Abstract
Plant growth-promoting rhizobacteria (PGPR) are known to stimulate plant growth because of their versatility in nutrient transformation. However, the success of PGPR inoculation depends not only on their ability to promote plant growth but also on their capacity to metabolize substrates that can be used as energy for the development and survival of the crops. Given the important influence of seed germination and vigor on crop yield, this study investigated the biochemical characteristics and effectiveness of multi-trait PGPR isolates in enhancing upland rice seedling growth and vigor. Biochemical identification was done using Biolog GEN III Microbial Identification System. Isolates were characterized based on their ability to metabolize all major classes of biochemicals in the carbon source utilization and chemical sensitivity assays. Identified rhizobacterial isolates were tested in vitro to evaluate their inoculation effects on the growth of PSB Rc23 upland rice seedlings. Biochemical identification results showed that rhizobacterial isolates have extensive metabolic activities in a wide range of carbon sources. Inoculation effects revealed that isolate IBBw1a was the most effective in enhancing root length and vigor index of rice seedlings in vitro, yielding a significant increase of 60% and 53%, respectively, over the uninoculated control. This study suggests that rhizobacterial isolates from upland rice may have commercial significance to improve seedling growth and vigor. These isolates will undergo a further assessment of their effectiveness in actual upland rice field conditions as they were already proven effective growth promoters in laboratory and screenhouse conditions. Such future activity can uncover their efficacy as potential biofertilizers in the actual soil environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is the most important cereal crop and staple food of more than half of the global population. It is the second widely cultivated cereal crop in the world and is a significant income source for millions of Asians (Timsina et al. 2011). Consumption of this economically important crop has been increasing in recent years, along with the rising population and demand (Prasad et al. 2017). However, its productivity is not at pace with this trend due to various factors such as decreasing soil fertility and poor soil management practices (Purwanto and Alam 2020). In upland rice field conditions, these problems are among the factors identified limiting crop yields (Serraj et al. 2011). To maintain productivity, farmers rely on higher amounts of mineral fertilizers and agrochemicals, which negatively impact humans, soil, and the environment. Such situations emphasize the need for sustainable technologies to preserve the soil’s long-term ecological balance while ensuring adequate crop yields (Sammauria et al. 2020). Prior researches have considered the use of root-colonizing microorganisms as inoculants, and have proven these amendments effective in enhancing the plant’s ability to capture nutrients and promoting crop health (Cruz et al. 2015; Nascente et al. 2019; Sharma et al. 2015). These microorganisms are widely recognized as plant growth-promoting rhizobacteria (PGPR).
PGPR are known to stimulate plant growth because of their versatility in nutrient transformation, mobilization, and solubilization (Dutta and Podile 2010; Mustafa et al. 2019). PGPR have been widely developed as plant growth regulators due to their direct and indirect beneficial effects on plant and soil environment (Gouda et al. 2018). Direct plant growth regulation involves providing the plant with growth-promoting substances such as indole-3-acetic acid (IAA) and siderophores. These substances are synthesized by the bacterium and facilitate the uptake of certain plant nutrients from the soil environment. On the other hand, indirect plant growth promotion involves reducing harmful effects by plant pathogens by producing defense enzymes that serve as antagonistic agents (Ahemad and Kibret 2014). PGPR also have the capacity to promote plant growth under stress conditions through secretion of 1-aminocyclopropane-1-carboxylate (ACC) deaminase in response to various plant stresses (Goswami and Deka 2020).
Several studies have recently shown that PGPR inoculation is a promising approach to improving upland rice growth and yield. Nascente et al. (2019) studied Serratia spp. in upland rice, which revealed an increase in dry matter and grain yield under field conditions. Cruz et al. (2015) found that actinomycete inoculation improved upland rice growth through a significant increase in P uptake and grain yield under screenhouse conditions. Guyasa et al. (2018) reported that inoculation of Pseudomonas fluorescens could increase the relative growth rate and vigor index of upland rice seeds. However, the success of PGPR inoculation depends not only on their ability to promote plant growth but also on their capacity to metabolize substrates that can be used as energy, essential to the development and survival of the crops (Khan et al. 2019).
Hence, this study was conducted to evaluate the biochemical characteristics and effectiveness of multi-trait PGPR isolates in enhancing upland rice (PSB Rc23) seedling growth in vitro. Given the important influence of seed germination and vigor on crop yield, it is thus useful to investigate the mechanisms behind growth promotion through PGPR inoculation. The ability of rhizobacterial isolates to improve upland rice seeds and vigor is an important factor in upland rice production, most especially that this farming system is usually constrained by poor nutrient and water acquisition due to drought (Nascente et al. 2019). This can be an economically promising alternative to address marginal rice farmers' production problems in the upland areas.
Materials and methods
Rhizobacterial isolates used
In the previous study of Cavite et al. (2020), bacteria were isolated from upland rice rhizosphere and screened for growth-promoting activities in vitro. These rhizobacteria were used as the source of inoculant for this experiment. Four isolates were selected on the basis of their growth-promoting activities—IBBw1a, IBBy1, IBBw2e, and IBBy2d. These isolates reportedly exhibited IAA production, tricalcium phosphate solubilization, ACC deaminase activity, siderophore production, and starch hydrolysis.
Biochemical identification
The biochemical identification process of selected isolates was done using the Biolog GEN III Microbial Identification System analysis. The GEN III MicroPlate test panel was used, which consisted of 94 phenotypic tests—71 of which are carbon source utilization assays and 23 of which are chemical sensitivity assays. Isolates were initially grown on Burks agar medium and then suspended in a special “gelling” inoculating fluid (IF). The cell suspension was inoculated into the GEN III MicroPlate and was incubated for 24 h to allow phenotypic fingerprint to form (Cavite et al. 2020). Results were read at the Natural Sciences Research Institute (NSRI), University of the Philippines (UP) Diliman, Quezon City, Philippines, using the Biolog’s Microbial Identification System software.
For the characterization process, the patterns produced by the software’s carbon source utilization technology were used to interpret and analyze the isolates’ biochemical properties. Isolates were characterized based on their ability to metabolize all major classes of biochemicals (Wong et al. 2015). Characterization parameters for the carbon source utilization assays include sugars, Hexose-PO4s, Amino acids, Hexose acids, and Carboxylic acids. For the chemical sensitivity assays, the GEN III MicroPlate can characterize the bacteria’s pH, salinity level, reducing power, and Gram-negative or positive characteristics (Chojniak et al. 2015).
Biochemical identification revealed that only two of the isolates had passed the similarity value criteria. These were isolates IBBy1 and IBBy2d, which were identified as Ralstonia pickettii and Acidovorax delafieldii, respectively. The other two identifications, Rhizobium rhizogenes (IBBw1a) and Burkholderia pyrrocinia (IBBw2e), were considered as “NO ID” and were supported by Wozniak et al. (2019) and Wong et al. (2015) as far as acceptable similarity values are concerned. Such isolate codes were retained for use in succeeding experiments. These results were reported in a recently published article by the same authors (Cavite et al. 2020).
Evaluation of rhizobacterial isolates
Biochemically identified rhizobacterial isolates were tested under laboratory conditions to evaluate their inoculation effects on the growth of PSB Rc23 upland rice seedlings.
Seed surface sterilization
Seeds were washed with sterile distilled water and subsequently soaked in 2.5% Sodium hypochlorite solution (w/w) for 20 min and then in 70% ethanol for 30 s. Seeds were washed afterward with sterile distilled water three times.
Inoculation and sowing
Prior to sowing, selected isolates were grown in Burks medium agar and incubated for 5 days at room temperature. Bacterial suspension [106 cell mL−1 with a population of more than 3.0 × 109 colony-forming units (CFU) 0.1 mL−1] was prepared in sterile distilled water following the methods of Ma et al. (2011). Inoculation of bacteria was done by soaking the seeds in the bacterial suspension for 2 h. Twenty-five surface inoculum-coated PSB Rc23 seeds were sown in a sterile Petri plate with moist filter paper maintained for 7 days under room temperature.
Treatments and experimental design
The set-up consisted of five inoculation treatments (presented below) replicated thrice. The experiment was arranged in a completely randomized design (CRD).
-
T1.
Uninoculated (control).
-
T2.
IBBw1a.
-
T3.
Ralstonia picketii.
-
T4.
IBBw2e.
-
T5.
Acidovorax delafieldii.
Data analysis
The number of germinated seeds was counted 7 days after sowing (DAS). Seeds were considered germinated when the radicle is ≥ 2 mm (He and Yang 2013). Root and shoot length of randomly selected seedlings (three seedlings per replicate) were also measured at seven DAS. Additionally, seed vigor index of treated seeds was determined after the computation of the percent germination. The above two parameters were computed using Eqs. 1 and 2 by Velmurugu et al. (2009). Data were analyzed by analysis of variance (ANOVA) using SAS Software, and treatment means were compared relative to the control (uninoculated) following the Least Significant Difference (LSD). Unless otherwise indicated, differences were only considered when significant at P < 0.05
Results
Biochemical characteristics of isolates
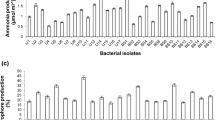
Biolog GEN III microbial identification system results showed that selected bacterial isolates have extensive metabolic activities in a wide range of carbon sources. Results of the chemical sensitivity assays are summarized in Table 1, and interpretation of the significance of these results is presented in the succeeding section.
Efficiency of isolates in accelerating germination and improving seed vigor
Selected rhizobacterial isolates were inoculated on upland rice seeds to examine their effects on shoot and root length and seed vigor index. It was observed that bacterial inoculation showed varied effects on seed germination, shoot length and root length, and seedling vigor index (Table 2). At seven DAS, longest seedling shoot of 4.19 cm was obtained with R. pickettii inoculation, recording a significant increase of 23% relative to the uninoculated treatment. Meanwhile, isolate IBBw1a gave rise to the highest root length of 5.00 cm, equivalent to a 60.14% increase relative to the control.
Further evaluation revealed that isolate IBBw1a was the most effective in enhancing the vigor index of PSB Rc23 rice seedlings, showing the highest value of 839.30 cm, which was equivalent to a 53% increase. Likewise, this isolate was found to have the highest germination rate of 94.67 and root length as described above. Figure 1 shows the effect of IBBw1a inoculation on PSB Rc23 rice seedlings seven days after sowing.
Discussion
Biochemical characteristics of isolates
One of the most important steps in the development of microbial inoculants is the identification of bacteria found to have promising growth-promoting activities (Wozniak et al. 2019). The most common identification methods employed in previous studies involve the technique of 16S rRNA sequencing (Al-Sadi et al. 2016; Damodaran et al. 2019; Lunsmann et al. 2016). Meanwhile, the present study identified the selected isolates using the Biolog GEN III MicroPlate-based technique, a latest innovation in bacterial identification that allows testing of both Gram-negative and Gram-positive bacteria in the same panel (Fernando and Cruz 2019). The software dissects and analyzes the cell’s capacity to metabolize any large class of biochemicals, and determines other essential physiological properties such as pH, salt, and lactic acid tolerance, reducing power, and chemical sensitivity (Guenoun et al. 2019).
The above-identified rhizobacteria have been found to be reported in several bacteriology and microbiology literature as Gram-negative found in soils. A recent study by Al-Sadi et al. (2016) reported Ralstonia pickettii as a soil bacteria present in commercial potting media products and found out its potential as biocontrol species. Bulut (2013) identified R. pickettii as a nitrogen-fixing asymbiotic bacteria and inoculated in wheat plants. The experiment revealed the potential of this strain in organic wheat farming. In the case of Acidovorax delafieldii, Gurley and Zdor (2005) examined its effects on rhizosphere colonization, and found significant effects suggesting its effectiveness in controlling weed growth with appropriate formulation methods with other beneficial strains.
Consistent with prior literature, the chemical sensitivity assays revealed the Gram-negative nature of all bacteria. In addition, all isolates were found to be lactic acid-tolerant and can thrive at pH 6. Salinity tolerance of the isolates is at 1% level except for A. delafieldii, which showed negative. Several studies have reported the use of Biolog microplate assay in PGPR, particularly in defining their specific carbon sources. Guenoun et al. (2019) and Wozniak et al. (2019) reported that the Biolog system provides high accuracy and can provide a useful verification of bacteria at the species level. Moreover, in a study by Fernando and Cruz (2019), the Biolog system provided clear indications of the degree of ambiguity for identifying bacteria at the genus and species level. The study further argued that Biolog system and 16S rDNA techniques could be used simultaneously for effective and correct verification of bacteria ID.
The utilization profiles of substrates and chemical sensitivity assays varied among selected isolates. Isolate IBBw2e showed the highest metabolic activities (67%), while lowest was observed in R. pickettii (50%). In the sugar test, most isolates grew in the presence of sucrose, α-d-Glucose, d-mannose, d-Fructose, and l-Rhamnose, which is common among nitrogen-fixing bacteria (Dhevendaran et al. 2013). It has been reported that these soluble sugars serve as a primary energy source and an important source of carbon for soil microorganisms in the vicinity of growing roots (Vidal et al. 2018). In amino acid test, most isolates were able to metabolize l-Alanine, l-Aspartic acid, l-Glutamic acid, l-Histidine, l-Pyroglutamic acid, and l-Serine. This result agrees with the literature that free-living nitrogen-fixing bacteria are well known for their ability to produce and metabolize significant amounts of amino acids, especially of the genera Azotobacter and Azospirillum (Gonzalez-Lopez et al. 1995). Similar results have recently been reported by Wozniak et al. (2019) on endophytic bacteria.
Carboxylic acid test of the Biolog GEN III system showed that selected bacteria were able to metabolize l-malic acid, y-amino-butyric acid, and acetic acid substrates. Utilization of these organic acids is one of the mechanisms of rhizobacteria in plant growth promotion as microorganisms use these compounds as substrates, leading to increased microbial biomass and activity around the roots—the so-called rhizosphere effect (Doornbos et al. 2012). Current evidence indicates that some components present in these organic acids are involved in a variety of functions, including modulating nutrient availability, increasing heavy metal tolerance, or attracting rhizobacteria (Carvalhais et al. 2011).
The use of these organic acid substrates in rhizobacteria is also seen as a root exudation mechanism associated with the release of compounds during rhizodeposition (Dey et al. 2012). Consequently, certain nutrient elements may be comparatively more accessible for plant absorption due to the released compounds. The component of plant root exudates not only serves as a source of carbon substratum for microbial growth but also contains chemical molecules which promote rhizosphere chemotaxis of soil microbes (Olanrewaju et al. 2019). These functions, which root exudates perform, ultimately affect plant acquisition of nutrients (Ankati and Podile 2019).
Inoculation effects on upland rice seedlings
Bacterial inoculation can impact seedling growth and influence seedling establishment (Maheshwari 2012). It has been widely documented in several studies that there is a strong relationship between rhizobacterial capabilities and seedling growth (Gupta and Pandey 2019; Shabanamol et al. 2018; Tamreiha et al. 2019). The current results revealed similar findings showing the significant seedling growth enhancement caused rhizobacterial inoculation. Isolate IBBw1a was the most effective among treatments with more evident effects in seed germination and root length. Such growth promotion can be attributed to the IAA-producing capacity of the bacteria. The compound IAA can stimulate germination and increase root surface area through lateral and adventitious root formation (Zažímalová et al. 2014). This consequently provides greater capacity for water and nutrient assimilation and positively affects cell division and shoot growth. Similar results were also found out by Mariano et al. (2018) through inoculation of IAA-producing actinobacteria Streptomyces sp. which significantly improved rice seedling growth. The use of indigenous PGPR from rice rhizosphere was also highlighted by Saengsanga (2018) to have beneficial effects on the early growth of Thai Jasmine Rice (KMDL105).
Seedling vigor is also one of the important considerations in seed quality determination and seedling establishment. In the present study, IBBw1a inoculation resulted in higher seedling vigor index suggesting its great potential to promote better seedling establishment of PSB Rc23 rice seeds. This is an essential characteristic for seedlings to survive under adverse external conditions (Bandurski et al. 2012). Enhancement in seedling vigor is also reported in recent studies. For instance, Damodaran et al. (2019) observed increased seedling vigor in rice and wheat genotypes through Lysinibacillus sp. inoculation. Meanwhile, Guyasa et al. (2018) recently found out that inoculation of Bacillus spp. and Pseudomonas flourescens can increase seedling vigor and relative growth rate of upland rice.
Conclusion
The present study has revealed the biochemical characteristics of four upland rice-associated rhizobacterial strains, which were recently reported to produce growth-promoting compounds and proven to stimulate and enhance upland rice growth under screenhouse conditions. Among four selected isolates, IBBw1a was found to be the most effective in improving seed germination percentage, root length, and vigor index of PSB Rc23 rice seedlings in vitro. This bacterium may have the commercial value to be developed as a potential bio-inoculant, which may enhance upland rice seedling growth and vigor. The next move to investigate these growth-promoting strains is to test their effectiveness under actual upland field conditions to uncover their efficacy as microbial inoculants. Moreover, 16 s rRNA gene sequencing is suggested as the Biolog GEN III system is based solely on the bacteria's phenotypic profiles. This may further improve the identification of selected isolates.
References
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J King Saud Univ Sci 26(1):1–20. https://doi.org/10.1016/j.jksus.2013.05.001
Al-Sadi AM, Al-Zakwani HA, Nasehi A, Al-Mazroui SS, Al-Mahmooli IH (2016) Analysis of bacterial communities associated with potting media. Springerplus 5:5. https://doi.org/10.1186/s40064-016-1729-0
Ankati S, Podile AR (2019) Metabolites in the root exudates of groundnut change during interaction with plant growth promoting rhizobacteria in a strain-specific manner. J Plant Physiol 243:8. https://doi.org/10.1016/j.jplph.2019.153057
Bandurski RS, Desrosiers MF, Jensen P, Pawlak M, Schulze A (2012) Genetics, chemistry, and biochemical physiology in the study of hormonal homeostasis. In: Karssen CM, van Loon LC, Vreugdenhil D (eds) Progress in plant growth regulation. Springer Netherlands, pp 1–12
Bulut S (2013) Evaluation of efficiency parameters of phosphorous-solubilizing and N-fixing bacteria inoculations in wheat (Triticum aestivum L.). Turk J Agric For 37(6):734–743. https://doi.org/10.3906/tar-1301-112
Carvalhais L, Fedoseyenko D, Haijirezai M, Borriss R, von Wiren N (2011) Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorous, potassium, iron deficiency. J Soil Sci Plant Nutr 174:3–11
Cavite HJM, Mactal AG, Evangelista EV, Cruz JA (2020) Growth and yield response of upland rice to application of plant growth-promoting rhizobacteria. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10114-3
Chojniak J, Jalowiecki L, Dorgeloh E, Hegedusova B, Ejhed H, Magner J, Plaza G (2015) Application of the BIOLOG system for characterization of Serratia marcescens ss marcescens isolated from onsite wastewater technology (OSWT). Acta Biochim Pol 62(4):799–805
Cruz J, Delfin E, Paterno E (2015) Enhancement of growth and yield of upland rice (Oryza sativa L.) by Actinomycetes. Asia Life Sci 24(2):575–584
Damodaran T, Mishra VK, Jha SK, Pankaj U, Gupta G, Gopal R (2019) Identification of rhizosphere bacterial diversity with promising salt tolerance, PGP traits and their exploitation for seed germination enhancement in sodic soil. Agric Res 8(1):36–43. https://doi.org/10.1007/s40003-018-0343-5
Dey R, Pal KK, Tilak K (2012) Influence of soil and plant types on diversity of rhizobacteria. Proc Natl A Sci India B 82(3):341–352. https://doi.org/10.1007/s40011-012-0030-4
Dhevendaran K, Preetha G, Vedha Hari BN (2013) Studies on nitrogen fixing bacteria and their application on the growth of seedling of Ocimum sanctum. Phcog J 5(2):60–65. https://doi.org/10.1016/j.phcgj.2013.03.003
Doornbos RF, van Loon LC, Bakker PAHM (2012) Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere: a review. Agron Sustain Dev 32(1):227–243. https://doi.org/10.1007/s13593-011-0028-y
Dutta S, Podile AR (2010) Plant growth promoting rhizobacteria (PGPR): the bugs to debug the root zone. Crit Rev Microbiol 36(3):232–244. https://doi.org/10.3109/10408411003766806
Fernando TC, Cruz JA (2019) Profiling and biochemical identification of potential plant growth-promoting endophytic bacteria from Nypa fruticans. Philipp J Crop Sci 44(2):77–85
Gonzalez-Lopez J, Martinez-Toledo MV, Rodelas B, Pozo C, Salmeron V (1995) Production of amino acids by free-living heterotrophic nitrogen-fixing bacteria. Amino Acids 8(1):15–21. https://doi.org/10.1007/bf00806540
Goswami M, Deka S (2020) Plant growth-promoting rhizobacteria-alleviators of abiotic stresses in soil: a review. Pedosphere 30(1):40–61. https://doi.org/10.1016/s1002-0160(19)60839-8
Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS, Patra JK (2018) Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res 206:131–140. https://doi.org/10.1016/j.micres.2017.08.016
Guenoun K, Chattaoui M, Bouri M, Rhouma A, Naghmouchi K, Raies A (2019) Biological control of growth promoting rhizobacteria against verticillium wilt of pepper plant. Biologia 74(3):237–250. https://doi.org/10.2478/s11756-018-00169-9
Gupta S, Pandey S (2019) ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in french bean (Phaseolus vulgaris) plants. Front Microbiol 10:17. https://doi.org/10.3389/fmicb.2019.01506
Gurley HG, Zdor RE (2005) Differential rhizosphere establishment and cyanide production by alginate-formulated weed-deleterious rhizobacteria. Curr Microbiol 50(3):167–171. https://doi.org/10.1007/s00284-004-4422-4
Guyasa IM, Sadimantara GR, Khaeruni A, Sutariati GAK (2018) Isolation of Bacillus spp. and Pseudomonas fluorescens from upland rice rhizosphere and its potential as plant growth promoting rhizobacteria for local upland rice (Oryza sativa L.). Biosci Res 15(4):3231–3239
He DL, Yang PF (2013) Proteomics of rice seed germination. Front Plant Sci 4:9. https://doi.org/10.3389/fpls.2013.00246
Khan N, Bano A, Babar MDA (2019) Metabolic and physiological changes induced by plant growth regulators and plant growth promoting rhizobacteria and their impact on drought tolerance in Cicer arietinum L. PLoS ONE. https://doi.org/10.1371/journal.pone.0213040
Lunsmann V, Kappelmeyer U, Benndorf R, Martinez-Lavanchy PM, Taubert A, Adrian L, Duarte M, Pieper DH, von Bergen M, Muller JA, Heipieper HJ, Jehmlich N (2016) In situ protein-SIP highlights Burkholderiaceae as key players degrading toluene by para ring hydroxylation in a constructed wetland model. Environ Microbiol 18(4):1176–1186. https://doi.org/10.1111/1462-2920.13133
Ma Y, Rajkumar M, Luo Y, Freitas H (2011) Inoculation of endophytic bacteria on host and non-host plants–effects on plant growth and Ni uptake. J Hazard Mater 195:230–237. https://doi.org/10.1016/j.jhazmat.2011.08.034
Maheshwari DK (2012) Potential use of soil microbial community in agriculture. In: Maheshwari DK (ed) Bacteria in agrobiology: Plant probiotics. Springer-Verlag, Berlin, pp 45–67
Mariano KJB, Santos AJC, Evangelista EV, Cruz JA (2018) Enhancement of NSIC Rc 192 seedling growth by soil-based and carbonized rice hull (CRH)-based actinomycete inoculants. Mindanao J Sci Technol 16:1–10
Mustafa S, Kabir S, Shabbir U, Batool R (2019) Plant growth promoting rhizobacteria in sustainable agriculture: from theoretical to pragmatic approach. Symbiosis 78(2):115–123. https://doi.org/10.1007/s13199-019-00602-w
Nascente AS, Lanna AC, de Sousa TP, Chaibub AA, de Souza ACA, de Filippi MCC (2019) N fertilizer dose-dependent efficiency of Serratia spp. for improving growth and yield of upland rice (Oryza sativa L.). Int J Plant Prod 13(3):217–226. https://doi.org/10.1007/s42106-019-00049-5
Olanrewaju OS, Ayangbenro AS, Glick BR, Babalola OO (2019) Plant health: feedback effect of root exudates-rhizobiome interactions. Appl Microbiol 103(3):1155–1166. https://doi.org/10.1007/s00253-018-9556-6
Prasad R, Shivay YS, Kumar D (2017) Current status, challenges, and opportunities in rice production. In: Chauhan BS, Jabran K, Mahajan G (eds) Rice production worldwide. Springer International Publishing, New York, pp 1–32
Purwanto BH, Alam S (2020) Impact of intensive agricultural management on carbon and nitrogen dynamics in the humid tropics. Soil Sci Plant Nutr 66(1):50–59. https://doi.org/10.1080/00380768.2019.1705182
Saengsanga T (2018) Isolation and characterization of indigenous plant growth-promoting rhizobacteria and their effects on growth at the early stage of Thai Jasmine Rice (Oryza sativa L. KDML105). Arab J Sci Eng 43(7):3359–3369. https://doi.org/10.1007/s13369-017-2999-8
Sammauria R, Kumawat S, Kumawat P, Singh J, Jatwa TK (2020) Microbial inoculants: Potential tool for sustainability of agricultural production systems. Arch Microbiol 202:667–693. https://doi.org/10.1007/s00203-019-01795-w
Serraj R, McNally KL, Slamet-Loedin I, Kohli A, Haefele SM, Atlin G, Kumar A (2011) Drought resistance improvement in rice: an integrated genetic and resource management strategy. Plant Prod Sci 14(1):1–14. https://doi.org/10.1626/pps.14.1
Shabanamol S, Divya K, George TK, Rishad KS, Sreekumar TS, Jisha MS (2018) Characterization and in planta nitrogen fixation of plant growth promoting endophytic diazotrophic Lysinibacillus sphaericus isolated from rice (Oryza sativa). Physiol Mol Plant Pathol 102:46–54. https://doi.org/10.1016/j.pmpp.2017.11.003
Sharma A, Patni B, Shankhdhar D, Shankhdhar SC (2015) Evaluation of different PGPR strains for yield enhancement and higher Zn content in different genotypes of rice (Oryza sativa L.). J Plant Nutr 38(3):456–472. https://doi.org/10.1080/01904167.2014.934475
Tamreiha K, Nimaichand S, Chanu SB, Devi KA, Lynda R, Jeeniita N, Ningthoujam DS, Roy SS (2019) Streptomyces manipurensis MBRL 201(T) as potential candidate for biocontrol and plant growth promoting agent for rice. Indian J Exp Biol 57(10):741–749
Timsina J, Buresh RJ, Dixon J (2011) Rice-maize systems in Asia: current situation and potential. International Rice Research Institute, Los Baños
Velmurugu J, Wijeratnam S, Wijesundera R (2009) Trichoderma as a seed treatment to control helminthosporium leaf spot disease of Chrysalidocarpus lutescens. World J Agric Sci 5(6):720–728
Vidal A, Hirte J, Bender SF, Mayer J, Gattinger A, Höschen C, Schädler S, Iqbal TM, Mueller CW (2018) Linking 3D soil structure and plant-microbe-soil carbon transfer in the rhizosphere. Front Environ Sci 6(9):1–13. https://doi.org/10.3389/fenvs.2018.00009
Wong WZ, H’Ng PS, Chin KL, Sajap AS, Tan GH, Paridah MT, Othman S, Chai EW, Go WZ (2015) Preferential use of carbon sources in culturable aerobic mesophilic bacteria of Coptotermes curvignathus’s (Isoptera: Rhinotermitidae) gut and its foraging area. Environ Entomol 44(5):1367–1374. https://doi.org/10.1093/ee/nvv115
Wozniak M, Galazka A, Tyskiewicz R, Jaroszuk-Scisel J (2019) Endophytic bacteria potentially promote plant growth by synthesizing different metabolites and their phenotypic/physiological profiles in the Biolog GEN III microplate(TM) test. Int J Mol Sci 20(21):24. https://doi.org/10.3390/ijms20215283
Zažímalová E, Petrášek J, Benková E (2014) Auxin and the interaction between plants and microorganisms. In: Ludwig-Muller J (ed) Auxin and its role in plant development. Springer-Verlag, Vienna, pp 413–434
Acknowledgements
This work was supported by the Department of Science and Technology-Science Education Institute, Philippines (DOST-SEI-Philippines) through the Accelerated Science and Technology Human Resource and Development Program (ASTHRDP) and the Philippine Rice Research Institute (PhilRice) Central Experiment Station, Science City of Muñoz, Nueva Ecija, Philippines. King Mongkut’s Institute of Technology Ladkrabang (KMITL) is also acknowledged for its access to scholarly publications reviewed in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cavite, H.J.M., Mactal, A.G., Evangelista, E.V. et al. Biochemical characteristics and inoculation effects of multi-trait plant growth-promoting rhizobacteria on upland rice (Oryza sativa L. cv PSB Rc23) seedling growth. Arch Microbiol 203, 3533–3540 (2021). https://doi.org/10.1007/s00203-021-02337-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-021-02337-z