Abstract
The annual production of plastics has doubled over the past 15 years and, consequently, a large amount of plastic has accumulated in the environment generating ecological problems. In this study, a Paenibacillus sp. isolate was obtained from a landfill from Brazil and it presented the alkane hydroxylase gene (alkB). Weight loss of low-density polyethylene (LDPE) was measured and a significant difference in final weight compared to initial weight was assessed. Some chemical characteristics, such as bond scissions and formation of new functional groups [carboxylic acids (3300–2500 cm−1), esters (1210–1163 cm−1), and ethers (1075–1020 cm−1)], were detected by Fourier-transform infrared spectroscopy. Bacterial colonization on the plastic surface and physical changes, as formation of cracks and pits, was visualized by scanning electron microscopy. This isolate was susceptible to all the antimicrobials tested. Therefore, this isolate possesses great potential to degrade polyethylene and become an option for LDPE bioremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bioremediation is a process in which living organisms are used to remove or reduce pollutants in the environment. This process is considered a viable alternative for the treatment of different contaminated environments, such as soil, water, and industrial effluents. The bioremediation process can be applied in situ, in which the treatment is carried out directly in the contaminated or ex situ, in which it is necessary for the excavation and the removal of the contaminated region from its place of origin. This technique can be used to remove different pollutants, including plastics (Lee et al. 2011; Lü et al. 2011).
Polyethylene (PE) is a linear and thermoplastic hydrocarbon polymer, which consist of long chains of ethylene monomers (Usha et al. 2011). This type of plastic is the main commercially produced synthetic polymers and has been widely used in the manufacturing plastic bags, disposable containers, and bottles (Byuntae et al. 1991; Balasubramanian et al. 2010; Gajendiran et al. 2016). Low-density polyethylene (LDPE) is normally not reactive at room temperature, except when strong oxidizing agents are used, it has a density range of 0.910–0.940 g × cm−3 and can withstand high temperatures (Pramila and Ramesh 2011).
The use of polyethylene is increasing worldwide at a rate of 12% per year and approximately 140 million tons are produced worldwide each year (Sharma et al. 2015). Due to this large amount of polyethylene, it accumulates in the environment, generating plastic waste, which needs thousands of years to be degraded (Usha et al. 2011). There are different methods of plastics disposal, such as incineration, recycling, and landfills; however, each one has its own inherent limitations. Thus, the role of microorganisms is very important for the plastic degradation (Sharma and Sharma 2004; Deepika and Jaya 2015).
Paenibacillus is a bacterial genus classified as facultative anaerobic, endospore-forming, and originally included in the Bacillus genus. Bacteria belonging to this genus have already been detected in several sources, such as soil, water, vegetables, animals, and humans. Some Paenibacillus species produce antimicrobials compounds and enzymes involved in the bioremediation process (Grady et al. 2016).
Many bacteria and fungi degrade different groups of plastics, and the potential of polyethylene degrading microorganisms has been investigated since the year 1961 (Fuhs 1961; Raziyafathima et al. 2016). Studies using an n-alkane-degrading enzyme for the study of low-molecular-weight polyethylene (LMWPE) have already been performed. However, there are a few reports of environmental bacteria codifying genes for polyethylene degradation (Yoon et al. 2012). In this study, the ability to degrade LDPE films was investigated by a bacterium isolated from a landfill from Brazil.
Materials and methods
Soil samples
Four samples were collected randomly from a landfill and solid-waste incinerator at Ribeirão Preto, São Paulo, Brazil. They were obtained from the superficial layer of soil (5–10 cm) and were transferred to sterile plastic bags. After that, the samples were stored at 4 °C for further experiments.
Bacterial isolation
Isolation of the LDPE degrading bacteria was performed according to Yoon et al. (2012) with modifications. The minimum salt medium (MSM) was composed by K2HPO4 0.5 g L−1; KH2PO4 0.04 g L−1; NaCl 0.1 g L−1; CaCl2.2H2O 0.002 g L−1; (NH4)2SO4 0.2 g L−1; MgSO4.7H2O 0.02 g L−1; FeSO4 0.001 g L−1. The final pH was adjusted to 7.8.
From each soil sample collected, 1 g was added in 10 mL of Brain Heart Infusion (BHI) (Oxoid, United Kingdom). After 24 h of incubation at 37 °C, 10 mL of the culture were added in an Erlenmeyer flask with 90 mL of MSM containing the polyethylene bags. After 1 week of incubation, 200 µL were withdrawn and inoculated into BHI agar plates. This procedure was repeated for 4 weeks.
Detection of the alkB gene
The genomic DNA was extracted using the using the QIAamp DNA Mini Kit (QIAGEN) according to the manufacturer’s recommendations. PCR reactions were performed to detect alkB gene in the isolates obtained from the landfill using the primers and conditions described by Yang et al. (2015). All reactions were performed using 100 ng of genomic DNA and the amplicons were sequenced for confirmation.
Bacterial identification
Bacteria carrying the alkB gene were identified by the conventional biochemical tests and molecularly by sequencing the 16S rRNA gene using primers fd1_fow (5′-AGAGTTTGATCCTGGCTCAG-3′) and rp2_rev (5′-ACGGCTACCTTGTTACGACTT-3′) according to Weisburg et al. (1991). The obtained sequences were compared to those available in GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Films of polyethylene bags
Polythene bags were collected from supermarkets and cut in pieces of 5 cm in diameter, which were disinfected with ethanol solution 70%. One pre-treatment of polyethylene was carried out to study polyethylene biodegradation. The polyethylene bags were transferred to a solution containing 10 mL of bleach, 70 mL of Tween 80, and 983 mL of distilled water, which were kept stirring during 30–60 min (El-Shafei et al. 1998). The strips were stirred for 1 h into a beaker containing distilled water and, posteriorly, they were aseptically transferred for an ethanol solution 70% v/v for 30 min. Then, the polyethylene strips were incubated at 45–50 °C overnight. Polyethylene bags without any treatment were also used.
Microbial degradation of polyethylene bags and weight measurement
The same procedure of microbial isolation was used for microbial degradation of the polyethylene bags. The polyethylene bags chemically treated and also those without any treatment were inserted aseptically in different flasks containing 90 mL of MSM. Then, the bacterial isolate was added, and the flasks incubated at 37 °C in an incubator shaker for 3 months. After incubation of the polyethylene bags, they were washed with sterile distilled water and alcohol, and then, they were air-dried. Posteriorly, they were weighed (final weight). Percentage of polyethylene degradation was determined by the following:
Fourier-transform infrared spectroscopy (FT-IR) analysis
Chemical changes, such as appearance or disappearance of bond scissions and new functional groups in the LDPE surface, were analyzed by Fourier-transform infrared spectroscopy (FTIR). Structural changes in the polyethylene strips were investigated using the EQUINOX 55 FT-IR spectrometer with a spectrum from 400 to 4000 wavenumbers cm−1.
Scanning electron microscopy (SEM) analysis
Scanning electron microscopy (SEM) analyzed some physical properties of the LDPE film, such as micro-cracks, surface changes, pits, and holes. The samples were metallized using gold particles (three discharges of 40 mA/50 s in argon atmosphere), a high vacuum metalizator (Bal-Tec SCD 005), and the SEM (Leo, 435VF, U.K.) at 15.00 kV EHT. Three successive magnifications (2.0, 5.0, and 10.0 KX) were performed.
Antimicrobial susceptibility testing
The resistance profile was determined by minimum inhibitory concentration (MIC) as recommended by the Clinical Laboratory Standards Institute (CLSI 2016). A total of ten antimicrobials were tested, being ampicillin, cefotaxime, ceftazidime, ceftriaxone, imipenem, gentamicin, tetracycline, ciprofloxacin, levofloxacin, and chloramphenicol. Staphylococcus aureus ATCC 29213 strain was used as control in this experiment.
Results and discussion
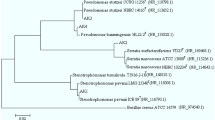
To isolate bacteria capable of degrading polythene, soil samples were collected from a landfill and five isolates were obtained, which were analyzed for the presence of alkB gene. Among these isolates, just one presented the alkB gene (GenBank accession number MK045309). This isolate was identified as Paenibacillus sp. (GenBank accession number MK053775) and named DK1.
Kohno et al. (2002) detected the alkB gene in bacterial isolates belonging to at least nine different genera and suggested that these bacteria are common in alkane-degrading environments. Yoon et al. (2012) stated that alkane hydroxylase, encoded by the alkB gene, is a key enzyme that catalyzes the first step in the alkane degradation reaction, so this same type of enzyme could also be involved in the polyethylene degradation.
Heiss-Blanquet et al. (2005) reported the alkB gene in soil samples and observed that, in soil contaminated with hydrocarbons, the quantity of the alkB gene was larger. Many studies have concluded that naturally growing soil microorganisms can degrade polyethylene (Deepika and Jaya 2015; Gajendiran et al. 2016; Gnanavel et al. 2016; Singh et al. 2016; Peixoto et al. 2017). Although the alkB gene has been detected in different bacterial genera, there are no reports of this gene in bacteria belonging to the genus Paenibacilllus.
Many studies have investigated the biodegradation of LPDE in different bacterial genera, including Bacillus and Lysinibacillus (Esmaeili et al. 2013; Singh et al. 2016). To our knowledge, there are no reports of Paenibacillus species degrading polyethylene as a single bacterium, although there is one report of Paenibacillus macerans colonizing polyethylene surface as a member of a bacterial consortium (Nowak et al. 2011).
A significant difference in the final weight compared to the initial weight was obtained. The percent of weight loss after incubation with polyethylene bags chemically treated was more than twice, comparing to those bags with no treatment (Table 1). The weight loss of the polythene films can be associated with the breakdown of carbon backbone, which probably occurs due to enzymatic degradation by the studied bacterium. Some studies have also shown weight loss results similar to those found in the present study (Kyaw et al. 2012; Deepika and Jaya 2015; Singh et al. 2016).
The potential of DK1 isolate towards biodegradation of LDPE was analyzed by FTIR (Figs. 1, 2). It was observed variation in the intensity of bands in different regions in the presence of Paenibacillus sp. in both cases. Absorption bands were assigned at 719 cm−1, 730 cm−1 (C–H bend-mono), 1462 cm−1, 1472 cm−1 (C=C stretch) and 2920, 2850 cm−1 (both due to C–H stretch) for control spectrum (Figs. 1, 2).
In the case of films with chemical treatment, new absorption bands between 1000 and 1700 cm−1 (1029 and 1371 cm−1) of the spectra are possibly due to the oxidized fractions, such as moieties containing –OH groups, which result from the biodegradation by the selected microorganisms (Corti et al. 2010). The carboxylic acids (3300–2500 cm−1), esters (1210–1163 cm−1), and ethers (1075–1020 cm−1) were also formed at different frequencies. Terminal doubles bonds (1650 cm−1) were also formed. In the case of no-treated film, it could be observed the formation of a peak at 970 cm−1, indicating the occurrence of internal double bonds (Figs. 1, 2). Our findings are consistent with the study of other authors, which have demonstrated the spectra of the polyethylene films incubated in soil with several new bands due to the degradation process (Gajendiran et al. 2016; Divyalakshmi and Subhashini 2016; Esmaeili et al. 2013).
Since most bacterial surfaces are hydrophilic, the hydrophobicity of polyethylene regularly interferes with bacterial adhesion to the surface. Microbial degradation of polymers, such as polyethylene, demands the formation of biofilm on its surface, permitting that bacteria utilize the non-soluble substrate (Gilan et al. 2004). Besides, the synthesis of biofilms by bacteria favors their adhesion to surfaces and survival in environments with low nutrient (Linos et al. 2000). This fact can explain the better biodegradation of polyethylene in the case of film with chemical treatment, when measured by weight loss and FTIR. In contrast, film without treatment did not produce a significant biofilm and was less biodegraded, since it was less hydrophilic.
SEM investigated the changes in the surface of the LDPE films. The control film of polyethylene bag without treatment revealed smooth and homogeneous morphology (Fig. 3a); however, no special features were detected after chemical treatment in the SEM micrograph of the films (Fig. 3b). After incubating the film with MSM, superficial salts attached can be observed (Fig. 3c, d). In the case of LDPE film incubated with the Paenibacillus sp. DK1, it was observed the surface deformation after 90 days of incubation in both cases (Fig. 3E and 3F; Fig. 3g). Bacteria were also noticed on the film surface, indicating their strong adhering capabilities and capacity to use LDPE (Fig. 3f–h).
In the film chemically treated, the bacterium attached to the polyethylene film was seen in higher quantity when compared to the film without treatment, and a larger number of holes and ruptures were detected. These findings are consistent with FTIR and lose weight experiments. Degradation marks can be observed in both cases at places where the bacterium was attached along with the pits and pockets (Fig. 3e–h). Our results are consistent with the other authors reporting the formation of cavities and the presence of biofilm (Bonhomme et al. 2003; Kyaw et al. 2012).
Bacterial resistance to antimicrobials has become a public health problem and the environment acts as a reservoir of multi-drug-resistant bacteria and antimicrobial resistance genes (Berendonk et al. 2015; Furlan and Stehling 2017). The antimicrobial resistance profile, the DK1 isolate, was susceptible to all antimicrobials, providing an additional benefit for its use in bioremediation.
Conclusions
In this study, a bacterial isolate identified as Paenibacillus sp. was obtained from a landfill and solid-waste incinerator from Brazil as pure culture. This isolate presented ability to degrade LDPE, and it was able to modify and colonize LDPE as carbon source after 3 months of incubation. Besides that, DK1 present a low resistance profile. Therefore, this isolate could be used for bioremediation as a promising tool for polythene degradation.
References
Balasubramanian V, Natarajan K, Hemambika B, Ramesh N, Sumathi CS, Kottaimuthu R, Rajesh Kannan V (2010) High-density polyethylene (HDPE)-degrading potential bacteria from marine ecosystem of Gulf of Mannar, India. Lett App Microbiol 51:205–211
Berendonk TU, Manaia CM, Merlin C, Fatta-Kassinos D, Cytryn E, Walsh F, Bürgmann H, Sørum H, Norström M, Pons MN, Kreuzinger N, Huovinen P, Stefani S, Schwartz T, Kisand V, Baquero F, Martinez JL (2015) Tackling antibiotic resistance: the environmental framework. Nat Rev Microbiol 13:310–317
Bonhomme S, Cuer A, Delort AM, Lemaire J, Sancelme M, Scott G (2003) Environmental biodegradation of polyethylene. Polym Degrad Stab 81:441–452
Byuntae L, Anthony LP, Alfred F, Theodore BB (1991) Biodegradation of degradable plastic polyethylene by Phanerocheate and Streptomyces species. Appl Environ Microbiol 57:678–688
CLSI (2016) Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria, 3rd edn. CLSI guideline M45. Clinical and Laboratory Standards Institute, Wayne
Corti A, Muniyasami S, Vitali M, Imam SH, Chiellini E (2010) Oxidation and biodegradation of polyethylene films containing pro-oxidant additives: synergistic effects of sunlight exposure, thermal aging and fungal biodegradation. Polym Degrad Stab 95:1106–1114
Deepika S, Jaya MR (2015) Biodegradation of low density polyethylene by microorganisms from garbage soil. J Exp Biol Agric Sci 3:1–5
Divyalakshmi S, Subhashini A (2016) Screening and isolation of polyethylene degrading bacteria from various soil environments. IOSR J Environ Sci Toxicol Food Technol 10:1–7
El-Shafei HA, El-Nasser NHA, Kansoh AL, Ali AM (1998) Biodegradation of disposable polyethylene by fungi and Streptomyces species. Polym Degrad Stab 62:361–365
Esmaeili A, Pourbabaee AA, Alikhani HA, Shabani F, Esmaeili E (2013) Biodegradation of low density polyethylene (LDPE) by mixed culture of Lysinibacillus xylanilyticus and Aspergillus niger in soil. PLoS One 9:717–720
Fuhs GW (1961) Der mikrobielle Abbau von Kohlenwasserstoffen. Arch Mikrobiol 39:374–422
Furlan JPF, Stehling EG (2017) Presence of β-lactamases encoding genes in soil samples from different origins. Water Air Soil Pollut 228:125
Gajendiran A, Krishnamoorthy S, Abraham J (2016) Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil. Biotech 6:52–58
Gilan Y, Hadar A, Sivan (2004) Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus rubber. Appl Microbiol Biotechnol 65:97–104
Gnanavel G, Thirumarimurugan M, Valli MJ (2016) Biodegradation of oxo polyethylene: an approach using soil compost degraders. Int J Adv Eng Technol 2:140–144
Grady EN, MacDonald J, Liu L, Richman A, Yuan ZC (2016) Current knowledge and perspectives of Paenibacillus: a review. Microb Cell Fact 15:203
Heiss-Blanquet S, Benoit Y, Maréchaux C, Monot F (2005) Assessing the role of alkane hydroxylase genotypes in environmental samples by competitive PCR. J Appl Microbiol 99:1392–1403
Kohno T, Sugimoto Y, Sei K, Mori K (2002) Design of PCR Primers and gene probes for general detection of alkane-degrading bacteria. Microb Environ 17:114–121
Kyaw BM, Champakalakshmi R, Sakharkar MK, Lim CS, Sakharkar KR (2012) Biodegradation of low density polythene (LDPE) by Pseudomonas species. Indian J Microbiol 3:411–419
Lee EH, Kang YS, Cho KS (2011) Bioremediation of diesel-contaminated soils by natural attenuation, biostimulation and bioaugmentation employing Rhodococcus sp. Korean J Microbiol Biotechnol 39:83–89
Linos A, Berekaa MM, Reichelt R, Keller U, Scmitt J, Flemming HC, Kroppenstedt RM, Steinbuchel A (2000) Biodegradation of cis-1,4-polyisoprene rubbers by distinct actinomycetes: microbial strategies and detailed surface analysis. Appl Environ Microbiol 66:1639–1645
Lü JC, Li ZT, Hussain K, Yang GK (2011) Bioremediation: the new directions of oil spill cleanup. Middle East J Sci Res 7:738–740
Nowak B, Pajak J, Drozd-Bratkowicz M, Rymarz G (2011) Microorganisms participating in the biodegradation of modified polyethylene films in different soils under laboratory conditions. Int Biodeterior Biodegrad 65:757–767
Peixoto J, Silva LP, Krüger RH (2017) Brazilian Cerrado soil reveals an untapped microbial potential for unpretreated polyethylene biodegradation. J Hazard Mat 324:634–644
Pramila R, Ramesh KV (2011) Biodegradation of low density polyethylene (LDPE) by fungi isolated from marine water—a SEM analysis. Afr J Microbiol Res 28:5013–5018
Raziyafathima M, Praseetha PK, Rimal IRS (2016) Microbial degradation of plastic waste: a review. J Pharm Chem Biol Sci 2:231–242
Sharma A, Sharma A (2004) Degradation assessment of low density polythene (LDP) and polythene (PP) by an indigenous isolate of Pseudomonas stutzeri. J Sci Ind Res 63:293–296
Sharma J, Gurung T, Upadhyay A, Nandy K, Agnihotri P, Mitra AK (2015) Isolation and characterization of plastic degrading bacteria from soil collected from the dumping grounds of an industrial area. Int J Adv Innov Res 3:2278–7844
Singh G, Singh AK, Bhatt K (2016) Biodegradation of polythenes by bacteria isolated from soil. Int J Res Dev Pharm Life Sci 2:2056–2062
Usha R, Sangeetha T, Palaniswamy M (2011) Screening of polyethylene degrading microorganisms from garbage soil. Lib Agric Res Cent J Int 4:200–204
Weisburg WG, Barns SM, Pelletier BA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Yang Y, Wang J, Liao J, Xie S, Huang Y (2015) Abundance and diversity of soil petroleum hydrocarbon-degrading microbial communities in oil exploring areas. Appl Microbiol Biotechnol 99:1935–1946
Yoon MG, Jeon HJ, Kim MN (2012) Biodegradation of Polyethylene by a Soil Bacterium and AlkB Cloned Recombinant Cell. J Bioremed Biodegrad 3:145–152
Funding
This work was supported by São Paulo Research Foundation—FAPESP (Grant number 2015/18990-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Ethical approval
Not required.
Additional information
Communicated by Erko Stackebrandt.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bardají, D.K.R., Furlan, J.P.R. & Stehling, E.G. Isolation of a polyethylene degrading Paenibacillus sp. from a landfill in Brazil. Arch Microbiol 201, 699–704 (2019). https://doi.org/10.1007/s00203-019-01637-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-019-01637-9