Abstract
The present study focused on the characterization of plant growth promoting rhizospheric (R) and endophytic (E) bacteria and their impact on wheat cultivars growth. In this study, 400 strains were isolated from the rhizosphere soil (250 isolates) and surface-sterilized roots (150 isolates) of wheat and screened for their ability to plant growth promotion (PGP) traits. Four R isolates and four E isolates with different ability were selected to investigate the interaction between R and B bacteria associated with wheat cultivars under in vitro and greenhouse conditions. Plant growth parameters were found to be enhanced by the combined inoculation of two groups of R and E bacteria compared to individual inoculations (respectively 33.7 and 37.8% increase in root and shoot dry weight), suggesting that PGP rhizobacteria acted synergistically with PGP endophytes in phosphate solubilization. Compared to inoculation with phosphate-solubilizing bacteria (PSB) or indole-3-acetic acid producer bacteria (IAA-PB), inoculation by bacteria with multiple PGP properties (PSB and IAA-PS) showed higher promotion capacity. Also, in greenhouse assay, bacterial inoculation had a positive effect on the soil dehydrogenase (70.2%) and phosphatase (52.2%) activity. It seems PGP traits do not work independently of each other but additively as it was suggested in the “synergistic hypothesis” that multiple mechanisms are responsible for the plant growth promotion and increased yield. Findings of this study could improve the current bio-fertilizer production procedure in research and related industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphate (P) deficiency is a critical nutritional disorder of wheat, particularly in the alkaline soils that contain great amounts of calcium carbonate minerals. The P uptake in wheat ranged from 2.8 to 4.5 kg P/t with average about 4 kg P/t (Tandon 1987). Use of P fertilizer is vital in the cultivation of wheat to attain and to maintain profitable crop production. According to a report by the International Fertilizer Association (IFA), the world’s P fertilizer demand was expected to reach 43.5 Mt P2O5 by 2018/2019 (Heffer and Prud’homme 2014). Most of the P fertilizers contain some of the toxic elements like fluorine (F), lead (Pb), cadmium (Cd), arsenic (As), mercury (Hg), chromium (Cr), and radium (Ra) (Hegedűs et al. 2017). Besides, the recovery of P fertilizer in the soil is quite low (15–20%) and the residual P is fixed as insoluble P fractions in soils (Hemwall 1957). Transport of soil particles loaded with P into lakes and surface waters cause eutrophication (Carpenter 2005). Process of P fixation decreases the fertilization efficiency as well as crop profitability, resulting in economic loss and ecological problems. Such environmental concerns and high fixation of P in soils have led to search eco-friendly and economically feasible alternative ways for improving crop production in P-deficient soils.
Plant growth-promoting bacteria (PGPB), which interact and promote plant growth and nutrients, are considered one such accessible viable strategy (Ji et al. 2014). The mechanisms for PGPB to promote the growth of agricultural crops are biological nitrogen fixation (BNF), synthesis of plant growth hormones (IAA, gibberellic acid, cytokinins, and ethylene), production of siderophores, and solubilization of minerals such as phosphorus, potassium, and zinc (Hu et al. 2017; Dinesh et al. 2015). Symbiotic and free-living bacteria are able to fix nitrogen and provide it to plants. The enzyme nitrogenase complex carries out the BNF by reducing molecular nitrogen (N2) to ammonium (NH4+) and reduces acetylene to ethylene (Masson-Boivin and Sachs 2018). Plant hormones are chemical messengers that regulate plant cell processes. They act by regulating plant growth processes, like seed growth, the formation of leaf and flowers, elongation of stems, and development and ripening of fruit. Microorganisms may also produce plant hormones and affect the plant’s hormonal balance (Tsukanova et al. 2017). Microorganisms produce various enzymes and metabolites that serve as attachment structures to mineral or rock surface and release (or solubilize) nutrients (Etesami et al. 2017; Rafi et al. 2019). PGP trait has been reported in strains belonging to the numerous genera, such as Acinetobacter, Azospirillum, Azotobacter, Arthrobacter, Bacillus, Beijerinckia, Burkholderia, Clostridium, Erwinia, Enterobacter, Flavobacterium, Hydrogenophaga, Pseudomonas, Rhizobium, and Serratia (Singh et al. 2015).
Among PGP bacteria, due to the deficiency of plant available P in many agricultural soils, PSB are considered as prospective bio-fertilizers (Emami et al. 2018). There were more reports of growth promotion on crop plants inoculated with PSB (Fernández et al. 2007; Dey et al. 2004; Chabot et al. 1996); the related research projects mainly focused on the isolation of PSB and test their P-solubilizing activity. Valetti et al. (2018) reported that application of Bacillus, Serratia, Arthrobacter, and Pantoea (PSB) increased rapeseed growth. Formation of clear halo around the bacterial colonies on solid agar (contain an insoluble form of P) has considered as a universal indicator for phosphate solubilization by PSB for over half a century (Bashan et al. 2013). However, subsequent empirical studies show that most of the isolates screened based on this factor are not effective. Recent resource surveys show that bio-solubilization of phosphate is a very complex phenomenon affected by several factors (variable soil constituents and environmental factors) where each cannot be evaluated and tested separately (Bashan et al. 2013; Musarrat and Khan 2014).
The rate of root growth and expansion of root architecture in the soil are important for solubilization and absorption of nutrients. IAA-PB can play a direct role in root growth and development. Production of IAA by bacteria has been directly linked to the development of the plant host root system and increased root growth and branching (Spaepen et al. 2007). Increased root branching has promoted nutrient acquisition (Jia et al. 2018). There is little information about the IAA-PB, development of the root system, and P absorption by plants (Emami et al. 2018). Nevertheless, to the authors’ knowledge, no experimental work has been reported on inoculation with IAA-PB for evaluation role of root expansion in P availability and their effect on growth promotion of plants. In addition, although the PGP traits among R and E bacteria may be similar, most of the studies have been done about rhizosphere bacteria.
Therefore, the present study was undertaken with the following objectives: (i) isolation of the R and E bacteria from rhizosphere soil and inside roots of wheat plant, (ii) comparison between the ability of R and E bacteria to stimulation of wheat plant growth as a single and co-inoculation, (ii) evaluation potentiality of PSB and IAA-PB in growth promotion and P uptake.
Materials and methods
Sampling and isolation of R and E bacteria of wheat roots
To isolate and screening the R and E bacterial isolates of wheat (Triticum aestivum L.) roots, a number of healthy wheat plants and associated rhizosphere soil (30 samples) were randomly collected from wheat field of Campus of Agriculture and Natural Resources of the Tehran University, Iran (35° 48′ 30.98″ N, 50° 57′ 29.47″ E) during the stem elongation. Physical, chemical, and biological properties of wheat field soil included soil texture (27.4% clay, 29.2% silt, and 43.4% sand), sandy loam, pH 7.9; electrical conductivity (EC), 1.1 dS m−1; organic carbon, 4.2 g kg−1; total nitrogen, 4.1 g kg−1; calcium carbonate equivalent, 93 g kg−1; available potassium, 260 mg kg−1; available phosphorus, 11.4 mg kg−1; and bacterial population, 1.33 × 106 colony forming unit (Okalebo et al. 2002).
According to a previous standard procedure (Robinson et al. 2016) and using Nutrient Agar medium (NA), the endophytic bacteria were isolated and purified (Emami et al. 2018). Briefly, roots were washed with running water to remove surface adhering soil. Plant roots were sectioned into 2-cm nodal root sections with a sterile blade. Roots were twice vortexed in sterile distilled water (SDW) before sterilization using an optimized surface sterilization procedure: a 1-min wash in 95% ethanol, a 14-min wash with agitation in sodium hypochlorite solution (1.4% active chlorine), a 10-s wash in 95% ethanol, followed by 10 rinses in SDW with agitation. Validation of the surface sterilization procedure was done by stamping the surface-sterilized roots onto nutrient agar medium (touch inoculation) and culturing aliquots of water from the last rinsing onto nutrient agar. The plates were transferred to the incubator as control of the test sample. Using sterile mortar, root samples (previously sterilized) were macerated, diluted, and plated. The cultivable in vitro bacteria in the rhizosphere soil of wheat were isolated by the tenfold serial dilutions method (Johnson and Curl 1972). After incubation at 28 ± 2 °C for 96 h, bacterial isolates phenotypically (color, opacity, and rate of growth) were selected for the further experiment.
Screening of isolates for PGP traits
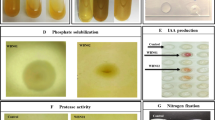
All bacterial isolates were screened for IAA production based on the method described by Bric et al. (1991) with minor modifications. Bacterial isolates were grown in the nutrient broth supplemented with l-tryptophan (1 mg mL−1 of broth) at 30 °C for 72 h. Culture supernatants from each of these isolates were mixed with Salkowski reagent (1 mL of 0.5 M FeCl3 in 50 mL of 35% HClO4) in the ratio of 1:1. Observation of pink color indicates the production of IAA and its optical density was recorded at 530 nm. The ability of phosphate solubilizing of the bacterial isolates (organic and inorganic phosphate) was determined on Sperber agar medium (Sperber 1958). The bio-oxidation of glycine to hydrogen cyanide (HCN) by isolates was tested qualitatively following the method of Lorck (1948). Siderophore production and Fe3+ reduction to Fe2+ was determined by chrome azurol sulfonate medium (Schwyn and Neilands 1987).
Identification of selected isolates
The genomic DNA extraction of the isolates (four R isolates: R63, R115, R120, R185, and four E isolates: E111, E149, E203, E240) grown in nutrient broth medium was performed using isolation kit (Promega, Madison, WI, USA), and the 16S rRNA gene was amplified using the general bacterial primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). The purified PCR product (with purification kit) was sequenced by DNA sequencer of the South Korean Macrogen Corporation (by ABI system 3730 XL). The nucleotide sequences were edited using the ChromasLite software and compared with the nucleotide sequences found in GenBank. The phylogenetic tree was constructed in RaxMl implemented in Geneious IR9 with the Tamura-Nei model and 1000 bootstrapping procedure. The nucleotide sequences assigned to this study were sent to the GeneBank database and registered with the accession numbers MF521965, MF521968, MF579599, MF521970, MF521967, MF579600, MF579598, and MF522213 for strains R115, R63, R185, R120, E149, E111, E240, and E203, respectively.
In vitro assay of the response of wheat cultivars to P-deficient in the presence of R and E bacteria
In vitro experiment was done in a 14 × 2 factorial completely randomized block strategy with three replications within test tubes. The fourteen inoculant treatments are as follows: (1) rhizospheric IAA-PB (isolate R115), (2) endophytic IAA-PB (isolate E149), (3) co-inoculation of rhizospheric and endophytic IAA-PB (isolates R115 and E149), (4) rhizospheric PSB (isolate R63), (5) endophytic PSB (isolate E111), (6) co-inoculation of rhizospheric and endophytic PSB (isolates R63 and E111), (7) rhizospheric IAA-PB and PSB (isolate R185), (8) endophytic IAA-PB and PSB (isolate E240), (9) co-inoculation of rhizospheric and endophytic IAA-PB and PSB (isolates R185 and E240), (10) R bacteria without PGP ability (isolate R120), (11) E bacteria without PGP ability (isolate E203), (12) co-inoculation of R and E bacteria without PGP ability (isolates R120 and E203), (13) negative control (contain the insoluble source of P), and (14) positive control (contain the soluble source of P). The two wheat cultivar treatments were Roshan and Marvdasht cultivars. Seeds were collected from national plant gene bank of Iran, seed and plant improvement institute.
Seeds were surface-sterilized and germinated under sterile conditions into the plate containing 1% agar. After germination, seeds were transferred under sterile conditions into the culture tubes containing Hoagland’s plant growth media (1 L of Hoagland solution was prepared by using 7.5 mL of 1 M calcium nitrate tetrahydrate (Ca [NO3]2·4H2O), 20 mL of 0.1 M calcium sulfate dihydrate (CaSO4·2H2O), 5 mL of 0.5 M potassium sulfate (K2SO4), 2 mL of 1 M magnesium sulfate heptahydrate (MgSO4·7H2O), 2 mL of Fe-EDTA, and 1 mL of micronutrient stock) (Adesemoye et al. 2009) and supplemented with 10 mL of 0.05 M tri-calcium phosphate (Ca3(PO4)2) as insoluble P source. Plants with the addition of soluble (monocalcium phosphate Ca[HPO4]2) and insoluble P (tri-calcium phosphate Ca3(PO4)2) source without inoculation were used as positive and negative controls, respectively. Emerging roots of 7 days-old plants were inoculated with 100 μL of the selected bacterial culture, previously grown at 27 °C during 48 h (108 CFU mL−1). Plants were grown in a greenhouse at 25 ± 2 °C and 14/10-h photoperiod (Fig. 1). After 90 days, plants were removed from the agar, and then shoot and roots were dried in 70 °C and weighed for shoot and root dry weight. The concentration of P in plant samples was examined using the vanadomolybdate method (Westerman 1990). Also, chlorophyll content in plant samples was measured by chlorophyll meter (Yadava 1986).
Setting up the greenhouse experiment
In order to investigate the effect of PGPB on plant growth under greenhouse condition, a bacterial consortium of R185 and E240 was selected based on their PGP properties under in vitro condition. The greenhouse experiment was performed based on the in vitro assay with the efficient bacterial inoculant and cultivar in potted soil with sandy clay loam texture, low available content of P (8.4 mg/kg), cation exchange capacity (CEC) of 17.5 (cmolc kg−1), and the minor population of PSB. Wheat seeds were surface-sterilized by soaking in 75% ethyl alcohol for 10 s, immersed in 5% NaOCl for 5 mins, and finally rinsed ten times with sterile water. Afterwards, 10 seeds were transplanted to a plastic pot (0.22-m diameter, 0.18-m depth) containing 3-kg soil. Plants were grown in a glasshouse at a temperature of 28 ± 2 °C, soil moisture of 75% of the field capacity, and 45% relative humidity in a day–night cycle of 14-h natural light. After appearing seedlings of soil, the number of wheat seedling has been reduced to five seedlings. Seven days after germination, pots were inoculated with bacterial inoculants (bacterial consortium) using a root drenching method (Wei et al. 2013). Briefly, 5 wheat plants per pot were drenched with 5 mL of bacterial inoculants at an initial concentration of 5 × 108 CFU mL−1. Control pots were inoculated by sterilized inoculum that had been sterilized by autoclaving. Plants were uprooted after 100 days of inoculation. The biomass of all plant samples was dried at 75 °C for 2 days. Then, phosphate content was determined in plant material (root, shoot, and grain) by the molybdovanadate spectrophotometric method. Available P in soils was extracted using the Olsen method (Olsen et al. 1954). Soil dehydrogenase and phosphatase activity were determined according to the methods initially described by Ohlinger (1996) and Tabatabai (1982) respectively.
Data analysis
All experiments (in vitro and pot) were done in triplicates and result values are shown as mean ± standard deviation. Means were compared by Duncan’s Multiple Range test at 1% probability level. In addition, a normality test was also conducted on data, which were normal. In the greenhouse, the pots with different treatments were planned in a completely randomized design.
Results
Isolation, identification, and characterization of the PGP bacteria
Based on cultural and morphological characteristics, 400 bacterial strains were isolated from the rhizosphere (250 isolates) and surface-sterilized roots (150 isolates) of wheat and screened for their ability for PGP traits (Fig. 2). Rhizosphere had more (88%) IAA-PB than surface-sterilized roots (72%). One hundred forty-eight out of 250 R isolates were able to produce siderophore while only seventy-eight out of 150 E isolates produced halos in CAS-agar medium. The ability of the isolates to organic and inorganic phosphate solubilization was detected by the formation of clear halo around the colony on medium containing inositol phosphate and tri-calcium phosphate respectively. Twenty-four percent of R isolates and 28% of E isolates were able to solubilize tri-calcium phosphate whereas over 38% and 34.6% of isolates obtained from the rhizosphere and surface-sterilized roots of the wheat plant were inositol phosphate solubilizes. Production of HCN was detected in 18% R isolates and 10% E isolates. The purpose of this experiment was to compare the ability of the PSB and IAA-PB in promotion of the plant’s ability to absorb P under in vitro condition. So based on IAA production and phosphate-solubilizing ability, we included four R isolates: R115 (IAA+: IAA producer), R63 (PS+: phosphate solubilizer), R185 (IAA+, PS+), R120 (IAA−, PS−), and four E isolates: E149 (IAA+), E111 (PS+), E240 (IAA+, PS+), E203 (IAA−, PS−) in our study system (Table 1).
The 16S rRNA gene sequences of the isolates R115, R63, R185, R120, E149, E111, E240, and E203 were closely similar to strains Stenotrophomonas sp., Serratia marcescens, Pseudomonas sp., Nocardia fluminea, Stenotrophomonas maltophilia, Bacillus zhangzhouensis, Pseudomonas mosselii, and Microbacterium sp., on the BLAST search in the NCBI (National Center for Biotechnology Information), respectively. The result of the phylogenetic analysis of the isolates is shown in Fig. 3.
In vitro culture response of wheat cultivars with inoculation
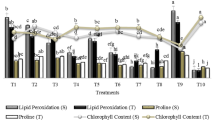
The shoot dry weight of the plants varied from a minimum of 0.71 g (registered in the negative control) to a maximum value of 1.1 g (registered in the positive control) (Fig. 4). Root and shoot dry weight of wheat significantly affected by bacterial strains (P ≤ 0.01). Single inoculation with PGP R and E bacteria significantly increased root and shoot dry weight than control. Similar trends were also found in co-inoculation with R and E isolates. However, for dual inoculation with R and E isolates, comparatively higher root and shoot dry weight was observed under in vitro condition. Among isolates used in our study, R isolate Pseudomonas sp. and E isolate Pseudomonas mosselii (IAA-PB and PSB) were more efficient than R and E isolates with the single ability (IAA-PB or PSB). Under deficient P conditions, the differences between Roshan and Marvdasht cultivars were statistically significant. In the negative and positive control of Roshan cultivar, the chlorophyll meter reading ranged from 45 to 47.5 respectively (Fig. 5). Individual addition of R and E isolates with IAA production ability caused a slight increment in chlorophyll content of wheat plant compared with control. Nevertheless, inoculation with single R and E isolates significantly increased plant growth parameters. The highest results were obtained on co-inoculation by R and E isolates by numerous PGP properties. The greatest P uptake (6.88 mg plant−1) was recorded in plants treated with the R isolate Pseudomonas sp. and E isolate Pseudomonas mosselii (Fig. 6). No significant differences in P uptake was observed in R and E isolates with IAA production ability treatments. PSB also showed a significant increase in P uptake in all the R and E inoculated treatments, whereas all the other treatments (negative control and IAA− + PS−) failed to show any significant increase in P uptake. Given that the maximum plant growth promotion was determined by co-inoculation of R185 and E240 isolates, this treat was selected for greenhouse experiment.
Effect of rhizospheric and endophytic bacterial inoculation with different PGP traits on the shoot and root dry weight of wheat cultivars under in vitro condition. The error bars represent the least significant difference among treatments at P ≤ 0.01. Statistical significance was determined by analysis of variance (ANOVA) followed by Duncan’s Multiple Range test; different lower case letters indicate significant differences (P ≤ 0.01) for each parameter
Effect of rhizospheric and endophytic bacterial inoculation with different PGP traits on chlorophyll content of wheat cultivars under in vitro condition. The error bars represent the least significant difference among treatments at P ≤ 0.01. Statistical significance was determined by analysis of variance (ANOVA) followed by Duncan’s Multiple Range test; different lower case letters indicate significant differences (P ≤ 0.01) for each parameter
Effect of rhizospheric and endophytic bacterial inoculation with different PGP traits on phosphorus concentration of wheat cultivars under in vitro condition (90 days after sprouting within test tubes) compared to un-inoculated control plants (negative control). The error bars represent the least significant difference among treatments at P ≤ 0.01. Statistical significance was determined by analysis of variance (ANOVA) followed by Duncan’s Multiple Range test; different lower case letters indicate significant differences (P ≤ 0.01) for each parameter
Greenhouse culture response of wheat with inoculation
In order to investigate the effect of PGPB on plant growth, a bacterial consortium of R185 and E240 were selected based on their potential PGP properties under in vitro condition. Analysis of variance indicated that bacterial inoculation significantly (P ≤ 0.01) increased shoot P compared to the control. In addition, bacterial consortium caused significant (P ≤ 0.01) increase in the grain P content in contrast to non-inoculated treatments. Root P content did not show any significant differences among the bacterial consortium with the control. Inoculation with a consortium of R and E isolates led to enzyme activity stimulation. The average enzyme activity recorded was 15.153 μg TPF g−1 24 h−1 (70.2% increase, P ≤ 0.01) and 450.67 μg pNP g−1 h−1 (52.2% increase, P ≤ 0.01) for dehydrogenase and phosphatase activity respectively compared to the control (Fig. 7).
Discussion
Currently, the excessive application of P fertilizers in the agriculture field is under debate due to environmental concerns. Therefore, it is important to find alternative strategies to reduce the harmful effects of farming practices. In the present study, wheat rhizosphere and roots were selected for isolation of PGP rhizobacteria and endophytes. Then, in vitro assays were carried out to evaluate PGP traits such as IAA production and phosphate solubilization by isolates. IAA production was observed in the majority of R and E isolates. Zakharova et al. (1999) reported that 80% of bacteria are able to produce IAA. If IAA producing bacteria colonize the rhizosphere and inside roots, the IAA levels in these areas may locally increase. This is likely to affect the architecture of the developing root system and nutrient absorption especially P. However, microbial IAA increases the lateral and adventitious rooting leading to enhanced mineral and nutrient uptake (Shaikh and Saraf, 2016). In this study, a large number of R and E isolates were able to produce siderophore. Under the aerobic and alkaline condition, iron exists in an insoluble from in soil, which is not available simply for plants. Therefore, bacterial isolates have evolved specific tools to chelate insoluble form iron by the production of siderophores. Soil bacteria from different genera including Pseudomonas, Bacillus, Azospirillum, Azotobacter, Burkholderia, Enterobacter, and Rhizobium helps in the production of siderophores and absorption of iron by plants (Sujatha and Ammani, 2013). Jahanian et al. (2012) and Gouda et al. (2018) reported that siderophore-producing bacteria significantly influence the availability of Fe, Zn, and Cu and help in growth and yield improvement of the plant. Due to the limited solubility of phosphorus in many soils, its availability in soil is rarely sufficient for the growth and development of plants (Malhotra et al. 2018). The PGPB are versatile organisms that solubilize insoluble phosphorus through the production of organic acids and acid phosphatases, which increase the availability of phosphorus for the plant. Phosphate-solubilizing ability of studied isolates was screened by plate assay method using Sperber agar. In the present study, 24% of R isolates and 28% of E isolates were P solubilizer. Solanki et al. (2018) reported that P-solubilizing microorganisms constitute 20–40% of the cultivable population of soil microorganisms. The R and E bacteria with phosphate-solubilizing ability can convert tri-calcium phosphate in the medium from insoluble to soluble forms. This leads to increased P availability, which finally improves plant P uptake (Pande et al. 2017).
Assessment of in vitro data for wheat plants shows that R and E isolates with multiple PGP traits (IAA-PB and PSB) were more efficient than R and E isolates with the single ability (IAA-PB or PSB). PGP bacteria, having multiple activities as phosphate solubilization, phytohormone, HCN, siderophore and ammonia production, nitrogenase activity, have shown interesting results in different crop studies. Some examples of PGP rhizobacteria are Agrobacterium, Arthrobacter, Azotobacter, Azospirillum, Bacillus, Burkholderia, Caulobacter, Chromobacterium, Erwinia, Flavobacterium, Micrococcus, Pseudomonas, and Serratia (Bhattacharyya and Jha 2012). Similarly, some examples of the PGP endophytes are Pseudomonas, Bacillus, Burkholderia, Stenotrophomonas, Micrococcus, Pantoea, and Microbacterium (Shi et al. 2014; Sun et al. 2009). From the results of the present study, it could be reaffirmed that R and E isolates were involved in promoting plant growth with PGP activity. Plant growth parameters were enhanced by the combined inoculation of two groups of R and E bacteria compared to individual inoculations, suggesting that PGP rhizobacteria acted synergistically with PGP endophytes in phosphate solubilization. Compared to inoculation with IAA-PB or PSB, inoculation with IAA-PB and PSB showed higher promotion ability. The increase in growth parameters due to the application of R and E isolates could be attributed to IAA production and P solubilization. It seems PGP traits do not work independently of each other but additively as it was suggested in the “synergistic hypothesis” that multiple mechanisms are responsible for the plant growth promotion and increased yield. Nevertheless, some researchers concluded that inoculation with PSB could stimulate plant growth through the production of organic acids (Bakhshandeh et al. 2017; Kaur and Reddy 2015). Hussain et al. (2013) showed that PSB Burkholderia sp. PS-01, Bacillus sp. PS-12, Pseudomonas sp. PS-32, Flavobacterium sp. PS-41, and Pseudomonas sp. PS-51 significantly increased plant growth parameters and grain yield up to 16, 11, 42, 29, and 33%, respectively, compared with control. In addition to phosphate solubilization, IAA released by R and E isolates enables the plant to increase both the root surface area and length by which uptake of nutrients become more efficient. Consequently, using IAA-PB for enhancing plant root development could play a significant role in improving nutrient uptake, especially if applied in combination with bio-fertilization such as PSB. The uptake of P by wheat cultivars followed a similar trend to growth parameters.
Wheat cultivars responded to bacterial inoculation by increasing the percentage of total plant P. Increased the supply of P to shoots resulted in better growth of aboveground parts in the inoculated plant compared with non-inoculated plants. In the absence of bacterial inoculation, Roshan cultivar had a higher proportion of total plant P allocated to the plant systems than Marvdasht cultivar. The results further showed that inoculation with R and E bacterial consortium with multiple PGP traits have a better impact on plant growth regardless of cultivar types. In P limited condition, plants have developed several mechanisms to overcome P deficiency, such as improving the ability of a plant to take up more P in a low P condition, and the ability of a plant to produce greater biomass per unit P taken up. Expansion of root system (Rubio et al. 2001), increasing root exudates (Bhattacharyya et al. 2013), and association with mycorrhiza (Miyasaka and Habte 2001) are mechanisms that enhance plant P uptake efficiency. Similar to in vitro assay, we found that use of the bacterial consortium in greenhouse assay increased assimilation of P into the plant tissue and soil enzyme activity. The bacterial consortium had a positive effect on the concentration of P in grain and shoot of P wheat, while no effect was found on the concentration of P in the root tissue. PGP could be mechanistically related to solubilization of P and enhanced production of IAA by the R and E isolates. Bacteria (most commonly Bacillus and Pseudomonas) have been identified as being able to release soluble P from fixed phosphate in soil minerals by releasing organic acid and proton phosphatases and cation-chelating compounds (Richardson et al. 2011). Given that only 15–20% of the applied P in fertilizers is taken up by the plant and the remaining P fixed as insoluble P fractions in soils, there seems to be an opportunity for further improvement in P bio-fertilizer.
Hence, co-inoculation of R and E bacteria with multiple PGP traits might stimulate plant growth by phosphate solubilization and plant hormones. Finally, the results suggest that co-inoculation of R and E bacterial inoculant with multi-PGP traits can develop plant growth compared to single inoculation of R or E bacterial inoculant.
References
Adesemoye AO, Torbert HA, Kloepper JW (2009) Plant growth promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb Ecol 58:921–929
Bakhshandeh E, Pirdashti H, Shahsavarpour K (2017) Phosphate and potassium solubilizing bacteria effect on the growth of rice. Ecol Eng 103:164–169
Bashan Y, Kamnev AA, De-Bashan LE (2013) Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: a proposal for an alternative procedure. Biol Fertil Soils 49:465–479
Bhattacharyya PN, Jha DK (2012) Plant growth promoting rhizobacteria: emergence in agriculture. World J Microbiol Biotechnol 28:1327–1350
Bhattacharyya P, Das S, Adhya TK (2013) Root exudates of rice cultivars affect rhizospheric phosphorus dynamics in soils with different phosphorus statuses. Commun Soil Sci Plant Anal 44:1643–1658
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in-situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Carpenter SR (2005) Eutrophication of aquatic ecosystems: bistability and soil phosphorus. Proc Natl Acad Sci U S A 102:10002–10005
Chabot R, Antoun H, Cescas MP (1996) Growth promotion of maize and lettuce by phosphate-solubilizing Rhizobium leguminosarum biovar Phaseoli. Plant Soil 184:311–321
Dey R, Pal KK, Bhatt DM, Chauhan SM (2004) Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth promoting rhizobacteria. Microbiol Res 159:371–394
Dinesh R, Anandaraj M, Kumar A, Bini YK, Subila KP, Aravind R (2015) Isolation, characterization and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiol Res 173:34–43
Emami S, Alikhani HA, Pourbabaei AA, Etesami H, Motashare Zadeh B, Sarmadian F (2018) Improved growth and nutrient acquisition of wheat genotypes in phosphorus deficient soils by plant growth-promoting rhizospheric and endophytic bacteria. Soil Sci Plant Nutr Article In Press
Etesami H, Emami S, Alikhani HA (2017) Potassium solubilizing bacteria (KSB): mechanisms, promotion of plant growth, and future prospects - a review. J of Soil Sci and Plant Nutri 17(4):897–911
Fernández LA, Zalba P, Gómez MA, Sagardoy MA (2007) Phosphate-solubilization activity of bacterial strains in soil and their effect on soybean growth under greenhouse conditions. Biol Fertil Soils 43:805–809
Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS, Patra PJ (2018) Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res 206:131–140
Heffer P, Prud’homme M (2014) Fertilizer Outlook 2014–2018. International Fertilizer Industry Association (IFA), Paris
Hegedűs M, Tóth-Bodroghi E, Németh SZ, Somlai J, Kovács T (2017) Radiological investigation of phosphate fertilizers: leaching studies. J Environ Radioact 173:34–43
Hemwall JB (1957) The fixation of phosphorus by soils. Adv Agron 9:95–112
Hu J, Wei Z, Weidner S, Friman VP, Xu YC, Shen QR, Jousset A (2017) Probiotic Pseudomonas communities enhance plant growth and nutrient assimilation via diversity-mediated ecosystem functioning. Soil Biol Biochem 113:122–129
Hussain MI, Asghar HN, Akhtar MJ, Arshad M (2013) Impact of phosphate solubilizing bacteria on growth and yield of maize. Plant Soil Environ 32:71–78 https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Impact+of+phosphate+solubilizing+bacteria+on+growth+and+yield+of+maize+&btnG
Jahanian A, Chaichi MR, Rezaei K, Rezayazdi K, Khavazi K (2012) The effect of plant growth promoting rhizobacteria (PGPR) on germination and primary growth of artichoke (Cynaras colymus). Int J Agric Crop Sci 4:923–929
Ji SH, Gururani MA, Chun S (2014) Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol Res 169:83–98
Jia X, Liu P, Lynch JP (2018) Greater lateral root branching density in maize improves phosphorus acquisition from low phosphorus soil. J Exp Bot 69(20):4961–4970
Johnson F, Curl EA (1972) Methods for the research on ecology of soil borne plant pathogens. Burgess Publishing Co, Minneapolis
Kaur G, Reddy MS (2015) Effects of phosphate solubilizing bacteria, rock phosphate and chemical fertilizers on maize-wheat cropping cycle and economics. Pedosphere 25:428–437
Lorck H (1948) Production of hydrocyanic acid by bacteria. Physiol Plant 1:142–146
Malhotra H, Vandana SS, Pandey R (2018) Phosphorus nutrition: plant growth in response to deficiency and excess. In: Hasanuzzaman M, Fujita M, Oku H, Nahar K, Hawrylak-Nowak B (eds) Plant nutrients and abiotic stress tolerance. Springer, Singapore
Masson-Boivin C, Sachs JL (2018) Symbiotic nitrogen fixation by rhizobia-the roots of a success story. Curr Opin Plant Biol 44:7–15
Miyasaka S, Habte M (2001) Plant mechanisms and mycorrhizal symbioses to increase phosphorus uptake efficiency. Commun Soil Sci Plant Anal 32:1101–1147
Musarrat J, Khan MS (2014) Factors affecting phosphate-solubilizing activity of microbes: current status. In: Khan M, Zaidi A, Musarrat J (eds) Phosphate solubilizing microorganisms. Springer International Publishing, pp 63–85
Ohlinger R (1996) Dehydrogenase activity with the substrate TTC. In: Schinner F, Ohlinger R, Kandeler E, Margesin R (eds) Methods in soil biology. Springer Verlag, Berlin
Okalebo JR, Gathua KW, Woomer PL (2002) Laboratory methods of soil and plant analysis: a working manual, 2nd edn. Sacred Africa, Nairobi
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with NaHCO3. USDA Cir. 939, U.S. Washington
Pande A, Pandey P, Mehra S, Singh M, Kaushik S (2017) Phenotypic and genotypic characterization of phosphate solubilizing bacteria and their efficiency on the growth of maize. J Genet Eng Biotechnol 15:379–391
Rafi MM, Krishnaveni MS, Charyulu PBBN (2019) Chapter 17 - Phosphate-Solubilizing Microorganisms and Their Emerging Role in Sustainable Agriculture. In: BuddollaV (eds) Recent developments in applied microbiology and biochemistry. Academic Press, pp 223–233. https://doi.org/10.1016/B978-0-12-816328-3.00017-9
Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey P, Ryan MH, Veneklaas EJ, Lambers H, Oberson A, Culvenor RA, Simpson RJ (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349:121–156
Robinson RJ, Fraaije BA, Clark IM, Jackson RW, Hirsch PR, Mauchline TH (2016) Endophytic bacterial community composition in wheat (Triticum aestivum) is determined by plant tissue type, developmental stage and soil nutrient availability. Plant Soil 405:381–396
Rubio G, Walk T, Ge Z, Yan X, Liao H, Lynch J (2001) Root gravitropism and below ground competition among neighboring plants: a modelling approach. Ann Bot 88:929–940
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Shaikh S, Saraf M (2016) Biofortification of Triticum aestivum through the inoculation of zinc solubilizing plant growth promoting rhizobacteria in field experiment. Biocatal Agric Biotechnol 9:120–126
Shi Y, Yang H, Zhang T, Sun J, Lou K (2014) Illumina-based analysis of endophytic bacterial diversity and space-time dynamics in sugar beet on the north slope of Tianshan Mountain. Appl Microbiol Biotechnol 98:6375–6385
Singh NP, Singh RK, Meena VS, Meena RK (2015) Can we use maize (Zea mays L.) rhizobacteria as plant growth promoter? Vegetos 28:86–99
Solanki M, Kundu BS, Nehra K (2018) Molecular diversity of phosphate solubilizing bacteria isolated from the rhizosphere of chickpea, mustard and wheat. Ann Agrar Sci 16:458–463
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31(4):425–448
Sperber JI (1958) The incidence of apatite-solubilizing organisms in the rhizosphere and soil. J Agric Res 9:778–781
Sujatha N, Ammani K (2013) Siderophore production by the isolates of fluorescent pseudomonads. Int J Curr Res Rev 5:1–7
Sun Y, Cheng Z, Glick BR (2009) The presence of a 1-aminocyclopropane-1-carboxylate (ACC) deaminase deletion mutation alters the physiology of the endophytic plant growth-promoting bacterium Burkholderia phytofirmans PsJN. FEMS Microbiol Lett 296:131–136
Tabatabai MA (1982) Soil enzymes. In: Methods of soil analysis, part 2. Soil Science Society of America and American Society of Agronomy, Madison, pp 903–947
Tandon HLS (1987) Phosphorus research and agricultural production in India. Fertiliser Development and Consultation Organisation, New Delhi, pp 50–120
Tsukanova KA, Сhеbоtаr VK, Meyer JJ, Bibikova TN (2017) Effect of plant growth-promoting rhizobacteria on plant hormone homeostasis. S Afr J Bot 113:91–102
Valetti L, Iriarte L, Fabra A (2018) Growth promotion of rapeseed (Brassica napus) associated with the inoculation of phosphate solubilizing bacteria. Appl Soil Ecol 132:1–10
Wei Z, Huang JF, Tan SY, Mei XL, Shen QR, Xu YC (2013) The congeneric strain Ralstonia pickettii QL-A6 of Ralstonia solanacearum as an effective biocontrol agent for bacterial wilt of tomato. Biol Control 65:278–285
Westerman RL (1990) Soil testing and plant analysis. Soil Science Society of America book series
Yadava UL (1986) A rapid and nondestructive method to determine chlorophyll in intact leaves. HortScience 21(6):1449–1450 https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=A+rapid+and+nondestructive+method+to+determine+chlorophyll+in+intact+leaves&btnG
Zakharova E, Shcherbakov A, Brudnik V, Skripko N, Bulkhin N, Ignatov V (1999) Biosynthesis of indole-3-acetic acid in Azospirillum brasilense insights from quantum chemistry. Eur J Biochem 259:572–576
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Emami, S., Alikhani, H.A., Pourbabaei, A.A. et al. Effect of rhizospheric and endophytic bacteria with multiple plant growth promoting traits on wheat growth. Environ Sci Pollut Res 26, 19804–19813 (2019). https://doi.org/10.1007/s11356-019-05284-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05284-x