisms acclimatizing to such variations over time enhance microbial coexistence, resulting in seasonal abundance and fluctuations in fungal and bacterial community composition (Bardgett et al. 1999, 2005; Smith et al. 2013). However, there is no adequate data to fully explain the relation of microbial communal with the atmospheric process. Therefore, investigating the microbial response to environmental fluctuations during different seasons is a key knowledge gap that is promising for identifying factors important for temporally structuring the microbial community.
The major goal of the present study is to examine seasonal differences in airborne microbial communities over a year collected samples in two different filter membranes (Quartz and PTFE) in an urban Lanzhou city of Gansu, China. PTFE filter membrane possesses a meshnet structure, is strong and resistant to acids, bases, and solvents have a low background, and is ideal for aerosol sampling, especially in water vapor environments. In contrast, quartz membrane is compact and suitable for sampling PM and chemical pollutants. Furthermore, Scaap suggested that the PTFE filter was more vulnerable than the quartz filter at temperatures below 21 °C in terms of evaporation loss. In comparison, the quartz filter was more vulnerable than the PTFE filter at temperatures exceeding 21 °C (Aikawa and Hiraki 2010). Hence, this study also tries to observe the more efficient filter membrane that can be used to analyze the biological component of aerosols as the chemical pollutants and meteorological parameters greatly influence them. Lanzhou (capital of Gansu Province, Northwest China) is situated in a curved-narrow river valley with surrounding mountains blocking a free airflow. Furthermore, Lanzhou lies in the temperate zone with a semi-arid climate (Sharma Ghimire et al. 2020). Therefore, samples from the present study were collected on the rooftop of the building to understand the impact of local sources on the predominance over microorganisms originating regionally. For this purpose, we evaluated the usefulness of two different air filters for studying airborne communities and compared the results in terms of seasonal, temporal and other meteorological parameters in an urban air shed.
Materials and methods
Sampling locations and aerosol collection
Aerosol monitoring was performed at Lanzhou station in Gansu, China. Lanzhou is the largest urban city in northwest China (population = 3.7 million within 13,087 km2) located around the yellow riverbank, including a traffic-influenced residential site and a key regional transportation hub, connecting areas further west by rail to the country's eastern half. A medium-volume aerosol sampler (Laoying 2030, China) (100 L min−1) (Sharma Ghimire et al. 2020) was located on the building roof of Northwest Institute of Eco-Environment and Resources. Fine particulate samples (PM2.5) were collected every six days over 23 h (9: a.m. to 8:00 a.m. on the next day) sampling periods spanning 12 months starting in September 2018 till August 2019. In addition, field blanks were collected concomitantly every month. The samples were collected on the quartz filter membrane (90 mm diameter with pore size 0.22 µm, Whatman™, GE Healthcare, USA) and PTFE filter membrane (Whatman™, GE Healthcare, USA). Samples were collected during the sampling period on both filter membranes using two samplers simultaneously. The sample holders were cleaned and washed using 75% ethanol between two sampling intervals and all filters were sterilized before use (Sharma Ghimire et al. 2020). The collected samples were stored at − 20 °C until further analyses. The details on sampling procedures have been provided elsewhere (Sharma Ghimire et al. 2020).
DNA extraction and PCR amplification
DNA was extracted from each filter sample following a method explained previously with some modification (Cao et al. 2014; Du et al. 2018a, b). Briefly, a quarter of filter punches were aseptically loaded into sterile mortar-pistil, ground into powder, and extracted with 1X phosphate-buffered saline. The extracts of each sample from the same month were combined and filtered with a 0.22 µm sterile vented filter unit (Sterivex™-GV, USA), which was then cut into pieces and used for DNA extraction using DNeasy Powersoil Kit (Qiagen, Germany). All of the tools used in the pretreatment process were sterilized. A blank filter was treated simultaneously using the same operation used for the samples. All the steps in each extraction were performed according to standard Qiagen soil DNA isolation protocol, as described by the manufacturer.
Small-subunit rRNA was amplified using a portion of the 16S rRNA gene designed by GENEWIZ (GENEWIZ, Inc., South Plainfield, NJ, USA) from bacteria and internal transcribed spacer (ITS) region of 18S rRNA in fungi (Sajjad et al. 2018). Briefly, the V3 and V4 region of the 16S rRNA gene was amplified with primers Fpb- “CCTACGGRRBGCASCAGKVRVGAAT” and Rpb- “GGACTACNVGGGTWTCTAATCC”. Indexed adapters were also added to the ends of the 16S rRNA amplicons to generate indexed libraries ready for downstream sequencing on Illumina Miseq. Similarly, oligonucleotide primers were designed to anneal to the relatively conserved sequences spanning fungi ITS gene regions. ITS region was amplified using a forward Fpf-"GTGAATCATCGARTC" and a reverse Rpf-"TCCTCCGCTTATTGAT" primer designed by GENEWIZ (GENEWIZ, USA). Adaptor sequences were added to the ITS target-specific primers to allow uniform amplification of the library with high complexity on the Illumina Miseq platform. PCR cycling reactions were performed in triplicate with 25 μL mixture volume containing 2.5 μL of TransStart Buffer, 2 μL of dNTPs, 1 μL of each primer, and 20 ng of template DNA. Amplicons were pooled at equal concentrations, and PCR clean-up was performed on the pooled DNA using the UltraClean PCR clean-up kit (MoBio Laboratories, Carlsbad, CA). The DNA library was quantified to 10 nM, multiplexed, and loaded on an Illumina MiSeq instrument according to the manufacturer’s instructions (Illumina, San Diego, CA, USA). Sequencing was performed using PE250/300 paired-end; image analysis and base calling were conducted by the MiSeq Control Software (MCS) embedded in the MiSeq instrument.
Microbial enumeration
The viable cell count and culture identification were performed for the quartz and PTFE filter membrane samples. As explained earlier, a grounded filter membrane was mixed with 10 mL sterilized normal saline (0.9% w/v of NaCl), and the suspension was diluted up to 10–7 times. For the enumeration of bacterial and fungal loads, 100 µL from each suspension was inoculated on nutrient agar medium for bacteria and potato dextrose agar (PDA) plates for fungi (Srivastava et al. 2012; Sharma Ghimire et al. 2020). The nutrient agar plates were incubated at 37 °C for 48 h and PDA plates were incubated at 25 °C for 72 h. The colonies were counted on each plate and calculated for the colony-forming unit (CFU/mL). The colonies were isolated and further processed for identification.
Measurement of PM2.5 mass
The collected PM2.5 filter weight was measured twice before and after the sampling. The net accumulation mass for each filter was calculated as the difference between the pre and post-sampling weight microbalance after equilibration at constant temperature and humidity (20 °C, 39%) for 24 h. Field blank filters were also collected through exposure to the sampler with no air drawn.
Measurement of ionic composition
The ionic composition was measured using a punch of the filter (3.14 cm2), which was first extracted with 45 mL of deionized distilled water (18.2 M Ω/cm resistivity), sonicated for 30 min, and filtered through a 0.45-μm filter. The water-soluble ionic components (Cl−, NO2−, SO42−, NO3−, Ca2+, Na+ K+, Mg2+, and NH4+) were analyzed using ion chromatography. The methods for ionic composition and analysis were performed following previous studies (Sharma Ghimire et al. 2020; Tripathee et al. 2021).
Statistical analyses
A direct gradient approach was used to examine relationships between microbial community structure and physicochemical parameters, canonical correspondence analysis (CCA) using XLSTAT. CCA creates an ordination plot, which indicates an array of variation in community composition and the influence of environmental variables. The ordination axes show linear combinations of the physicochemical parameters such as major ions and meteorological parameters such as temperature, pressure, and microbial composition data. Additionally, Spearman’s pairwise correlations between the environmental parameters and microbial taxa have been done, which aided in defining their impact for further ecological analysis.
Results
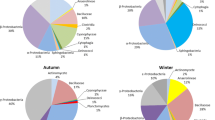
A total of 182 bacterial and 1374 fungal OTUs were identified with a 97% sequence similarity. Overall, the total sequence count obtained in this study for bacteria was 692,874 and for fungal was 1,175,128. The numbers of shared bacterial and fungal OTUs were 49 172, respectively. The numbers of fungal OTUs were higher than bacterial. The fungal OTUs were observed in descending order as Autumn-PTFE > Winter-quartz > Summer-PTFE > Summer-quartz > Winter-PTFE > Autumn-quartz > Spring-PTFE > Spring-quartz. However, no such trends were noted in the case of the bacterial community (Winter-quartz > Spring-quartz = Spring-PTFE = Autumn-PTFE > Winter-PTFE > Summer-quartz = Summer-PTFE.
Concentration and temporal variations of PM2.5 chemical compositions
The annual average concentration and seasonal variation of chemical components measured in PM2.5 are presented in Table 1. The annual average PM2.5 mass was observed to be 90.80 ± 56.82 μg m−3. Carbonaceous species (OC, EC, and WSOC) dominated the fine particulate composition in Lanzhou, with the highest concentration in winter and lowest in summer. Similar levels of carbonaceous species were found during autumn and spring, inferring to the similar sources and emissions during this period. Anthropogenically derived ions such as SO42− and NO3− followed the similar seasonal pattern in Table 1. In contrast, crustal-derived aerosols (Ca2+, IC) had higher concentrations during autumn and spring than in winter, suggesting favorable occurrence during those dry periods. The highest concentrations of K+ (biomass burning tracer) were found during winter, which must be related to heating. The microbial load (fungi and bacteria) showed similar seasonal variation as by the autumn and spring, suggesting the influence of crustal dust sources on bioaerosol variation.
Microbial loadings for different seasons
Figure 1 represents the average concentrations of bacterial and fungal loadings in the outdoor air in Lanzhou during the study period. The first observation is that the sample collected in the PTFE filter membrane is slightly higher for bacterial and fungal loadings than that collected in the quartz membrane. The average concentration of the total bacterial collected in the outdoor air is higher than fungal loadings throughout all seasons. In quartz membrane, the average levels of bacterial loadings in the outdoor air ranged from approximately 2 × 105 to 1.1 × 106 CFU m−3, whereas the average levels of fungal loadings ranged from approximately 1.3 × 104 to 2.5 × 104 CFU m−3. In the case of the PTFE membrane, the bacterial load ranged between 5 × 105 to 1.4 × 106 CFU m−3 while the average levels of fungal loadings ranged from 1.5 × 104 to 3.5 × 104 CFU m−3. The minimum bacterial loadings were observed for both membranes during spring (March, April, May). In contrast, maximum loadings were observed during Autumn (September, October, November) followed by winter (December, January, February) (Fig. 1a). Similarly, the fungal loadings were lowest during winter and highest during autumn, followed by summer (June, July, August) (Fig. 1b).
Average concentration (CFU/m3) of bacterial (a) and fungal (b) loadings on PM2.5 bioaerosols collected for 1 year during different seasons. The red-bar graph indicates average concentrations of bacterial bioaerosols on the quartz filter membrane. The blue-line graph indicates average concentrations of fungal bioaerosols on the PTFE filter membrane
Bacterial compositions amongst taxonomical levels
The experiments were done on quartz and PTFE filter membranes across 4 seasons amongst the class, genus, and family taxonomical levels. As shown in Fig. 2, on the PTFE membrane, the abundance of members belonging to Clostridia was the most dominant class ranging from around 20% to 35% in each season. Similarly, members of Gammaproteobacteria and Coriobacteria were also found to be relatively abundant in each season comprising around 20–30% of the total sequences in all seasons, followed by, the members of Erysipelotrichia, Negativicutes, and Fusobacteria. Similarly, the members of Actinobacteria and Bacilli showed 17–20% abundance in spring and winter, with a very small proportion during autumn (Fig. 2a). On the quartz filter, members of Clostridia, Gammaproteobacteria, Bacilli, and Actinobacteria were dominant in all seasons and accounted for more than 60% of the total sequences. Members of Clostridia were highly abundant in winter and spring (~ 20%) but very low during summer and autumn. Members belonging to Gammaproteobacteria were abundantly present (~ 70%) during the summer season while moderate during the rest of the seasons. Species of Actinobacteria were found highest during autumn and lowest during summer and the other two seasons. The abundance of Bacilli members was nearly constant in all seasons ranging from 5 to 10%. Similarly, members of Oxyphotobacteria, Coriobacteria, and Alphaproteobacterial were relatively abundant in total sequences. However, bacteria such as Nitrososphaeria, Holophagae and Deinococci were minutely present in all seasons. The heatmap of the bacterial community at the class level revealed that some classes of bacteria such as Firmicutes, Negativiticus and Fusobacteriia were observed higher during summer and autumn on the PTFE membrane.
Relative abundance and heatmap illustration of 16S rRNA gene sequences for bacteria classified at the class level: top 19 class. a Relative abundance. b Heatmap illustrates the abundance of the 19 most abundant bacterial classes collected for 1 year during different seasons using quartz and PTFE filter membrane. A blue–white color gradient indicates heatmap’s relative abundance from white to dark blue, with white representing low abundance and dark blue representing high abundance
In comparison, members of Chloroflexia and Acidimicrobiia were observed during autumn on quartz membranes. The bacterial community composition for both quartz and PTFE membranes was similar among winter and spring seasons (Fig. 2b). At the family level (Fig. 3a), members of Peptostreptococcaceae were highly abundant in spring and winter on PTFE membrane (~ 17%) as compared to those on quartz membrane (~ 8%). Similarly, species of Cariobacteriales and Enterobacteriaceae were also present with almost similar abundance as the members of Peptostreptococcaceae. Similarly, members of Enterobacteriaceae concentrations were almost evenly distributed across all the seasons except summer on quartz filter, where their abundance significantly increased by around 40%. Members of families such as Erysipelotrichaceae, Moraxellaceae, Lachnospiraceae were found in all seasons. At the family level (Fig. 3b), members of Peptostreptococcaceae were the dominant family of all sequences. They were available with the highest abundance during summer and autumn on a PTFE filter. However, their concentrations were absent during summer and autumn on quartz filters. Similarly, species of Escherichia and Erysipelotrichaceae were also relatively abundant, the latter being highest during summer on quartz membranes.
The taxon plot of 16 rRNA sequence depicts the dominant bacterial community's composition and relative abundance at a different taxonomic level during different seasons for the samples collected in quartz and PTFE filter membrane. a Relative abundances of dominant bacterial community composition at family taxonomic levels across all samples. b There are relative abundances of dominant bacterial community composition at genus taxonomic levels across all samples
Fungal compositions amongst taxonomical levels
During summer and winter, members of Dothideomycetes and Agaricomycetes showed higher dominance (50 and 20%, respectively) on the PTFE filter, followed by the members of Saccharomycetes with around (~ 8%). While on quartz filter, most distributions were almost similar to that of the PTFE filter; however, members of Dothideomycetes abundance dropped by 30% while members of Agaricomycetes rose by 10% during summer. Similarly, members of Cladosporiaceae, Pleosporaceae, Polyporaceae, Psathyrellaceae, Schizophyllaceae, and Aspergillaceae showed similar abundance in both PTFE and quartz membranes (Fig. 4a). Figure 4b shows that members of fungal classes such as Agaricomycetes and Dothidiomycetes, although present in all the seasons, but maximum during spring and winter respectively, for both filter membranes. Similarly, members of Ascomycota, Malasseziomycetes and Pucciniomycetes were comparatively higher during autumn for the PTFE membrane. At the family level (Fig. 5a), members of Schizoporaceae were the most dominant family in both filter membranes, followed by the members of Tricholomataceae. Other abundant families were Cladosporiaceae, Psathyrellaceae, Pleosporaceae, with a relative abundance of more than 5%. Most of the fungal distributions were almost similar except members of Debaryoomycetaceae, which were more than 5% on PTFE but negligible in quartz membrane during spring. At the genus level (Fig. 5b), Cladosporium was the most abundant in PTFE and quartz filters. The abundance of genus Trametes and Debaryomyces was slightly higher in the quartz during summer. Cladosporiaceae was the most dominant family of fungi in both quartz and PTFE membranes during autumn, whereas Schizophyllaceae and Malasseziaceae were the lowest. Both PTFE and quartz membranes showed similar abundance for class Dothideomycetes (~ 50%) followed by Agaricomycetes (~ 20%), family Cladosporiaceae and genus Cladosporium (~ 20%) during winter. Whereas other genera such as Alternaria, Pleurotus, and Schizophyllum were also abundant during winter. Agaricomycetes were the most dominant class on quartz and PTFE membranes, followed by Dothideomycetes. Saccharomycetes and Tremellomycetes (~ 12%) were significantly higher in the PTFE filter than those obtained from quartz membranes during the spring season.
Relative abundance and heatmap illustration of ITS gene sequences for fungi classified at the class level: top 29 class. a Relative abundance. b Heatmap illustrating the relative abundance of the 29 most abundant bacterial classes collected for 1 year during different seasons using quartz and PTFE filter membrane. A blue–white color gradient indicates heatmap’s relative abundance from white to dark blue, with white representing low abundance and dark blue representing high abundance
Taxon plot of ITS sequence depicting the composition and relative abundance of the dominant fungal community at a different taxonomic level during different seasons for the samples collected in quartz and PTFE filter membrane. a Relative abundances of dominant fungal community composition at family taxonomic levels across all samples. b There are relative abundances of dominant fungal community composition at genus taxonomic levels across all samples
Correlation among the microbial community and with physicochemical parameters
The correlation analyses between bacteria showed that most of the bacteria were positively correlated (p < 0.05), some of which include members of Acinetobacter, Planococcus, and Paracoccus. The positivity indicates that the increment of one bacterium level results in the increment of the others. On the other hand, these positively correlated bacterial levels were negatively correlated with the bacteria such as members of Peptostreptococcaceae, Erysipelotrichaceae, Coriobacteriales, Lachnospiraceae, Atopobium, Veillonella, Peptoniphilus, and Peptococcus. In the case of fungal genera, some genus such as Cladosporium shows a positive correlation with Alternaria, Fusarium, Rhizopus, Penicellium and Clitopilus. On the other hand, some fungi such as Xylodon, Psathyrellaceae, and Bortrytis showed a highly negative correlation with other genera. This suggests that some genera are in symbiosis with some microorganisms, whereas some are rarely present and depend on the abundance of microbial community or vice versa. Refer to the supplementary files for detail.
CCA analyses was carried out to recognize the possible relationship between microbial community structure and environmental parameters (Fig. 6). Based on parameters we used in the study: PM2.5, EC, OC, the ionic composition including NO2, SO4, Cl, Na, K, and Ca were selected in the CCA biplot. The length of an environmental parameter arrow in the ordination plot indicates the strength of the relationship of that parameter to community composition. Based on the relationship between environmental factors and bacterial community composition in samples of the different seasons (Fig. 6a), the environmental factors on the first- and second-ordinal axes accounted for 12.05 and 87.95% between species and environmental factors. Similarly, the first- and second-ordinal axes accounted for 37.63 and 62.37% between species and environmental factors. The result showed that PM2.5, SO4, NO2, Na, EC, and OC are important environmental parameters that could influence the seasonal microbial community for bacteria. However, no significant regression coefficient was observed. However, a positive relation was observed for the fungal community for SO4 and Na, but the relation was not significant.
Further, the results were statistically analyzed for correlation (presented in Supplementary Files) and showed some bacteria such as Escherichia-shigella, Pseudomonas, Acidovorax, Limnobacter and Tumebacillus showed a positive correlation with PM2.5. Some bacteria showed a significant correlation (p < 0.05) with physical factors such as Planomicrobium and Brachybacterium showed a significant correlation with temperature, Kocuria with wind speed, Acinetobacter, Enterococcus, Sphingobacterium and Streptococcus with pressure, Planococcus with OC and EC and Arthrobacter with NO2. Fungi such as Cladosporium showed a positive correlation with EC, Cl, Na and K, whereas Pleurotus was negatively correlated with NO2. Similarly, the pathogenic fungi Aspergillus showed a negative correlation with most of the factors, including NO2, SO4, NO3, Na and K; however, Alternaria was found to be positively correlated with OC, EC, Cl, NO3, Na and K. The relation of the microbiome with the environment cannot be confidently defined because the physiological factors of the microbiome and the environmental factors are changeable and yet interrelated. Hence, the relationship between the microbiome and the environment is highly unpredictable in the free outdoor atmosphere.
Discussion
The current data show that microbial community changes during different seasons and differs with filter membranes in an outdoor setting. Hence, we have concluded that our long-term analysis reliably showed evidence of the airborne microbiome's unique and repeated taxonomic composition coupled to seasons and collection medium. Furthermore, the relative abundance of taxa was recurrently detected in the long-term monitoring of bioaerosol at an urban site located in Lanzhou, China. Thus, the ambient microbial community can be considered ubiquitous taxa, common inhabitants in the atmosphere.
Several past studies have observed seasonal variation of microbial composition for airborne bacterial and fungal communities (Franzetti et al. 2011; Bowers et al. 2013; Uetake et al. 2019). These observations are reliable with the current study, which presented no steady free troposphere microbial community throughout the year of sampling at Lanzhou, with a major microbiome consisting of 19 bacterial and 29 fungal classes. A total of 183 bacterial and 1374 fungal OTUs were identified with a 97% sequence similarity. Many microbiome genera have previously been identified as indicator species for urban outdoor air (Els et al. 2019). Culture-dependent microbial concentration was also different for the four sampling periods. The maximum bacterial loads were observed during autumn and lowest during spring for both PTFE and quartz membranes, whereas the maximum fungal load was observed during autumn and lowest during winter for both filter membranes. However, the magnitude of the microbial loads obtained in the PTFE membrane is higher than the quartz membrane for bacteria and fungi cultures. Several studies have reported that the temperature and relative humidity variation during different seasons of the year, collection medium, sampling period, and geographic location have a greater impact on microbial loadings on aerosols (Sharma Ghimire et al. 2020; Gulshan et al. 2021). A past study also showed a similar magnitude of microbial loads with the highest monthly mean concentrations were observed during November or September (refer to autumn), while the lowest concentrations were observed during December, January, or February (refer to winter) (Gulshan et al. 2021). A different source, regions, transport, and annual variation have been shown to impact the microbial composition of the atmosphere (Burrows et al. 2009; Zweifel et al. 2012; DeLeon-Rodriguez et al. 2013; Sharma Ghimire et al. 2019). One of the mechanisms that might influence airborne microbial composition is thermal convection and air mass mixing in the boundary layer (Zweifel et al. 2012). For instance, the lowest amount of distinctive bacterial genera was found in August (in August, the thermal convection and mixing are active) but the highest number of such genera was found in May (Zweifel et al. 2012). High bacterial loads in spring and fall were previously reported at high elevations sites (Bowers et al. 2012). Similarly, the urban site in Bangladesh showed the occurrence of total airborne bacterial as winter > spring > summer > rainy, whereas the total fungal spore concentrations occurred in the following descending order: summer > spring > winter > rainy (Gulshan et al. 2021). Our study showed a similar pattern for highest bacteria loadings during autumn and winter whereas lowest during spring for both PTFE and quartz filter membranes. In the case of fungi, the highest loadings were observed during autumn for both filter membranes. This result suggests that the PTFE filter membrane showed more efficiency for culture-dependent microbial load identification than the quartz membrane. One similar study compared five different filter membranes for PVDF, MCE, PES, PA, and PTFE filters with < 1, 48, 234, 86, 92, and 113%, respectively (Jeong and Kim 2021). The study pointed out that filter materials can be important parameters for microbial study, including membrane structure, gravimetric porosity, pore size and distribution, tortuosity, surface roughness, and thickness, which differs among commercially available filter membranes. Hence, it cannot convincingly conclude the direct link of filter efficiencies to the properties of filter materials (Jan et al. 2014; Jeong and Kim 2021). In addition, the same study also provided that fungal OTUs substantially exceeded the bacterial OTUs, showing that fungi were more abundant (Jeong and Kim 2021). Fungal loadings and seasonal occurrences showed more variability, which might be due to their sporulation process (Jan et al. 2014). Further, Jeong and Kim (2021) also revealed that fungal and eukaryotic populations were greater by more than four times than bacterial populations in the air for PTFE, PA, and PES filters, followed by PA and PES filters. However, it is still difficult to define the actual interaction between the species in the air. Hence, this study somehow tried to highlight the importance of filter selection for the airborne microbial study.
The present study revealed the maximum occurrence of the members belongs to Gammaproteobacteria, Coriobacteria and Clostridia in all seasons on PTFE membrane, followed by, Erysipelotrichia, Negativicutes and Fusobacteria. Similarly, members of Actinobacteria and Bacilli showed the highest abundance in spring and winter, with a very small proportion during autumn. Members of Clostridia, Gammaproteobacteria, Bacilli, and Actinobacteria showed maximum abundance on the quartz filter in all the seasons. Similarly, on the PTFE filter, fungi such as Dothideomycetes and Agaricomycetes showed higher dominance, followed by Saccharomycetes during summer and winter. The microbial distribution and survival also depend on the physiological nature of microorganisms themselves. For example, the members of Actinobacteria bacteria and most fungal cells can form spores to withstand extreme environments by producing extracellular enzymes that decompose plant and animal residues and complex organic compounds in soils, water, or air (Eisenlord and Zak 2010; Sajjad et al. 2021).
On the other hand, Firmicutes are represented as desert microbes that can produce endospores and contribute to colonization in arid conditions (Bukar et al. 2019). Mycelial fungi such as Ascomycetes or Basidiomycetes cell produce hydrophobins proteins vital to growing aerial hyphae and are usually most active in summer, assisting in transporting higher up in the atmosphere (Chenu and Cosentino 2011). Such physiology may also alter according to seasonal conditions and hence contribute to reshaping the nature and composition of the microbial community.
The microbial communities studied at several urban sites in the world have been studied using 16S rRNA and ITS amplicon sequencing, and the data reveal the existence of similar dominant phyla, such as Actinobacteria, Bacillus, Proteobacteria, Bacteroidetes, and Firmicutes. However, the proportion of each phylum exhibited a difference at abundance level among the sites. To the best of our knowledge, this study is the first to compare the relative seasonal abundance of the outdoor microbiome in the urban city of Lanzhou. Evidently, seasonality has shown a significant role in reshaping the microbiome profiles in the environments (Ruiz-Gil et al. 2020a, b). The trend of publics migrating from rural to urban areas and the rapid changes environmental factors and seasonal variation. The present study provides essential observations to facilitate further research on the understanding interrelationships of the environmental and seasonal microbiome in urban cities. For example, the taxonomic profile of airborne bacteria (Table 2) has shown the Proteobacteria (Pseudomonas, Acinetobacter, Methylobacterium, Acetobacter and Sphingomonas) as the most abundant phylum in the air. Similarly, the Firmicutes (Bacillales and Lactobacillales), Actinobacteria (Corynebacteriales and Micrococcales), and Bacteroidetes (Sphingobacteriales) are also representatively found in air samples collected at several urban sites (Du et al. 2018a, b; Núñez et al. 2019). Ascomycota and Basidiomycota are the most common fungal phyla commonly found in urban sites of South Korea and Xian, China (Kumari and Choi 2014; Wang et al. 2021). Furthermore, the geographic location and sampling area also greatly influence the microbiome composition, characterized by the relative offerings of the different bioaerosol emission sources at different urbanization levels (Bowers et al. 2013; Gandolfi et al. 2013; Sharma Ghimire et al. 2020). For instance, abundant vegetation and soil influences bioaerosols in rural areas. In contrast, urban and suburban areas are characterized by physicochemical factors (PM10, PM2.5 and CO), gas, industrial, and dust. This enables a high relative abundance of pathogenic and pollutant-degrading bacteria (Wei et al. 2019), further influenced by the regional temperature, atmospheric pressure, humidity, precipitation, and other climatic factors. Past studies showed that the Actinomycetales, Rhizobiales, Sphingomonadales, Pseudomonadales and Enterobacteriales were more predominantly observed in samples collected after precipitation, where Burkholderiales, Lactobacillales and Clostridiales were less dominant (Jang et al. 2018). Similarly, plant-associated bacteria (e.g., Sphingomonadales) are sometimes observed during warm seasons, soil-inhabiting bacteria (such as Actinobacteria and Firmicutes) can more frequently prevail during dry and crop-harvesting seasons (Franzetti et al. 2011; Bowers et al. 2013). Another study revealed Burkholderiales and Actinomycetales as predominant orders in colder seasons while showing Rhodobacterales dominance during warmer seasons at two urban sites in Northern Italy (Gandolfi et al. 2015).
Conclusions
The present study was intended to disclose the variations in bacterial and fungal community composition and their relations with environmental factors in the outdoor air of urban Lanzhou. In our study, seasonal changes significantly impacted bacterial and fungal bioaerosol composition for a year-long period. In addition, meteorological parameters such as relative humidity and wind speed appeared to influence bioaerosol composition and concentration. It was observed that a higher microbial load for the samples collected in the PTFE filter medium than the quartz filter medium. Moreover, the microbial loadings and abundance were higher for fungi than bacteria. However, the bacterial loads are higher during autumn and winter, while fungal loads are higher during autumn and spring. Further studies on outdoor bioaerosols are needed to understand better the relation of atmospheric biological and physical processes in the future.
References
Aikawa M, Hiraki T (2010) Difference in the use of a quartz filter and a PTFE filter as first-stage filter in the four-stage filter-pack method. Water Air Soil Pollut 213(1):331–339
Barberán A, Ladau J, Leff JW et al (2015) Continental-scale distributions of dust-associated bacteria and fungi. PNAS 112(18):5756–5761
Bardgett RD, Lovell RD, Hobbs PJ, Jarvis SC (1999) Seasonal changes in soil microbial communities along a fertility gradient of temperate grasslands. Soil Biol Biochem 31(7):1021–1030
Bardgett RD, Bowman WD, Kaufmann R, Schmidt SK (2005) A temporal approach to linking aboveground and belowground ecology. Trends Ecol Evol 20(11):634–641. https://doi.org/10.1016/j.tree.2005.08.005
Bowers RM, McCubbin IB, Hallar AG, Fierer N (2012) Seasonal variability in airborne bacterial communities at a high-elevation site. Atmos Environ 50:41–49. https://doi.org/10.1016/j.atmosenv.2012.01.005
Bowers RM, Clements N, Emerson JB et al (2013) Seasonal variability in bacterial and fungal diversity of the near-surface atmosphere. Environ Sci Technol 47(21):12097–12106
Bukar M, Sodipo O, Dawkins K et al (2019) Microbiomes of top and sub-layers of semi-arid soils in North-Eastern Nigeria are rich in Firmicutes and Proteobacteria with surprisingly high diversity of rare species. Adv Microbiol 9(1):102–118
Burrows SM, Elbert W, Lawrence MG, Pöschl U (2009) Bacteria in the global atmosphere–Part 1: review and synthesis of literature data for different ecosystems. Atmos Chem Phys 9(23):9263–9280
Cao C, Jiang W, Wang B et al (2014) Inhalable microorganisms in Beijing’s PM2.5 and PM10 pollutants during a severe smog event. Environ Sci Technol 48(3):1499–1507. https://doi.org/10.1021/es4048472
Chenu C, Cosentino D (2011) Microbial regulation of soil structural dynamics. In: The architecture and biology of soils: life in inner space, pp 37–70
DeLeon-Rodriguez N et al (2013) Microbiome of the upper troposphere: species composition and prevalence, effects of tropical storms, and atmospheric implications. PNAS 110(7):2575–2580
Du P, Du R, Ren W, Lu Z, Fu P (2018a) Seasonal variation characteristic of inhalable microbial communities in PM2.5 in Beijing city. China Sci Total Environ 610:308–315
Du P, Du R, Ren W, Lu Z, Zhang Y, Fu P (2018b) Variations of bacteria and fungi in PM2. 5 in Beijing, China. Atmos Environ 172:55–64
Eduard W, Heederik D, Duchaine C, Green BJ (2012) Bioaerosol exposure assessment in the workplace: the past, present and recent advances. J Environ Monit 14(2):334–339. https://doi.org/10.1039/c2em10717a
Eisenlord SD, Zak DR (2010) Simulated atmospheric nitrogen deposition alters actinobacterial community composition in forest soils. Soil Sci Soc Am J 74(4):1157–1166
Els N, Baumann-Stanzer K, Larose C, Vogel TM, Sattler B (2019) Beyond the planetary boundary layer: bacterial and fungal vertical biogeography at Mount Sonnblick, Austria. Geo Geogr Environ 6(1):e00069
Franzetti A, Gandolfi I, Gaspari E, Ambrosini R, Bestetti G (2011) Seasonal variability of bacteria in fine and coarse urban air particulate matter. Appl Microbiol Biotechnol 90(2):745–753
Gandolfi I, Bertolini V, Ambrosini R, Bestetti G, Franzetti A (2013) Unravelling the bacterial diversity in the atmosphere. Appl Microbiol Biotechnol 97(11):4727–4736
Gandolfi I, Bertolini V, Bestetti G et al (2015) Spatio-temporal variability of airborne bacterial communities and their correlation with particulate matter chemical composition across two urban areas. Appl Microbiol Biotechnol 99(11):4867–4877
Ghimire PS, Tripathee L, Chen P, Kang S (2019) Linking the conventional and emerging detection techniques for ambient bioaerosols: a review. Rev Environ Sci BioTechnol 1–29
Ghimire PS, Tripathee L, Kang S (2022) Modification and coupled use of technologies are an essential envisioned need for Bioaerosol study-an emerging public health concern. Fundam Res 2:218–221
Gulshan JE, Hossain SA, Hossain ME et al (2021) Seasonal variations of microbes in particulate matter obtained from Dhaka City in Bangladesh. Environ Pollut Bioavailab 33(1):122–134
Gunthe S, Beig G, Sahu L (2016) Study of relationship between daily maxima in ozone and temperature in an urban site in India. Curr Sci 110:1994–1999
Haas D, Galler H, Luxner J et al (2013a) The concentrations of culturable microorganisms in relation to particulate matter in urban air. Atmos Environ 65:215–222. https://doi.org/10.1016/j.atmosenv.2012.10.031
Heederik D, Von Mutius E (2012) Does diversity of environmental microbial exposure matter for the occurrence of allergy and asthma? J Allergy Clin Immunol 130(1):44–50. https://doi.org/10.1016/j.jaci.2012.01.067
Hodges E, Tomcej V (2016) Is there a link between pollutant exposure and emerging infectious disease? Can Vet J 57(5):535
Innocente E, Squizzato S, Visin F et al (2017) Influence of seasonality, air mass origin and particulate matter chemical composition on airborne bacterial community structure in the Po Valley, Italy. Sci Total Environ 593:677–687
Jan D, Mir TA, Kamilli AN, Pandit AK, Aijaz S (2014) Relationship between fungal community and physico-chemical characteristics in the Hokersar Wetland, Kashmir Himalayas. Afr J Microbiol Res 8(4):368–374. https://doi.org/10.5897/AJMR12.1836
Jang GI, Hwang CY, Cho BC (2018) Effects of heavy rainfall on the composition of airborne bacterial communities. Front Environ Sci Eng 12(2):1–10
Jeong SY, Kim TG (2021) Comparison of five membrane filters to collect bioaerosols for airborne microbiome analysis. J Appl Microbiol 131(2):780–790
Kaarakainen P, Meklin T, Rintala H et al (2008) Seasonal variation in airborne microbial concentrations and diversity at landfill, urban and rural sites. Clean: Soil, Air, Water 36(7):556–563
Kim J (1994) Atmospheric environment of bioaerosols. Atmospheric microbial aerosols. Springer, Berlin, pp 28–67
Kumari P, Choi HL (2014) Seasonal variability in airborne biotic contaminants in swine confinement buildings. PLoS ONE 9(11):e112897
Li H, Zhou XY, Yang XR et al (2019) Spatial and seasonal variation of the airborne microbiome in a rapidly developing city of China. Sci Total Environ 665:61–68
Lighthart B, Stetzenbach LD (1994a) Distribution of microbial bioaerosol. Atmospheric microbial aerosols. Springer, Berlin, pp 68–98
Lighthart B, Stetzenbach LD (1994b) Distribution of microbial bioaerosol atmospheric microbial aerosols. Springer, Berlin, pp 68–98
Madhwal S, Prabhu V, Sundriyal S et al (2020) Ambient bioaerosol distribution and associated health risks at a high traffic density junction at Dehradun city, India. Environ Monit Assess 192(3):1–15
Mouli P, Mohan S, Reddy S (2005) Assessment of microbial(bacteria) concentrations of ambient air at semi-arid urban region: Influence of meteorological factors. Appl Ecol Environ Res 3(2):139–149
Núñez A, de Paz GA, Rastrojo A et al (2019) Temporal patterns of variability for prokaryotic and eukaryotic diversity in the urban air of Madrid (Spain). Atmos Environ 217:116972
Ruiz-Gil T, Acuña JJ, Fujiyoshi S et al (2020a) Airborne bacterial communities of outdoor environments and their associated influencing factors. Environ Int 145:106156. https://doi.org/10.1016/j.envint.2020.106156
Ruiz-Gil T, Acuña JJ, Fujiyoshi S et al (2020b) Airborne bacterial communities of outdoor environments and their associated influencing factors. Environ Int 145:106156
Sajjad W, Zheng G, Zhang G, Ma X, Xu W, Ali B, Rafiq M (2018) Diversity of prokaryotic communities indigenous to acid mine drainage and related rocks from Baiyin Open-Pit Copper Mine Stope, China. Geomicrobiol J 35(7):580–600. https://doi.org/10.1080/01490451.2018.1430873
Sajjad W, Rafiq M, Din G, Hasan F, Iqbal A, Zada S, Ali B, Hayat M, Irfan M, Kang S (2020) Resurrection of inactive microbes and resistome present in the natural frozen world: reality or myth? Sci Total Environ 735(2020):139275
Sajjad W, Ali B, Bahadur A, Ghimire PS, Kang S (2021) Bacterial diversity and communities structural dynamics in soil and meltwater runoff at the frontier of Baishui Glacier No. 1, China. Microb Ecol 81(2):370–384. https://doi.org/10.1007/s00248-020-01600-y
Sajjad W, Ilahi N, Kang S, Ali B, Zada S, Iqbal A (2022) Endolithic microbes of rocks, their community, function and survival strategies. Int Biodeterior Biodegrad 169(2022):105387. https://doi.org/10.1016/j.ibiod.2022.105387
Šantl-Temkiv T, Gosewinkel U, Starnawski P et al (2018) Aeolian dispersal of bacteria in southwest Greenland: their sources, abundance, diversity and physiological states. FEMS Microbiol Ecol 94(4):fiy031
Sharma Ghimire P, Tripathee L, Chen P, Kang S (2019) Linking the conventional and emerging detection techniques for ambient bioaerosols: a review. Rev Environ Sci Biotechnol 18(3):495–523. https://doi.org/10.1007/s11157-019-09506-z
Sharma Ghimire P, Kang S, Sajjad W et al (2020) Microbial community composition analysis in spring aerosols at urban and remote sites over the Tibetan Plateau. Atmosphere 11(5):527
Smith DJ, Timonen HJ, Jaffe DA et al (2013) Intercontinental dispersal of bacteria and archaea by transpacific winds. Appl Environ Microbiol 79(4):1134–1139. https://doi.org/10.1128/AEM.03029-12
Srivastava A, Singh M, Jain V (2012) Identification and characterization of size-segregated bioaerosols at Jawaharlal Nehru University, New Delhi. Nat Hazards 60(2):485–499
Tomasi C, Fuzzi S, Kokhanovsky A (2017) Atmospheric aerosols: life cycles and effects on air quality and climate. Wiley, Berlin
Tripathee L, Kang S, Chen P, Bhattarai GJ, Shrestha KL, Sharma CM, Ghimire PS, Huang J (2021) Water-soluble organic and inorganic nitrogen in ambient aerosols over the Himalayan middle hills: seasonality, sources, and transport pathways. Atmos Res 250:105376
Troutt C, Levetin E (2001) Correlation of spring spore concentrations and meteorological conditions in Tulsa, Oklahoma. Int J Biometeorol 45(2):64–74
Uetake J, Tobo Y, Uji Y et al (2019) Seasonal changes of airborne bacterial communities over Tokyo and influence of local meteorology. Front Microbiol 10:1572
Wang S, Liu W, Li J et al (2021) Seasonal variation characteristics of bacteria and fungi in PM2.5 in typical basin cities of Xi’an and Linfen, China. Atmosphere 12(7):809
Wei M, Xu C, Xu X, Zhu C, Li J, Lv G (2019) Characteristics of atmospheric bacterial and fungal communities in PM2.5 following biomass burning disturbance in a rural area of North China Plain. Sci Total Environ 651:2727–2739
Zhai Y, Li X, Wang T, Wang B, Li C, Zeng G (2018) A review on airborne microorganisms in particulate matters: composition, characteristics and influence factors. Environ Int 113:74–90. https://doi.org/10.1016/j.envint.2018.01.007
Zhong S, Zhang L, Jiang X, Gao P (2019) Comparison of chemical composition and airborne bacterial community structure in PM2.5 during haze and non-haze days in the winter in Guilin. China Sci Total Environ 655:202–210
Zweifel UL, Hagström Å, Holmfeldt K et al (2012) High bacterial 16S rRNA gene diversity above the atmospheric boundary layer. Aerobiologia 28(4):481–498
Acknowledgements
We acknowledge the support provided by the second Tibetan Plateau Scientific Expedition and Research Program (STEP) (2019QZKK0605), the National Natural Science Foundation of China (41630754, 41721091), and the State Key Laboratory of Cryospheric Science (SKLCS-ZZ-2018). Prakriti Sharma Ghimire is supported by a PIFI Fellowship from the Chinese Academy of Sciences (PIFI2018PC20021) and China Postdoctoral Science Funding (2019M663859).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All authors approve to submit and publication to the journal.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma Ghimire, P., Joshi, D.R., Tripathee, L. et al. Seasonal taxonomic composition of microbial communal shaping the bioaerosols milieu of the urban city of Lanzhou. Arch Microbiol 204, 222 (2022). https://doi.org/10.1007/s00203-022-02832-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02832-x