Abstract
Ambient levels of viable bioaerosol were measured at four different sites on the campus of Jawaharlal Nehru University (JNU), New Delhi, India. Most of the viable bioaerosol was associated with the fungal fraction with the remainder due to bacteria. The highest concentrations of fungi were found at a health center (1,293 cfu/m3). The highest concentrations of both gram-positive (338.8 cfu/m2) and gram-negative (614 cfu/m2) bacteria were found at a garbage dump site. Gram-negative bacteria were found in larger numbers than gram-positive, possibly due to the higher ambient temperatures during their sampling. Most of the fungal bioaerosol identified is associated with immunotoxic diseases such as sick building syndrome and allergic diseases and was found in respirable fractions. Since the bacterial fractions were identified on the basis of morphology, i.e. only to the level of genus, their relationship to health effects could not be established. Although most of the sampling occurred indoors in naturally ventilated buildings, external temperature and humidity did not vary significantly during the study. Thus, it is more likely that the types of organisms and levels observed had more to do with the sources (decomposing garbage, spores carried from wooded areas, etc.) than the ambient or indoor environmental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bioaerosols are airborne particles composed of living organisms (bacteria, fungi and viruses) or that originate from living organisms (pollen, cell fragments, etc.). They are ubiquitous, highly variable and complex and are natural or man-made in origin. The sampling and analysis of bioaerosols have received attention in recent years due to concerns with mold contamination in indoor environments, the threat of bioterrorism and the occurrence of associated health effects, including infectious diseases, acute toxic effects, allergies and cancer (Douwes et al. 2003; Riordan et al. 2004; Stetzenbach et al. 2004). Bioaerosols constitute 5–34% of all indoor air pollution (www.pollutionissues.com 2006; www.airqualitydirect.com 2007).
Bacterial cells and cellular fragments, fungal spores and by-products of microbial metabolism, present as particulate, liquid or volatile organic compounds, may be components of bioaerosols (Stetzenbach 2005). Air contains a significant number of bioaerosol particles. Inhalation, ingestion and dermal contact are the primary routes of human exposure with inhalation the most significant. The particles in a bioaerosol are generally 0.3–100 μm in diameter; however, the respirable size fraction of 1–10 μm is of primary concern (Cox and Wathes 1995). Bioaerosols ranging in size from 1.0 to 5.0 μm generally remain in the air, whereas larger particles are deposited on surfaces (Mohr 2002).
Bioaerosols, unlike chemicals, do not have established exposure limits. This makes it difficult to assess health impacts or toxic effects and is due in part to their complexity, variations in human response to them and difficulty in their sampling (Macher et al. 1999).
Both the development and application of bioaerosol sampling and assay methods have been studied (Reinthaler et al. 1997; Hameed and Awad 2007; Wang et al. 2010). Prior knowledge of the likely forms of bioaerosol present is necessary to properly design the sampling and analysis system to be used. This is especially true for microorganisms, where special media for colony growth are required.
2 Experimental
2.1 Bioaerosol sampling
Bioaerosol sampling was performed at Jawaharlal Nehru University (JNU), located in New Delhi, the capital of India. JNU is situated in a bush forest on a ridge of the Aravali Hills on about 1,000 acres (4km2) of land in southwest New Delhi. JNU has an enrollment of over 5,500 students, 550 faculty and more than 1,000 other staff. Four sampling sites representative of the entire campus were selected (Fig. 1). These locations were considered likely sources of bioaerosols and were expected to provide an indication of total exposure at the university. The sites were a garbage disposal area (Site A), the Jhelium mess—a cafeteria (Site B), the health center (Site C) and an experimental laboratory of the School of Environmental Sciences (Site D).
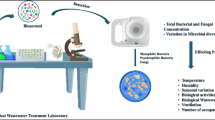
Air sampling was conducted near the center of each location, i.e. rooms nearest the center of the buildings or the garbage disposal area. Sampling was conducted on 3 days at each site in March and April 2010 from 11 a.m. to 6 p.m. (Table 1). This is a period of transition from winter to spring/summertime conditions with temperatures rising from the beginning of the period to the end. Fungal sampling was conducted on March 18, gram-positive bacteria sampling on April 6 and gram-negative bacteria sampling on April 23. Sampling was carried out using a six-stage viable cascade impactor sampler (Tisch Environmental, South Miami, OH, USA). This sampler collects both the total and what can be considered respirable bioaerosol fractions. Size fraction cutoffs for each stage and their association with the human respiratory system are given in Table 2. The sampler was located at a height of 1.4 m to simulate exposure in the human breathing zone. Sampling was carried out for 30 min at a flow rate of 28.3 l/min.
2.2 Culture media
2.2.1 Fungi
The fungal fraction of bioaerosol was collected over potato dextrose agar media. Potato dextrose agar (PDA M127) or PDA and potato dextrose broth (PDB) are common microbiological culture media made from potato infusion and dextrose (corn sugar). PDA is the most widely used medium for growing fungi and bacteria that infect living plants or decompose dead plant biomass (Eddleman 2005). The PDA medium was prepared using pre-made (purchased) PDA. A 2% (vol.) solution was made in distilled water and sterilized by autoclaving at 15psi for 15 min (Bacteriological Analytical Manual 1998). It was then poured into sterilized Petri dishes and allowed to solidify prior to use.
2.2.2 Bacteria
Gram-negative bacteria were collected on eosin methylene blue agar media (EMB agar media). EMB agar is a selective medium used for the isolation and differentiation of enteric bacilli, especially coli forms, in clinical specimens, water and dairy products. This is a selective-differential plating medium for the detection and isolation of gram-negative bacteria. Gram-positive bacteria were collected on blood agar using commercial Blood Agar Base Improved. This is used with blood for the isolation and cultivation of a wide variety of fastidious microorganisms, those that require nutrients to grow. Blood agar bases are typically supplemented with 5–10% sheep, rabbit or horse blood for use in isolating, cultivating and determining hemolytic reactions of fastidious pathogenic microorganisms. Without enrichment, blood agar bases can be used as general purpose media as was the case in this study.
2.2.3 Media blank
Laboratory media blanks (LMB) and field media blanks (FMB) were prepared. LMB were unexposed, fresh media samples. These samples were not taken into the field. Approximately five LMB’s were included with each sample set. FMB’s were unopened, fresh media samples that were treated the same as field samples, including labeling, except no air was drawn through them. Two field blanks for every 10 samples were prepared with a maximum of 10 field blanks for each sample set. All results were blank corrected.
2.3 Preparation, identification and enumeration of culturable bioaerosols
2.3.1 Sample preparation
Inoculated (exposed) agar plates were incubated at the appropriate temperature for times ranging from hours for a fast-growing bacterium to develop a microcolony to days for a fungus to develop into a visible colony, and perhaps sporulate to weeks for an organism such as drug-resistant Mycobacterium tuberculosis to produce visible colonies (ATS 1990). In this study, incubation was done for 2 h, at 25°C or room temperature with natural light for fungi and at 25–30°C for bacteria (ACGIH 1989; Baron and Finegold 1990; Smith 1990).
2.3.2 Enumeration
A colony is a macroscopically visible growth of microorganisms on a solid culture medium. Colonies that grew on the Petri dishes after incubation appeared as fixed circular spots. They were counted using a colony counter. The total concentration of culturable organisms was calculated by dividing the volume of air sampled into the total number of colonies counted as shown in the equation below. The concentration is reported as the number of colony-forming units (cfu) per unit volume of air.
Often, it is difficult to identify multiple colonies at one location on a plate because of the lack of differential colony morphology or because the chemicals secreted by one microorganism might inhibit the growth of other microorganisms at that same location (Burge et al. 1977). In addition, some organisms produce large, spreading colonies, while others produce microcolonies. Also, the morphology of the colony of one microorganism may completely obscure that of another, and a fast-grower might obscure a slow-grower. In these cases, a statistical adjustment of the observed number of colonies is needed to account for the probability that more than one particle impacted the same site (Andersen 1958; Leopold 1988; Macher 1989). The adjusted concentration of culturable microorganisms is calculated by following the reference http://www.skcinc.com/pdf/Multiple_Jet_Impactors.pdf.
2.3.3 Identification
A small portion of a fungus colony is taken with the help of inoculum loop and placed on to a slide containing 4% of NaCl. A drop of cotton blue stain is added over it immediately and left for about 1–2 min. The area is then covered by a cover slip and now it is ready for microscopic examination and visual identification.
For bacterial identification, a thin film of bacterial culture was fixed on a clean slide and stained for 1 min with ammonium oxalate crystal violet solution. The slide was then washed with water for less than 2 s to remove the excess stain, immersed in iodine solution for 1 min, washed with water and allowed to dry. It was then immersed in 95% ethanol for 30 s and safranin solution for 10 s. After a final water wash, glycerin was added and it was covered with a cover slip prior to microscopic examination and visual identification. Using this procedure, the gram-positive bacteria appear purple and the gram-negative pink to red. All bacterial genera were identified and classified on the basis of the colony morphology and their cellular shape by microscopic examination. No individual species could be determined using this method.
3 Results
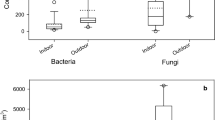
The number of microorganisms found in the air samples at JNU varied with the sampling location. The average number of colonies or colony-forming units at each sampling site for each bioaerosol fraction (fungal, bacterial) is shown in Figs. 2 and 3 and the concentrations in cfu/m3 in Figs. 4 and 5. The concentration of total viable airborne microorganisms ranged from 63.6 cfu/m3 at environmental laboratory (Site D) to 1,293.2 cfu/m3 at the health center (Site C).
3.1 Fungi
The concentration of fungi ranged from 755 to 1,293 cfu/m3 and also varied across impactor stages. As shown in Fig. 2a, the number of fungal colonies was greatest for impactor Stage 4 at all sites and lowest for Stage 6. The second highest concentrations were observed for Stage 3 and the second lowest for Stage 1. The concentrations by stage roughly follow a unimodal distribution, somewhat skewed toward the lower stages (the higher size ranges). The number of colonies also increased somewhat with decreasing particle size up to Stage 4 and then declined rapidly to almost zero for Stage 6. As indicated in Fig. 5, the maximum average concentration (1,293 cfu/m3) and mean variation across all stages (one SD: 210 ± 148.2 cfu/m3) were observed at Site C, the health center. The lowest concentrations were found at Site D, the environmental laboratory (755 cfu/m3; one SD: 94.4 ± 79.7 cfu/m3).
Fungi from 12 different genera were found in the JNU bioaerosol fractions (Table 3). Four of these constituted 78% of total colonies (Aspergillus 37%, Alterneria 16%, Ulocladium 13% and Rhizopus (12%). Aspergillus, Alterneria and Rhizopus are allergic and immunotoxic, while Ulocladium is allergic.
3.2 Gram-positive bacteria (GPB)
The concentration of gram-positive bacteria (GPB) ranged from 63.6 to 338.8 cfu/m3, but unlike that observed for the fungal fraction, did not follow a recognizable pattern across impactor stages either for the total number or concentration of colonies (Figs. 2b, 3, 4b, 5). The overall number and concentration of colonies were greatest for the garbage dumping site (Site A) and least for the environmental laboratory (Site D) with results for the other two sites similar to each other.
Ten different gram-positive bacterial genera were identified (Table 4), although this may underestimate the true number of strains present. Five of these (Bacillus 35.9%, Coccus 14.9%, Palisade 13.3%, Streptobacillus 7.5% and Streptococcus 7.3%) accounted for 79% of the total with the first three most frequently found in the different impactor stages.
3.3 Gram-negative bacteria (GNB)
The gram-negative bacteria identified also did not follow a recognizable pattern with respect to number of cfus or concentration relative to impactor stages (Figs. 2c, 3, 4c, 5). Once again, the garbage dumping site was the largest contributor and the environmental laboratory the smallest. The GNB concentration was about twice that observed for the GPB across all sites, ranging from 159 to 614 cfu/m3.
Eight different genera of GNB were identified and are listed in Table 5. Similar to the GPB, 5 of these Bacillus (31.1%), Coccus, (19.1%), Micrococcus (5.6%), Streptobacillus (8.2%) and Streptococcus (12%) accounted for 76% of total colonies.
4 Discussion
The fungal fraction of bioaerosol constituted 54–80% of the total collected (Table 6). As noted previously, the highest concentrations were found in samples from Site C, the health center (1,293 cfu/m3), possibly due to the presence of a pathology laboratory, a dressing room and a toilet which could be major sources of fungal bioaerosol for this location. The outside air is also a potential source with such factors as two canteens that are located in close proximity to the building, nearby traffic, a bus stop and a parking lot all creating turbulence that could cause dust containing spores to be suspended in the air and drawn into the building. Some of the aerosol could also be of natural origin due to dense vegetation surrounding the center. The fact that the room where the sampling was carried out is naturally ventilated supports some of the fungi originating from external sources. The lowest concentration observed (755 cfu/m3) at Site D is probably due to the fact the laboratory has limited exposure to both indoor and outdoor source of fungi.
Certain fungi have known undesirable human health effects. Among the 12 genera identified, Alternaria was found at three out of four of the sites and among the highest concentrations. It is known to be a frequent cause of inhalant diseases (Kool et al. 1999). Aspergillus, also an allergen as well as immunotoxic was also found at all sites. Botrytis and Epicoccum are also known allergens (Kirmeyer et al. 1993) along with Basidiomycetes of which Basidiospores was also found (Breathnach et al. 1998). Cladosporium was also detected.
The total bacterial concentration varied by an order of magnitude (64–614 cfu/m3) across the sites with the highest concentrations of both GPB (614 cfu/m3) and GNB (338 cfu/m3) measured in samples from the garbage dumping site (Site A). The high levels relative to the other sites can probably be explained by the constant input of waste along with the high ambient temperatures at the time of sampling and the fact that, this site is outdoors. As was the case with the fungal bioaerosol, the lowest levels of bacteria were found in the air from the environmental laboratory, 63 cfu/m3 for GPB and 159 cfu/m3 for GNB.
GNB were found at higher levels at each site than GPB, even though there is evidence that some GPB have greater resistance and survival ability than GNB (Traeger et al. 2002).
Although no measurements of temperature or relative humidity were taken at the sites during the sampling period, local temperature, relative humidity and wind speed were obtained from the Centre for Studies in Regional Development, JNU, and are shown in Table 1.
These factors may have influenced the absolute concentration of bioaerosol at each site. For example, bacterial and fungal growth is very much temperature-dependent, with the optimum temperature for bacterial growth 37°C (for both GPB and GNB) and 27°C for fungi. However, since conditions were nearly the same at all sites during the sampling periods, any relative effects from changing conditions cannot be discerned with the exception of the higher temperatures during the GNB sampling that could possibly explain its higher levels than GPB at the same sites. In general, it is more likely that the levels observed had more to do with the sources of bioaerosols (rotting garbage, spores carried by the wind from wooded areas, etc.) than the ambient conditions.
No attempt was made to correlate activities at the sampling sites with results. As fungal and bacterial sampling occurred on separate days, day-to-day differences cannot be ruled out and thus the results obtained represent at best a gross characterization of the bioaerosol composition from these sites. However, since ambient temperature, humidity and wind speed did not vary significantly during the sampling events, even though most of the sampling was conducted indoors, conditions in these non-air-conditioned facilities probably were not very different from one sampling episode to the next.
The human respiratory system acts somewhat like an aerodynamic classifying system for airborne particles, similar to the 6-stage impactor used to collect the samples in this study. Just like with the sampling impactor, the fraction of inhaled particles retained in the respiratory tract and the site of deposition vary with the physical properties (shape, size and density) of the particles. The maximum concentration of fungal bioaerosol was found for Stage 4 of Site C and the minimum for Stage 6 of Site D. So, if the stages of the bioaerosol sampler are analogous to regions of the human respiratory tract, then the maximum deposition or penetration of fungal bioaerosol is in the secondary bronchiole region (i.e. Stage 5). Similarly, for bacteria, the maximum concentration of GPB was observed for Stages 3 and 5 of Site A, and the minimum concentration was also for Stage 6 of Site A. Thus, the maximum deposition of GPB is likely to occur in the tracheal, primary bronchial and terminal bronchiole regions and the minimum in the alveoli. For GNB, the maximum deposition of bacteria would be in the tracheal and primary bronchial region, with the minimum in the nasal and pharyngeal region of the respiratory tract. Thus, a significant fraction of the bioaerosols from the various sites would be considered respirable, including those that could penetrate deep into the bronchioles of an exposed individual.
5 Conclusions
A size-segregated characterization of atmospheric bioaerosol was carried out at 4 different sites on the campus of Jawaharlal Nehru University (JNU), New Delhi, India. The highest concentration of the fungal fraction was found at the Health Center. The highest levels of bacterial bioaerosol (GPB and GNB) were found at a garbage dumping site. The fungal fraction was the largest component of the total bioaerosol. The GNB were in greater abundance than the GPB. Many of the 12 genera of fungi identified are associated with immunotoxic and allergic diseases, and most of the fungal bioaerosol found would be respirable. Since the bacterial fractions of bioaerosol were identified on the basis of morphology and cell shape and individual species were not determined, their potential pathogenicity is uncertain. Some environmental conditions, such as temperature may have influenced the growth of gram-negative bacteria, but no clear relationship was established for bioaerosols in general. However, this initial sampling of air from a major university provides some interesting baseline data that can serve as benchmarks in designing future studies.
References
ACGIH (1989) Step two: on-site investigation, 1–8; Fungi, 1–10; Bacteria, 1–7. Committee on bioaerosols. In: Guidelines for the assessment of bioaerosols in the indoor environment. American Conference of Governmental Industrial Hygienists, Cincinnati, OH
ATS (American Thoracic Society) (1990) Diagnostic standards and classification of tuberculosis. Am Rev Respir Dis 142(3):725–735
Andersen AA (1958) New sampler for the collection, sizing, and enumeration of viable airborne particles. J Bacteriol 76:471–484
Bacteriological Analytical Manual (1998) 8th edn, revision A
Baron EJ, Finegold SM (1990) Bailey & Scott’s diagnostic microbiology, 8th edn. The C.V. Mosby Company, St. Louis, pp 76, 79, 82, 100–126, 133
Breathnach AS, de Ruiter A, Holdworth GM, Bateman NT, O-Sullivan DG, Rees PJ (1998) An outbreak of multi-drug resistant tuberculosis in a London teaching hospital. J Hosp Infect 39:11–17
Burge HP, Boise JR, Rutherford JA, Solomon WR (1977) Comparative recoveries of airborne fungus spores by viable and non-viable modes of volumetric collection. Mycopathologia 61(1):27–33
Cox CS, Wathes CM (1995) Bioaerosols in the environment. Bioaerosols handbook. Lewis, Boca Raton, pp 11–14
Douwes J, Thorne P, Pearce N, Heederik D (2003) Bioaerosol health effects and exposure assessment: progress and prospects. Ann Occup Hyg 47:87–200
Eddleman H (2005) Bacteria media from potato. Ph.D. thesis. www.disknet.com/indiana
Hameed A, Awad A (2007) Airborne dust, bacteria, actinomycetes and fungi at a flourmill. Aerobiologia 23:59–69
http://www.airqualitydirect.com/store/index.cfm?page=bioaerosols
http://www.pollutionissues.com/Ho-Li/Indoor-Air-Pollution.html
Kirmeyer GJ, Foust GW, Pierson GL, Simmler JJ, Le Chevalier MW (1993) Optimizing Chloramine Treatment. Denver, American Water Works Research Foundation
Kool JL, Bergmire-Sweat D, Butler JC, Brown EW, Peabody DJ, Massi DS (1999) Hospital characteristics associated with colonization of water systems by Legionella and risk of nosocomial legionnaires disease: a cohort study of 15 hospitals. Infect Control Hosp Epidemiol 20:798–805
Leopold SS (1988) “Positive hole” statistical adjustment for a two-stage, 200-hole-per-stage Andersen air sampler. Am Ind Hyg Assoc 49(2):A88–A90
Macher JM (1989) Positive-hole correction of multiple-jet impactors for collecting viable microorganisms. Am Ind Hyg Assoc J 50(11):561–568
Macher J, Ammann HA, Burge HA, Milton DK, Morey PR (1999) Bioaerosols: assessment and control. American Conference of Governmental Industrial Hygienists, Cincinnati, pp 1–5
Meteorological Parameters (2010) Centre for studies on regional development (CSRD), JNU, New Delhi 2010, daily weather data
Mohr AJ (2002) Fate and transport of microorganisms in air, Chapter 74. Manual of environmental microbiology, 2nd ed In: Hurst CJ, Crawford RL, Knudsen G, McInerney M, Stetzenbach LD, (eds), ASM Press, Washington DC, pp 827–38
Reinthaler FF, Marth E, Eibel U, Enayat U, Feenstra O, Friedl H, Kock M, Semmelrock FPP, Pridnig G, Schlacher R (1997) The assessment of airborne microorganisms in large-scale composting facilities and their immediate surroundings. Aerobiologia 13:167–175
Riordan TG, Smaldone GC (2004) Respiratory medical societies and the threat of bioterrorism. Thorax 59:265–267
Smith EG (1990) Sampling and identifying allergenic pollens and molds. Blewstone Press 15, San Antonio, pp 41–49
Stetzenbach LD (2005) Bacteriology of air, Topley and Wilsons microbiology and microbial infections: bacteriology-I, 10th edn. In: Borriello PS, Murray PR, Funke G (eds) ASM Press, Washington, DC, pp 185–194
Stetzenbach LD, Buttner MP, Cruz P (2004) Detection and enumeration of airborne biocontaminants. Curr Opin Biotechnol 15:170–174
Traeger MS, Wiersma ST, Rosenstein NE, Malecki JM, Shepard CW, Raghunathan PL (2002) First case of bioterrorism-related inhalational anthrax in the United States, Palm Beach County, Florida, 2001. Emerg Infect Dis 8:1029–1034
Wang CC, Fang GC, Kuo CH (2010) Bioaerosols as contributors to poor air quality in Taichung City, Taiwan. Environ Monit Assess 166:1–9
Acknowledgments
Authors acknowledge the contributions of the anonymous reviewer and editor for their help in improving the quality of this manuscript. Thanks to Himanshu Lal and Rajesh Kushwaha for their help during sampling and analysis. Individuals who gave permission for and assisted in the sampling phase of the work are also thanked.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srivastava, A., Singh, M. & Jain, V.K. Identification and characterization of size-segregated bioaerosols at Jawaharlal Nehru University, New Delhi. Nat Hazards 60, 485–499 (2012). https://doi.org/10.1007/s11069-011-0022-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11069-011-0022-3