Abstract
P1B-type ATPases are involved in heavy metal transport across the plasma membrane. Some Mycobacterium tuberculosis P-type ATPases are induced during infection, suggesting that this type of transporter could play a critical role in mycobacterial survival. To date, the ion specificity of M. tuberculosis heavy metal-transporting P1B-ATPases is not well understood. In this work, we observed that, although divalent heavy metal cations such as Cu2+, Co2+, Ni2+, Zn2+ Cd2+ and Pb2+ stimulate the ATPase activity of the putative P1B-type ATPase CtpG in the plasma membrane, whole cells of M. smegmatis expressing CtpG only tolerate high levels of Cd2+ and Cu2+. As indicator of the catalytic constant, Michaelis–Menten kinetics showed that CtpG embedded in the mycobacterial cell membrane has a V max/K m ratio 7.4-fold higher for Cd2+ than for Cu2+ ions. Thus, although CtpG can accept different substrates in vitro, this P-type ATPase transports Cd2+ more efficiently than other heavy metal cations across the mycobacterial plasma membrane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is caused by the acid-fast bacillus Mycobacterium tuberculosis, and is a major public health issue, with 8.6 new cases and 1.3 million people dying from TB around the world in 2014 (WHO 2015). Currently, a third of the world’s population is infected with M. tuberculosis, which establishes a permanent infection reservoir and facilitates the spread of TB (WHO 2010). TB and HIV co-infection and the emergence of multi- and extensively drug-resistant TB (MDR- and XDR-TB) have delayed efficient TB control. Therefore, understanding the mechanisms used by tubercle bacilli for survival within host cells is essential for the development of new strategies to prevent latent and active M. tuberculosis infections.
Mycobacterium-infected cells increase toxic cation concentrations, which deleteriously react with mycobacterial lipids, proteins, and DNA (Agranoff and Krishna 1998). Increased concentrations of Cu2+, Zn2+, and Fe2+ produce an effective response mediated by inflammatory cytokines, such as TNF and INFɤ, inside phagosomes infected with M. tuberculosis (Wagner et al. 2005), indicating that high doses of heavy metals interfere with progression of infection. During infection, M. tuberculosis uses cell membrane transporters to avoid increased intracellular concentrations of heavy metal cations, such as Co2+, Cu2+, Ni2+, Fe2+, Zn2+, Mn2+and Cd2+. Thus, mycobacterial cell membrane transporters are critical for ion homeostasis and bacterial survival inside macrophages (Raimunda et al. 2012).
Cadmium does not have apparent specific biological functions in bacteria, however, it competes with cell storages of Mn2+ and Zn2+, and displaces Ca2+ and Zn2+ from proteins. In addition, Cd2+ interacts with nucleic acids, competes as a cofactor for proteins and makes bacteria susceptible to oxidative stress with the production of reactive oxygen species (Paulsen et al. 1996; Haney et al. 2005). In general, Cd2+ detoxification systems are activated by intracellular bacteria in response to the high concentration of this cation in phagocytic cells as a consequence of infection. P-type ATPase pumps, together with the cation diffusion facilitator (CDF) and resistance-nodulation-division (RND) transporter families are the main Cd2+ detoxification system in bacteria. These protein families are also used in bacteria to efflux other toxic cations such as Co2+, Cu2+, Ni2+, Ag+, Pb2+. In some cases, genes encoding Cd2+ detoxification are the same as those used in Zn2+ detoxification (Paulsen et al. 1996; Haney et al. 2005). CDF transporters are found in bacteria, archaea, and eukarya, and correspond to polypeptides of 400 kDa approximately, which contain six transmembrane segments (TMS) and perform cation/H + exchange chemoprocessing in the periplasmic space, such as the case of CzcD protein from Cupriavidus metallidurans (Haney et al. 2005). Likewise, RND is a superfamily of bacterial transporters, which form part of a multimeric complex in the plasma membrane. An example is the CzcCBA complex, an antiporter cation/H+ of toxic ions, including Cd2+ that is transported externally from the cytoplasm of C. metallidurans (Paulsen et al. 1996).
There are also detoxifying proteins, such as metallothioneins that sequester heavy metal cations and increasing bacterial tolerance to these toxic substances. For instance, the bacterial cytoplasmic metallothioneins, such as SmtA from Synechococcus and P. putida (Olafson et al. 1988; Blindauer et al. 2002) and metallo-chaperones (Blencowe and Morby 2003) detoxify bacteria from toxic metals such as Cd2+ and Zn2+.
P-type ATPases responsible for Cd2+ detoxification belong to the Zn2+/Cd2+/Pb2+ translocating P1B-type ATPases (Argüello 2003). P1B-type, as well as the other P-type ATPases, use the energy released in ATP hydrolysis to pump heavy metal cations (Argüello et al. 2007). The key steps in this process include: (1) metal binding on the cytosolic ATPase portion, (2) metal transfer to the ATPase transmembrane segment (TMS), (3) ATP hydrolysis and enzyme autophosphorylation of a conserved arginine residue, (4) metal release in the extracellular side, and (5) subsequent enzyme dephosphorylation to reinitiate the cycle. Specifically, P1B-type ATPases, also known as CPx-type ATPases, transport toxic heavy metal cations to the cellular periplasm (Sharma et al. 2006). The ion specificity of P1B -type ATPases is given by the metal binding domain that is located in the N-terminal cytoplasmic region (Argüello 2003). P1B-type ATPases are present in many bacterial species, including Escherichia coli (ZntA), Staphylococcus aureus (CadA), Pseudomonas putida, and C. metllidurans, among others (Nucifora et al. 1989; Rensing et al. 1997; Hu and Zhao 2007; Scherer and Nies 2009).
Interestingly, tubercle bacilli contain a high number of heavy metal P1B-type ATPase transporters compared to other intracellular pathogens, suggesting that these transporters could be critical for mycobacterial survival (Novoa-Aponte et al. 2012). M. tuberculosis contains twelve open-reading frames annotated as P-type ATPases; seven of which are putative P1B-type ATPases (CtpA, CtpB, CtpC, CtpD, CtpG, CtpJ, and CtpV). They are responsible for catalyzing the translocation of heavy metals across the cell membrane (Novoa-Aponte et al. 2012). The role of putative mycobacterial P1B-type ATPases for dealing with high doses of heavy metal ions is not well understood. However, it is known that P1B-type ATPases, such as CtpC and CtpD, are required for murine infection with M. tuberculosis (Sassetti and Rubin 2003); CtpA and CtpC are overexpressed during the infection processes (Botella et al. 2011); and CtpV is needed for M. tuberculosis virulence (Ward et al. 2010; Raimunda et al. 2014). In addition, we recently described that CtpA is stimulated by high doses of Cu+ in the mycobacterial plasma membrane (León-Torres et al. 2015).
In particular, CtpG is an interesting heavy metal transporter. This putative P-type ATPase has been associated with different mechanisms used for M. tuberculosis survival, for example, with a role in Zn2+ poisoning in human macrophages and survival in human phagocytic cells (Botella et al. 2011). Furthermore, CtpG is activated in response to the oxidizing agent diamide in oxidative stress processes (Hampshire et al. 2004) and to M. tuberculosis starvation (Betts et al. 2002; Hampshire et al. 2004). To date, the ion specificity and function of CtpG remains elusive. In this work, we performed bioinformatics predictions and estimated the tolerance of M. smegmatis cells expressing CtpG to sublethal doses of different heavy metal cations, together with the ATPase activity mediated by CtpG of plasma membrane vesicles from recombinant cells. The results obtained evidenced that CtpG preferentially transports Cd2+ across the mycobacterial plasma membrane.
Materials and methods
Strains, culture conditions, and genomic DNA isolation
M. smegmatis mc2155 (ATCC 700084) cells (Snapper et al. 1990) were grown in Luria–Bertani (LB) broth until an OD600 = 0.3 for electroporation experiments, 0.1 for cell viability assays and 0.4 for cell membrane isolation. For the viability assays, the cells were harvested and resuspended in LB containing 0.05% Tween 80, separately supplemented with heavy metal cations, and incubated at 37 °C. E. coli BL21 (Agilent Technologies, CA, USA) cells were grown at 37 °C in LB broth or plates. M. tuberculosis H37Rv (ATCC 27294) genomic DNA was isolated from cells cultured in 7H9-ADC broth (Somerville et al. 2005).
Expression of M. tuberculosis H37Rv CtpG in M. smegmatis cells
Plasmids and primers used are listed in Table 1. The ctpG (Rv1992c) gene was amplified by PCR from genomic DNA of M. tuberculosis H37Rv using the primer pairs ctpG-pMV Dir/ctpG-pMV Rev that respectively introduce the restriction sites EcoRI and SalI to the 5´ and 3´ ends of the ctpG gene. The amplimer obtained was inserted into the mycobacterial/E. coli shuttle vector pMV261 (Stover et al. 1991) to produce the pML01 plasmid (Table 1), which was subsequently transformed into E. coli BL21 cells (Agilent Technologies, CA, USA). The recombinant cells were screened by colony PCR using the primer pairs pMV comp-up/RT ctpG-Rev. The integrity of the ctpG gene was confirmed by sequencing of pML01, using the same primer pairs. Finally, M. smegmatis mc2155 cells were electropored with pML01 or pMV261 (control plasmid), and transformation was confirmed by colony PCR using the primer pairs pMVcomp Up/RT ctpG-Rev and Tm903A/Tm903B, respectively. Finally, the M. tuberculosis CtpG protein was expressed on the M. smegmatis cell membrane by incubating recombinant cells at 45 °C.
Cell membrane isolation
Differential centrifugation was used to isolate the cell membranes as previously described (Basu et al. 1992; Ayala et al. 2015). Mycobacterial cells were harvested, centrifuged, and resuspended in lysis buffer (10 mM MOPS, 1 mM EDTA, and 0.3 mM phenylmethylsulfonyl fluoride, pH 7.4), and cells were then lysed in a Mini Beadbeater-16 (Biospec, OK, USA). Cell debris was isolated by centrifugation at 25,000g for 30 min at 4 °C, and the cell membranes were obtained from the supernatant by centrifugation at 100,000g for 90 min at 4 °C. The membrane fraction was resuspended in buffer containing 10 mM MOPS and 250 mM sucrose (pH 7.4), and analyzed by SDS–PAGE.
ATPase activity
ATPase activity was measured for the plasma membrane vesicles from wild-type M. smegmatis mc2155 and cells transformed with pMV261 or pML01. The inorganic phosphate (Pi) release by the ATPase catalytic activity was quantified as previously described (Fiske and Subbarow 1925; Cariani et al. 2004; León-Torres et al. 2015). The enzymatic reactions (50 µL) were performed in incubation buffer (40 mM MOPS, 150 mM NaCl, 5 mM MgCl2, 5 mM KCl, 5 mM NaN3, 0.25 mM Na2MoO4, and 0.02% Brij-58, pH 7.4) using 8.0 μg membrane vesicles, and individually supplemented with 25 µM (final concentration) of each heavy metal cation: CoCl2, Cu SO4, ZnSO4, MnSO4, NiSO4, Pb(CH3COO)2 and CdCl2. Reactions supplemented with Cu+ were performed in the presence of DTT (2.5 μM) and cysteine (0.2 mM) (León-Torres et al. 2015). The reactions were subsequently initiated by adding 1 mM Na2ATP, then incubated at 37 °C for 30 min, and terminated by adding 100 µL of stop solution (3% ascorbic acid, 0.5% ammonium molybdate, and 3% SDS in 1.0 M HCl). Finally, 150 µL of 3.5% bismuth citrate and 3.5% sodium citrate in 2.0 M HCl were added, and samples were incubated at 37 °C for 10 min. The difference between the total ATPase activity and the activity obtained with no cations (basal activity) was considered the ATPase activity stimulated by the tested cations. In addition, the ATPase activity attributed to CtpG was estimated by subtracting the activity of the membrane vesicles from cells transformed with pMV261 to the activity obtained from cells transformed with pML01. The enzymatic activity was reported as nmol of Pi released/mg of protein × min, and assessed from three independent experiments.
Cell viability assay
Mycobacterial cells (M. smegmatis mc2155 transformed with pML01 or pMV261) were harvested and diluted in culture medium until an OD595 of 0.05 was reached. Then, 100 µL of bacterial suspension were separately mixed in 96-well plates with 100 µL of serial dilutions of heavy metal cations: Cu2+ (4.0 to 0.75 mM), Co2+ (1.2 to 0.4 mM), Mn2+ (100 to 10 mM), Ni2+ (2 to 0.4 mM), Zn2+ (4.0 to 1.0 mM), Cd2+ (0.3 to 0.035 mM), and Pb2+ (3.5 to 1.0 mM, pH = 5.5). Subsequently, the cultures were incubated at 37 °C for 72 h at 80 rpm. Finally, the OD595 of the cultures was measured in an iMARKTM Microplate Reader (Bio-Rad, CA, USA). Cultures supplemented with no cation and kanamycin were considered as 100 and 0% growth, respectively (Ayala-Torres et al. 2015; León-Torres et al. 2015).
Kinetic parameters of CtpG
The ATPase activity stimulated by the heavy metal cations in plasma membrane vesicles expressing M. tuberculosis CtpG was estimated as previously described (León-Torres et al. 2015). The optimum quantity of the membrane protein was estimated using 2–15 μg of membrane vesicles in the enzymatic reactions. The pH dependence of ion metal transport was evaluated in reactions performed with 8.0 μg of membrane vesicles and 25 µM of each cation, varying the pH from 5.9 to 8.7 using 10 mM MOPS (pH values below 7.9) and 10 mM TRIS (pH values above 8.2). The optimum temperature was evaluated in reactions at pH 7.5, by varying the temperature from 4 to 60 °C. All of the enzymatic reactions were performed for 30 min. The enzymatic activity was reported as nmol of Pi released/mg of protein × min and assessed from three independent experiments.
Bioinformatics analyses
The ctpG nucleotide sequence was retrieved from Tuberculist (Lew et al. 2011). The topology of the TMS was predicted with ExPASy (Gasteiger et al. 2005), TMHMM 2.0 (Zankari et al. 2013), TMpred (Hofmann and Stoffel 1993), HMMTOP (Tusnády and Simon 2001), Phobius (Käll et al. 2004), DAS (Cserzo et al. 1997), TMDET (Tusnády et al. 2005), and PPM server (Lomize et al. 2012). The tertiary structure and potential binding sites were predicted using Phyre2 (Kelley and Sternberg 2009). Modeling and validation of the tertiary structure was performed using Swiss Model (Biasini et al. 2014), What If (Vriend 1990), and PROCHECK (Laskowski et al.1993). The quality of the model was evaluated using PROSA (Wiederstein and Sippl 2007). ClustalW2 (Larkin et al. 2007) and BLASTP were used to align the CtpG amino acid sequence with the well characterized bacterial P-type ATPases ZntA from E. coli (P37617), ZntA of Shigella sonnei (Q3YW59), CopA (O29777) and CopB (O30085) from Archaeoglobus fulgidus (Mana-Capelli et al. 2003), and ZosA from Bacillus subtilis (O31668).
Results
Different divalent heavy metal cations are possibly transported by CtpG
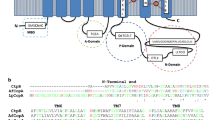
The ctpG gene (Rv1992c) is annotated in the M. tuberculosis H37Rv genome as a 771 amino acid (2316 bp) transmembrane protein that possibly catalyzes the transport of an undetermined metal cation with hydrolysis of ATP, and part of the P-type ATPases classified in the group of acid anhydride hydrolases (Lew et al. 2011). The predicted isoelectric point and molecular weight of CtpG are 5.55 and 79.3 kDa, respectively. In a previous study, we classified CtpG as a P1B-type ATPase, due to the presence of the WI (YE) (RG) motif located between positions 406 and 409 (TMS6), and the LS motif in TMS7 that it is associated to Zn2+ P-type ATPases (Futai et al. 2004; Lewinson et al. 2009). The hydrophobicity profile of M. tuberculosis CtpG is similar to that of ATPase pumps experimentally associated with Cd2+, Zn2+, and Co2+ transport (Novoa-Aponte et al. 2012). According to the PPM server, CtpG displays a topology typical of heavy metal transporting P1B-type ATPases, which includes eight TMS, whose function is to transport heavy-metal cations. In addition, the InterProScan server suggests that CtpG contains its phosphorylation site (DKTGTLT) within TMS6 and TMS7 (Fig. 1). Interestingly, an uncommon heavy metal binding motif APCAL was found in TMS6, suggesting that CtpG is an atypical P1B-type ATPases. On the other hand, the modelled 3D structure demonstrated that the transport domain of CtpG is typical of type I ATPases.
Prediction of metal-binding sites of M. tuberculosis CtpG. a Membrane type I topology of CtpG. b Model of the tertiary structure of CtpG (Ramachandran Z score − 0.581), constructed using the 4 templates: 4umv (35.3% identity). The model was validated using PROSA (Wiederstein and Sippl 2007) obtaining a Z score − 7.66, PROCHECK (Laskowski et al. 1993), and WHAT-IF (Vriend 1990) obtaining a Ramachandran Z score − 0.581
The M. tuberculosis CtpG amino acid sequence shows 38% identity with the M. smegmatis CtpD protein, a Co2+ transporting P1B-type ATPase (Raimunda et al. 2012). M. smegmatis CtpD is orthologous of M. tuberculosis CtpD, which is also a Co2+ transporting P1B-type ATPase in M. tuberculosis together with the paralogous heavy-metal transporter CtpJ. Although the amino acid sequences of all of the above-mentioned P-1B type ATPases contain the catalytic domains of heavy metal P1B-4 type ATPases, specifically CtpG does not show the SCP motif in the TMS6 (Novoa-Aponte et al. 2012). Therefore, CtpG could display cation transporting characteristics different to M. tuberculosis CtpJ, and CtpD from M. tuberculosis and M. smegmatis. According to the alignment with other heavy metal P1B-type ATPases, CtpG showed a similarity between 34 and 39% (query cover from 78 to 80%) with ZntA from E. coli, CopA and CopB of A. fulgidus, and ZosA from B. subtilis that are putative Cu+ and Cu2+ P-type ATPases, respectively. In conclusion, the bioinformatics analyses suggest that different heavy metal cations, such as Cd2+, Zn2+, Cu2+, and Co2+, are potentially transported by M. tuberculosis CtpG.
M. tuberculosis CtpG was heterologously expressed in the M. smegmatis mc2155 plasma membrane
The heterologous expression of CtpG in the cell membrane of the non-pathogenic and environmental M. smegmatis allows estimation of the activity and biological effect of CtpG embedded in the natural environment of the mycobacterial cell membrane. The EcoRI and SalI restriction sites introduced by PCR in the 5`and 3′-ends of the M. tuberculosis ctpG gene, respectively, allowed ctpG to be directionally ligated into the pMV261shuttle vector, which contains replication origins for E. coli (oriE) and one for mycobacteria (oriM). PCR amplification, enzyme digestion mapping, and DNA sequencing verified the integrity of ctpG and its insertion into the correct open-reading frame with the hsp60 promoter of pMV261 in the pML01 recombinant plasmid (Supplementary Fig. 1).
The ATPase activity associated with M. tuberculosis CtpG is stimulated by different heavy metal cations in the mycobacterial plasma membrane
Based on the bioinformatics predictions and previous works (Botella et al. 2011), we assessed the ATPase activity in plasma membrane vesicles enriched with CtpG and separately stimulated by different heavy metal cations (Co2+, Cu2+, Cu+, Zn2+, Mn2+, Ni2+, Pb2+and Cd2+), as a first approach to identify the possible ion specificity of CtpG. Since the ATPase activity was assessed on membrane vesicles containing ion transporters other than P-type ATPases, the enzymatic activity associated with CtpG was calculated by subtracting the ATPase activity value of membranes from M. smegmatis cells transformed with the expression vector pMV261 from the activity obtained from cell membranes expressing CtpG. First, the optimal quantity of protein in the enzymatic reactions was estimated. Therefore, the reaction samples were supplemented with at least 8.0 µg of membrane vesicles to produce values of ATPase activity independent of the amount of protein. The enzymatic reactions were also separately supplemented with 25 µM of each heavy metal cation, which ensured high enzymatic activity in every case (data not shown). Statistically, significant ATPase activities associated with M. tuberculosis CtpG were observed by stimulation with Cu2+ (9.42 ± 0.44 U/mg of protein × min), Co2+ (4.08 ± 0.22 U/mg of protein × min), Ni2+ (2.67 ± 0.69 U/mg of protein × min), Zn2+ (2.22 ± 0.44 U/mg of protein × min), Cd2+ (5.03 ± 1.69 U/mg of protein × min), Pb2+ (4.28 ± 0.70 U/mg of protein × min), and Mn2+ (1.83 ± 0.27 U/mg of protein × min). Only the presence of Cu+ ions did not stimulate the ATPase activity of CtpG. The obtained values of ATPase activity suggested that Cu2+, Co2+, Pb2+, and Cd2+ are the most likely substrates of CtpG; however, this transporter is able to transport other divalent heavy metal cations at a lower level across the mycobacterial cell membrane (Fig. 2).
ATPase activity of the mycobacterial cell membrane stimulated by heavy metal cations. The ATPase activity associated with M. tuberculosis CtpG was estimated as the difference between the activity stimulated by the heavy metal cations and the basal ATPase activity of cells expressing the recombinant plasmid pML01 and control cells. The ATPase activity corresponds to nmol of Pi released/mg of protein × min. The SD was calculated from triplicates of two independent experiments
CtpG is associated with Cu2+ and Cd2+ pumping across the mycobacterial plasma membrane
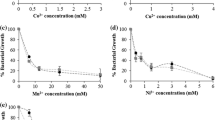
Cell viability assays were performed to evaluate the tolerance of CtpG-expressing M. smegmatis cells to toxic levels of the potentially transported cations, to gain insight of the actual heavy metal ions pumped by this cell membrane transporter. Since cultures supplemented with high doses of cations were sometimes colored, the percentage of growth was taken as the difference between the OD595 of culture growth in the presence and absence of heavy metal cations. As shown in Fig. 3, the cell viability of M. smegmatis transformed with pML01 or the control vector pMV261 did not tolerate toxic concentrations of Ni+, Zn2+, Mn2+, Co2+, and Pb2+. Conversely, the viability of M. smegmatis cells expressing CtpG was up to 4.4-fold higher under toxic levels of Cu2+ (1.5 mM) compared to the control cells. Similarly, the viability of recombinant cells was between 11 and 36-fold higher than the viability of control cells under toxic levels of Cd2+ (0.05 to 0.1 mM). Therefore, the cell viability assays strongly suggest that CtpG is associated with the tolerance of toxic concentrations of copper and/or cadmium.
Metal sensitivity of mycobacterial cells. The OD595 of M. smegmatis mc2155 expressing M. tuberculosis CtpG was compared with control cells transformed with the vector pMV261. Each data represent triplicates in three independent experiments. The dotted lines (**) show significant differences in cell growth between M. smegmatis mc2155 expressing M. tuberculosis CtpG and control cells (P < 0.05) cultured under toxic levels of Cu2+ (1.5 mM), Pb2+ (1.0 mM) and Cd2+ (0.05 to 0.1 mM)
CtpG preferentially transports Cd2+ across the mycobacterial plasma membrane
The enzymatic reactions were always supplemented with excess ATP to estimate the actual dependence of enzymatic activity on other experimental variables. Based on the results obtained in the cell viability assays, we estimated the dependence of the ATPase activity on Cu2+ and Cd2+ concentrations. We observed that the ATPase activity increased with the amount of membrane vesicles, up to a maximum of 6 µg of protein (Fig. 4). Regarding pH dependence, the optimal ATPase activity stimulated by the heavy metal cations ranged from pH 6.8 to 7.4. Additionally, the optimal temperature was always 37 °C (Fig. 4). The enzyme kinetics showed an apparent K m of 0.108 ± 0.007 µM and a V max of 0.856 ± 0.011 (nmol of Pi/mg of protein × min) for Cd2+, and K m of 0.981 ± 0.0422 µM and V max of 1.051 ± 0.155 (nmol of Pi/mg of protein × min) for Cu2+ (Fig. 5). Thus, CtpG displayed a V max/K m ratio 7.4-fold higher for Cd2+ compared with Cu2+ ions, suggesting that CtpG preferentially transports Cd2+ across the mycobacterial cell membrane.
Kinetic parameters of CtpG expressed in the M. smegmatis cell membrane. The kinetic parameters that were evaluated for CtpG-enriched membranes were the dependence in Cu2+ and Cd2+ ATPase activity of: a amount of membrane protein, b pH, and c Temperature. Bars represent the SD calculated from two independent experiments, each performed in triplicate
Discussion
The ion specificity assigned to bacterial P-type ATPases has been open to interpretation depending on the experimental approaches used to investigate it. The methods used in this work have been previously useful to ascertain the transport preferences of some mycobacterial P-type ATPases (Andreu et al. 2004; Ayala-Torres et al. 2015; León-Torres et al. 2015). The in silico hydrophobicity profile of the CtpG is similar to those of ATPases experimentally associated with Cu2+, Cd2+, Co2+, and Zn2+ efflux, and typical of P1B-type ATPases. Interestingly, the [WI (YE) (RG)] motif between positions 406 and 409 and the [LS] motif associates CtpG with Zn2+ transporters. Furthermore, most of the transport residues of CtpG are also found in the previously characterized P-type ATPase ZntA of S. sonnei (32.6% identity) suggesting that CtpG could be a Zn2+ transporting P-type ATPase. In agreement with this proposal are previous works that indicated the ctpG gene is upregulated as a consequence of Zn2+ poisoning in macrophages (Botella et al. 2011; Ward et al. 2008). The Zn2+ and Cu2+ intraphagosomal concentration increases 1 h after macrophages infection with M. tuberculosis (up to 37.8 and 426 µM respectively), but considerably diminish after 24 h of infection (Wagner et al. 2005). Therefore, tubercle bacilli need to activate detoxification systems to avoid heavy metal cation promotion of reactive oxygen species (ROS) and membrane destabilization during early infection (Ward et al. 2010). We observed that M. smegmatis cells expressing M. tuberculosis CtpG do not tolerate toxic levels of Zn2+. In contrast, the recombinant cells tolerate high doses of other divalent heavy metal cations, such as Cu2+ and Cd2+. This behavior suggests that CtpG contains the structural elements to bind different cations, which compete to be transported by this transporter. P-type ATPases recognizing different substrates are relatively common in bacteria; for example, the E. coli Zn2+ transporting P-type ATPase ZntA is able to bind other divalent cations such as Ni2+, Co2+, and Cu2+ with similar stoichiometric affinities (Liu et al. 2006). Regarding the Mycobacterium genus, the M. smegmatis Co2+ transporting P1B4-type ATPase CtpD is also activated by different divalent cations, such as Ni2+ and Zn2+ to a lesser extent (Raimunda et al. 2012).
If CtpG potentially recognizes different divalent heavy metal cations, it raises the question as to which is the preferred substrate for this P-type ATPase. Mycobacterial viability assays showed that although CtpG is associated with Cd2+ and Cu2+ pumping across the M. tuberculosis cell membrane, the Michaelis–Menten kinetics (V max/K m) indicate that Cd2+ is preferentially transported by CtpG. It is therefore not surprising that toxic levels of Cd2+ activate CtpG in vivo. On the other hand, CmtR is a DNA-binding repressor that senses Cd2+ and regulates genes involved in reducing the intracellular levels of this heavy metal ion in human alveolar macrophages infected with tubercle bacilli (Grasseschi et al. 2003). Cd2+ de-represses the regulator CmtR, allowing the transcription of the cmtR-Rv1993c-ctpG operon of M. tuberculosis (Chauhan et al. 2009) and the CtpG expression.
It is known that cadmium from pollution and cigarette smoke accumulates in the pulmonary alveoli (Grasseschi et al. 2003) and M. tuberculosis subsequently exposes to high concentrations of this heavy metal inside macrophages. Thus, M. tuberculosis must activate cadmium detoxification and efflux systems, as could be for as the P-type ATPase, CtpG. In consequence, CtpG may be relevant during the first hours of the M. tuberculosis infection (early infection) when the intrafagosomal concentration of Cd2+ and Cu2+ is elevated. In this context, the results obtained in this work could be significant because demonstrate that although Cd2+ and Cu2+ are potentially transported by CtpG, cadmium is the heavy metal more efficiently pumped outside mycobacterial cells; therefore, CtpG is a possible cadmium detoxification system belonging to the P-type ATPase family. If there was not an efficient cadmium detoxification system in mycobacterial cells, this toxic heavy metal could displace Ca2+ and Zn2+ from proteins and makes bacteria susceptible to oxidative stress (Paulsen et al. 1996; Haney et al. 2005).
On the other hand, regarding CtpG as a possible Cu2+ transporting P-type ATPase of M. tuberculosis, there are more relevant Cu2+ detoxification systems in M. tuberculosis (Rowland and Niederweis 2012) than CtpG; however, alternative copper transporters could be activated to preserve tubercle bacilli virulence. For instance, CtpV, which is associated to M. tuberculosis tolerance to toxic levels of copper ions, (Ward et al. 2010) displays the function of an alternative copper mycobacterial transporter. Therefore, it is not possible to exclude CtpG as an alternative copper transporter in M. tuberculosis.
References
Agranoff D, Krishna S (1998) Metal ion homeostasis and intracellular parasitism. Mol Microbiol 28:403–412
Andreu N, Soto CY, Roca I, Martín C, Gibert I (2004) Mycobacterium smegmatis displays the Mycobacterium tuberculosis virulence-related neutral red character when expressing the Rv0577 gene. FEMS Microbiol Lett 231(2):283–289
Argüello JM (2003) Identification of ion-selectivity determinants in heavy-metal transport P1B-type ATPases. J Membr Biol 195: 93–108
Argüello JM, Eren E, González-Guerrero M (2007) The structure and function of heavy metal transport P1B-ATPases. Biometals 20:233–248
Ayala-Torres C, Novoa-Aponte L, Soto CY (2015) Pma1 is an alkali/alkaline earth metal cation ATPase that preferentially transports Na(+) and K(+) across the Mycobacterium smegmatis plasma membrane. Microbiol Res 176:1–6
Basu J, Chattopadhyay R, Kundu M, Chakrabarti P (1992) Purification and partial characterization of a penicillin-binding protein from Mycobacterium smegmatis. J Bacteriol 174:4829–4832
Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K (2002) Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol 43:717–731
Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T (2014) SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42(Web Server issue):W252–W258
Blencowe DK, Morby AP (2003) Zn(II) metabolism in prokaryotes. FEMS Microbiol Rev 27:291–311
Blindauer CA, Harrison MD, Robinson AK, Parkinson JA, Bowness PW, Sadler PJ, Robinson NJ (2002) Multiple bacteria encode metallothioneins and SmtA-like zinc fingers. Mol Microbiol 45:1421–1432
Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charrière GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O (2011) Mycobacterial P 1-Type ATPases mediate resistance to Zinc poisoning in human macrophages. Cell Host Microbe 10:248–259
Cariani L, Thomas L, Brito J, del Castillo JR (2004) Bismuth citrate in the quantification of inorganic phosphate and its utility in the determination of membrane-bound phosphatases. Anal Biochem 324:79–83
Chauhan S, Kumar A, Singhal A, Tyagi JS, Prasad HK (2009) CmtR, a cadmium-sensing ArsR-SmtB repressor, cooperatively interacts with multiple operator sites to autorepress its transcription in Mycobacterium tuberculosis. FEBS J 276:3428–3439
Cserzo M, Wallin E, Simon I, Von Heijne G, Elofsson A (1997) Predictions of transmembrane alpha/helices in procariotic membrane proteins: the Dense Aligment Surface method. Prot Eng 10:673–676
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Futai M, Wada Y, Kaplan J (2004) Handbook of ATPases biochemistry, cell biology, pathopysiology, 1st edn. Wiley-VCH, Germany
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (ed) The proteomics protocols handbook. Humana Press, New York, pp 571–607
Grasseschi RM, Ramaswamy RB, Levine DJ, Klaassen CD, Wesselius LJ (2003) Cadmium accumulation and detoxification by alveolar macrophages of cigarette smokers. Chest 124:1924–1928
Hampshire T, Soneji S, Butcher P (2004) Stationary phase gene expression of Mycobacterium tuberculosis following a progressive nutrient depletion: A model for persistent organisms? Tuberculosis 84:228–238
Haney CJ, Grass G, Franke S, Rensing C (2005) New developments in the understanding of the cation diffusion facilitator family. J Ind Microbiol Biotechnol 32:215–226
Hofmann K, Stoffel W (1993) TMbase—a database of membrane spanning proteins segments. Biol Chem Hoppe-Seyler 374:166
Hu N, Zhao B (2007) Key genes involved in heavy-metal resistance in Pseudomonas putida CD2. FEMS Microbiol Lett 267:17–22
Käll L, Krogh A, Sonnhammer ELL (2004) A combined transmembrane topology and signal peptide prediction method. J Mol Biol 338:1027–1036
Kelley LA, Sternberg MJE (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4:363–371
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Laskowski RA, MacArthur M, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst 26:283–291
León-Torres A, Novoa-Aponte L, Soto CY (2015) CtpA, a putative Mycobacterium tuberculosis P-type ATPase, is stimulated by copper (I) in the mycobacterial plasma membrane. Biometals 28:713–724
Lew JM, Kapopoulou A, Jones LM, Cole ST (2011) TubercuList–10 years after. Tuberculosis (Edinb) 91:1–7
Lewinson O, Lee AT, Rees DC (2009) A P-type ATPase importer that discriminates between essential and toxic transition metals. Proc Natl Acad Sci USA 106:4677–4682
Liu J, Dutta SJ, Stemmler AJ, Mitra B (2006) Metal-binding affinity of the transmembrane site in ZntA: implications for metal selectivity. Biochemistry 45(3):763–772
Lomize MA, Pogosheva ID, Joo H, Mosberg HI, Lomize AL (2012) OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res 40:D370–D376
Mana-Capelli S, Mandal AK, Argüello JM (2003) Archaeoglobus fulgidus CopB is a thermophilic Cu2+-ATPase: functional role of its histidine-rich-N-terminal metal binding domain. J Biol Chem 278:40534–40541
Novoa-Aponte L, León-Torres A, Patiño-Ruiz M, Cuesta-Bernal J, Salazar LM, Landsman D, Mariño-Ramírez L, Soto CY (2012) In silico identification and characterization of the ion transport specificity for P-type ATPases in the Mycobacterium tuberculosis complex. BMC Struct Biol 12:25
Nucifora G, Chu L, Misra TK, Silver S (1989) Cadmium resistance from Staphylococcus aureus plasmid pI258 cadA gene results from a cadmium efflux ATPase. Proc Natl Acad Sci USA 86:3544–3548
Olafson RW, McCubbin WD, Kay CM (1988) Primary- and secondary-structural analysis of a unique prokaryotic metallothionein from a Synechococcus sp. cyanobacterium. Biochem J 251:691–699
Paulsen IT, Brown MH, Skurray RA (1996) Proton-dependent multidrug efflux systems. Microbiol Rev 60:575–608
Raimunda D, Long JE, Sassetti CM (2012) Role in metal homeostasis of CtpD, a Co2+ transporting P1B4-ATPase of Mycobacterium smegmatis. Mol Microbiol 84:1139–1149
Raimunda D, Long JE, Padilla-Benavides T, Sassetti CM, Arguello JM (2014) Differential roles for the Co2+/Ni2 + transporting ATPases, CtpD and CtpJ, in Mycobacterium tuberculosis virulence. Mol Microbiol 91:185–197
Rensing C, Mitra B, Rosen BP (1997) The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc Natl Acad Sci USA 94:14326–14331
Rowland J, Niederweis M (2012) Resistance mechanisms of Mycobacterium tuberculosis against phagosomal copper overload. Tuberculosis 92:202–210
Sassetti CM, Rubin EJ (2003) Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci USA 100:12989–12994
Scherer J, Nies DH (2009) CzcP is a novel efflux system contributing to transition metal resistance in Cupriavidus metallidurans CH34. Mol Microbiol 73:601–621
Snapper SB, Melton RE, Mustafa S (1990) Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol 4:1911–1919
Somerville W, Thibert L, Schwartzman K, Berh MA (2005) Extraction of Mycobacterium tuberculosis DNA: a Question of Containment. J Clin Microbiol 43:2996–2997
Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, Bansal GP, Young JF, Lee MH, Hatfull GF, Snapper SB, Barletta RG, Jacobs JR, Bloom WR BR (1991) New use of BCG for recombinant vaccines. Nature 351:456–460
Tusnády GE, Simon I (2001) The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849–850
Tusnády GE, Dosztányi Z, Simon I (2005) TMDET: Web server for detecting transmembrane regions of proteins by using their 3D coordinates. Bioinformatics 21:1276–1277
Vriend G (1990) WHAT IF: a molecular modeling and drug design program. J Mol Graph 8:52–56
Wagner D, Maser J, Moric I, Boechat N, Vogt S, Gicquel B, Lai B, Reyrat JM, Bermudez L (2005) Changes of the phagosomal elemental concentrations by Mycobacterium tuberculosis Mramp. Microbiology 151(Pt 1):323–332
Ward SK, Hoye EA, Talaat AM (2008) The global responses of Mycobacterium tuberculosis to physiological levels of copper. J Bacteriol 190:2939–2946
Ward SK, Abomoelak B, Hoye EA, Steinberg H, Talaat AM (2010) CtpV: A putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol Microbiol 77:1096–1110
Wiederstein M, Sippl MJ (2007) ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res 35(Web Server issue):W407–W410
World Health Organization (WHO) (2010) Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf. Accessed 1 May 2017
World Health Organization (WHO) (2015) Global Tuberculosis Report 2015. 20th edn, World Health Organization
Zankari E, Hasman H, Kaas RS, Seyfarth AM, Agersø Y, Lund O, Larsen MV, Aarestrup FM (2013) Genotyping using whole-genome sequencing is a realistic alternative to surveillance based on phenotypic antimicrobial susceptibility testing. J Antimicrob Chemother 68:771–777
Acknowledgements
This work was supported by the División de Investigación Bogotá (DIB)-Universidad Nacional de Colombia (Grant 27754) and Colciencias (Grant 110171250419). ML and LQ were fellows of the “Jóvenes Investigadores e Innovadores” Program, Colciencias, Colombia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
203_2017_1465_MOESM1_ESM.docx
Supplementary Fig. 1 M. tuberculosis ctpG cloning in the pMV261 mycobacterial/E. coli shuttle vector. a Schematic representation of the pML01 recombinant expression vector. b The ctpG gene was amplified from M. tuberculosis H37Rv genomic DNA using the ctpG pMV-Dir/ ctpG pMV-Rev primers. Lanes: (1) Molecular weight marker (GeneRuler 1kb DNA Ladder, Thermo Scientific, USA) and (2) ctpG amplimer (2383 pb). c Integrity and directionality of ctpG in pML01 was verified by restriction patterns. Lanes: (1) Molecular weight marker (GeneRuler 1kb DNA Ladder, Thermo Scientific, USA) (2) pML01 recombinant vector, and restriction enzyme patterns obtained with the (3) NheI, (4) BglII, (5), XhoI, (6) EcoRI and (7) EcoRI/SalI restriction enzymes (DOCX 63 KB)
Rights and permissions
About this article
Cite this article
López, M., Quitian, LV., Calderón, MN. et al. The P-type ATPase CtpG preferentially transports Cd2+ across the Mycobacterium tuberculosis plasma membrane. Arch Microbiol 200, 483–492 (2018). https://doi.org/10.1007/s00203-017-1465-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-017-1465-z