Abstract
The transport of heavy-metal ions across the plasma membrane is essential for mycobacterial intracellular survival; in this context, P-type ATPases are pivotal for maintenance of ionic gradients and the plasma membrane homeostasis of mycobacteria. To date, the copper ion transport that is mediated by P-type ATPases in mycobacteria is poorly understood. In this work, the ion-specific activation of CtpA, a putative plasma membrane Mycobacterium tuberculosis P-type ATPase, with different heavy-metal cations was assessed. Mycobacterium smegmatis mc2155 cells heterologously expressing the M. tuberculosis ctpA gene displayed an increased tolerance to toxic levels of the Cu2+ ion (4 mM) compared to control cells, suggesting that CtpA is possibly involved in the copper detoxification of mycobacterial cells. In contrast, the tolerance of M. smegmatis recombinant cells against other heavy-metal divalent cations, such as Co2+, Mn2+, Ni2+ and Zn2+, was not detected. In addition, the ATPase activity of plasma membrane vesicles that were obtained from M. smegmatis cells expressing CtpA was stimulated by Cu+ (4.9 nmol of Pi released/mg of protein.min) but not by Cu2+ ions; therefore, Cu2+ reduction to Cu+ inside mycobacterial cells is suggested. Finally, the plasma membrane vesicles of M. smegmatis that were enriched with CtpA exhibited an optimal activity at 37 °C and pH 7.9; the apparent kinetic parameters of the enzyme were a K 1/2 of 4.68 × 10−2 µM for Cu+, a V max of 10.3 U/mg of protein, and an h value of 1.91.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is one of the most important challenges in worldwide public health. According to the World Health Organization (WHO), there were 9 million new cases and 1.5 million deaths from TB in 2013 (WHO 2014). The emergence of multidrug and extensively drug-resistant (MDR and XDR, respectively) Mycobacterium tuberculosis strains and the lack of efficient drugs against latent TB infections (WHO 2014) makes the finding of novel therapeutic targets against mycobacteria a priority for the development of new anti-TB drugs. In this context, anti-TB targeting proteins of the cell membrane could be interesting because they avoid problems that are related to permeating the mycobacterial plasma membrane (Novoa-Aponte et al. 2012).
Metal-ion transport in bacteria is performed by ion channels and transporters, such as ATP-binding cassettes (ABC transporters), metal ion/H+-antiporters and P-type ATPases (Agranoff and Krishna 1998). Specifically, P-type ATPases help to maintain the ion gradients that are responsible for cell volume and nutrient transport across the cell membrane, hydrolysing ATP and releasing energy that is used for the transport of substances against electrochemical gradients (Kuhlbrandt 2004). The role of the P-type ATPases is pivotal for mycobacterial biology because these enzymes not only transport metal cations across the plasma membranes of the pathogen, but also generate electrochemical gradients that are necessary for the transport of other solutes and mycobacterial protection against heavy-metal cations in phagosomes (Chan et al. 2010; Palmgren and Nissen 2011; Soldati and Neyrolles 2012). P-type ATPases are grouped into five subfamilies that transport a wide range of cations and lipids (Bublitz et al. 2010; Thever and Saier 2009). These enzymes have three cytoplasmic domains (A, actuator; N, nucleotide binding and P, phosphorylation) and two transmembrane domains (T, transport and S, class specific support), each of them having different function through the catalytic cycle (Kuhlbrandt 2004; Palmgren and Nissen 2011). The large number of P-type ATPases that are contained in the M. tuberculosis genome suggests that these pumps could be key to face the increased level of heavy metals inside macrophages that are infected with the tubercle bacilli (Novoa-Aponte et al. 2012). In this context, there are increased Cu2+, Fe2+ and Zn2+ concentrations within the phagosomes of macrophages in response to infection with M. tuberculosis (Wagner et al. 2005), while no significant changes in the intra-phagosomal Mn2+ and Ni2+ concentrations have been detected (Wagner et al. 2005). Copper is involved in bacterial processes, such as the proliferation of reactive oxygen species (ROS) via oxidative stress, protein denaturation by the interaction of Cu+ with thiol groups, enzyme inactivation by competing against other biometals and membrane destabilisation (Ward et al. 2010). The Cu2+ concentration in macrophages that are infected with M. tuberculosis varies from 25 to 500 µM, which is toxic to the tubercle bacilli (Wagner et al. 2005); however, M. tuberculosis tolerates lower concentrations of Cu2+ (<24 µM) than do other bacteria (Hung et al. 2007; Wolschendorf et al. 2011).
Previous studies have reported that ctpA is part of a group of genes that are overexpressed during M. tuberculosis infection in vivo (Kumar et al. 2011); specifically, increased ctpA transcription in TB and extrapulmonary TB patients who are infected with MDR-TB and XDR-TB strains has been observed, suggesting that CtpA could be relevant for the TB infection progress (Kumar et al. 2011). Our previous in silico analyses have suggested that CtpA is a possible copper transporter across the mycobacterial plasma membrane (Novoa-Aponte et al. 2012). The aim of this work was to determine the possible ion-specific activation of CtpA in the mycobacterial plasma membrane. The results suggest that M. tuberculosis CtpA is associated to Cu2+ tolerance to Mycobacterium smegmatis mc2155 and that the presence of Cu+ (4.9 nmol of Pi released/mg of protein.min) stimulates ATPase activity in the plasma membrane vesicles of M. smegmatis that are enriched with the CtpA transporter.
Materials and methods
Bacterial strains and growth conditions
Mycobacteria cells were grown with agitation at 37 °C in Middlebrook 7H9 (Sigma-Aldrich, St. Louis, MO, United States) that was supplemented with 5 % w/v bovine albumin (fraction V), 2 % w/v dextrose and 0.2 % v/v glycerol until an OD600 of 0.3 for electroporation experiments and an OD600 of 0.4 for the extraction of the mycobacterial plasma membrane. E. coli BL21 (Agilent Technologies, Santa Clara, CA, United States) was used as a host for plasmid construction. In this case, the bacteria were cultured in LB broth or on LB agar plates at 37 °C; when required, the following concentrations of antibiotics or inducing supplement were used: 50 μg/mL kanamycin, 100 μg/mL ampicillin, 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and 80 μg/mL X-gal. Finally, the M. tuberculosis H37Rv genomic DNA was isolated as previously reported (Somerville et al. 2005).
Cloning and overexpression of M. tuberculosis H37Rv CtpA
The M. tuberculosis H37Rv ctpA gene was amplified using the ctpAsmDir (5′-gaggtgagtaatttggtc-3′) and ctpAsmRev (5′-cacgcggattttgactc-3′) primers and cloned in the pGEM-T easy vector (Promega Corporation, Madison, WI, United States) to obtain the pALT1 plasmid. Then, the ctpA gen was amplified from pALT1 by PCR using the ctpApMVdir (5′-ttttggatccgatgacgacggccgtgacc-3′) and ctpApMVrev (5′-ttttgaattctgatcggcgcggtcctgg-3′) primers that respectively introduced the BamHI and EcoRI restriction sites to the 5′ and 3′ ends of the gene and was cloned into the pMV261 shuttle vector (Stover et al. 1991) to generate the pALT4 recombinant plasmid. The ctpA gene sequence was obtained from pALT4 using the pMVcompUp (5′-cagcgaggacaacttgagc-3′) and pMVcompDown (5′-tatttgatgcctggcagtcg-3′) primers and compared to the data that were registered in TubercuList (http://genolist.pasteur.fr/TubercuList/) to corroborate gene integrity. Finally, pALT4 and pMV261 were separately electroporated in M. smegmatis mc2155, and the recombinant protein was expressed in incubating cells at 45 °C for 30 min (Zhang et al. 2009).
Assays of mycobacterial viability
To estimate the influence of different heavy-metal cations on the viability of M. smegmatis mc2155 cells, serial dilutions of metal cation (100 µL) were mixed with 100 µL of the bacterial suspension (OD595 of 0.1) of M. smegmatis wild type cells or separately transformed with the pALT4 or pMV261 plasmid in 96-well plates. The tested divalent metal cations concentrations were Cu2+ (4–0.125 mM), Co2+ (12–0.375 mM), Mn2+ (4–0.125 mM), Ni2+ (12–0.375 mM) and Zn2+ (4–0.125 mM). The cultures were incubated at 37 °C for 96 h, and the OD595 was considered the rate growth measure. The cells that were supplemented with no metal cations or kanamycin were used as positive and negative controls, respectively.
Mycobacterial plasma membrane isolation
Mycobacterial plasma membranes were isolated using the protocol that was reported by Basu and collaborators (Basu et al. 1992) with subtle modifications. In brief, 1.5 L of mycobacterial cell culture was grown until an OD595 of 0.4 and was centrifuged; the bacterial pellet was resuspended in MOPS buffer (10 mM MOPS, 1 mM EDTA and 0.3 mM phenylmethylsulfonyl fluoride, pH 7.4) and lysed by 8 pulses/1 min in a Mini Beadbeater-16 (Biospec, Bartlesville, OK, United States). The mycobacterial cell walls were then isolated by centrifugation at 25,000×g for 30 min at 4 °C; the supernatants containing the plasma membranes were centrifuged at 100,000×g for 90 min at 4 °C, and the resulting pellets that contained the plasma membrane fraction were resuspended in 0.1-mL aliquots of pH 7.4 buffer containing 10 mM MOPS and 0.08 g/mL sucrose and stored at −80 °C until further use. The protein concentration was determined by the Bradford-Zor-Selinger method (Bradford 1976) using fraction V of bovine serum albumin as a standard, and the plasma membrane fraction was analysed by 12 % SDS-PAGE, using ×5 SDS-PAGE loading buffer (0.25 M Tris–Cl, pH 6.8; 15 % SDS; 50 % glycerol; 2.5 % β-mercaptoethanol and 0.01 % bromophenol blue).
ATPase activity of the plasma membrane vesicles
The ATPase activity of the plasma membrane vesicles was determined by quantifying the inorganic phosphate (Pi) that was released during the P-type ATPase catalytic cycle using the Fiske-Subbarow (Fiske 1925) method and bismuth citrate according to the recommendations of Cariani (Cariani et al. 2004). Briefly, the enzymatic reactions (50 µL) were carried out in pH-7.5 incubation media (10 mM MOPS pH 7.5, 3 mM MgCl2, and 0.02 % Brij-58) using 4-μg plasma membrane vesicles that were obtained from wild type and M. smegmatis cells that were transformed with pMV261 or pALT4. The enzymatic reactions were supplemented with a 10 µM final concentration of the tested metal salts: CuCl2, CoCl2, ZnSO4, MnSO4 and NiSO4; the enzymatic reactions to test the ATPase activity dependence on Cu+ were performed using CuCl2 that was supplemented with 2.5 μM DTT and 0.3 mM cysteine to achieve reductive conditions. The samples were incubated at 37 °C for 5 min, and enzymatic reactions were initiated adding 3 mM Na2ATP; the samples were incubated at 37 °C for 30 min, and the reactions were stopped by adding 100 µL of stop solution (3 % ascorbic acid, 0.5 % ammonium molybdate, and 3 % SDS in 1 M HCl) and incubating the samples on ice for 10 min. Finally, 150 µL of 3.5 % bismuth citrate and 3.5 % sodium citrate in 2 M HCl was added, and the samples were incubated at 37 °C for an additional 10 min. The OD690 of the samples was measured using an iMark™ Microplate Absorbance Reader (Bio-Rad, Philadelphia, PA, United States) and interpolated in a calibration curve from 3 to 35 μM of NaH2PO4. The total enzymatic activity was reported as nmol of Pi released/mg of protein.min; the reactions were assessed in triplicate from three independent experiments.
Kinetic parameters
The Cu+-ATPase activity of the plasma membrane vesicles expressing M. tuberculosis CtpA was estimated by the Fiske-Subbarow method (Fiske 1925) as described above. In every case, to guarantee stimulation by Cu+, the enzymatic reactions were performed under reducing conditions using CuCl2 that was supplemented with 2.5 μM DTT. To establish the optimum quantity of the membrane protein, independent enzymatic reactions were performed using plasma membrane from 2 to 10 μg. To estimate the pH dependence of ion metal transport, enzymatic reactions were assessed using 4 μg of plasma membrane vesicles and 10 µM Cu+ (CuCl2/DTT) and varying the reaction pH from 5.9 to 8.7 using 10 mM MOPS for pH values below 7.9 and 10 mM TRIS for pH values above 8.2. To estimate the temperature dependence, the enzymatic reactions that contained 4 μg of plasma membrane were performed using incubation media at pH 7.5 and incubating samples from 4 to 60 °C. Finally, the dependence of the Cu+ concentration (CuCl2/DTT) was evaluated under the same conditions, but the ion metal concentration varied from 0.1 to 10 μM. All of the enzymatic reactions were performed for 30 min. Negative controls were performed under the same conditions, but 3 mM Na2ATP was added after the stop solution. The enzymatic activity was reported as nmol of Pi released/mg of protein.min and assessed in triplicate for three independent experiments.
Bioinformatic analyses
The amino acid sequence of M. tuberculosis H37Rv CtpA (P9WPU1) that was retrieved from the UniProt database was analysed with the Phobius (Kall et al. 2007) and TMHMM (Krogh et al. 2001) tools in order to establish the location of the transmembrane segments (M) of the CtpA protein. The ClustalW2 tool (Gonnet weight matrix with default options) (Larkin et al. 2007) was used to align the CtpA M with its counterparts in two well-characterised bacterial P-type ATPases: a Cu+-ATPase - CopA from Legionella pneumophila subsp. pneumophila (Q5ZWR1; PDB: 3rfu, 4bbj, 4bev) (Gourdon et al. 2011) and CopA from Archaeoglobus fulgidus (O29777); and a Cu2+-ATPase - CopB from Archaeoglobus fulgidus (O30085) (Mana-Capelli et al. 2003). Additionally, the tertiary structure of CtpA was constructed using a homology modelling strategy with the web server Phyre 2 (Kelley and Sternberg 2009) and 4 templates: c3rfuC (39 % identity) (Gourdon et al. 2011), c3j08A (38 % identity) (Allen et al. 2011), c4umwA (31 % identity) (Wang et al. 2014) and c3j09A (36 % identity) (Allen et al. 2011). The model was validated using the What If package (Vriend 1990). The quality of the model was evaluated by calculating the corrected Z-score with the Ramachandran plot evaluation tool (Vriend 1990). The PPM server (Lomize et al. 2011) was used to include the possible spatial arrangement of the protein model in a lipid bilayer (the polar head of the lipids are represented as dummies).
Results
M. smegmatis mc2155 cells expressing the M. tuberculosis CtpA protein exhibit increased tolerance against Cu2+ ions
Regarding the ctpA cloning in the pMV261 shuttle vector, the nucleotide sequence of the pALT4 recombinant plasmid showed two important evidences: (1) punctual mutations were not introduced into the ctpA gene by PCR; and (2) the M. tuberculosis ctpA gene was cloned in the right open reading frame. The recombinant CtpA was induced in M. smegmatis mc2155 cells at 45 °C for 30 min. Although CtpA was expressed under the control of an hsp60 promotor, detectable levels of recombinant protein were not detected by SDS-PAGE from cell lysates of mycobacterial cells; however, we were able to observe the recombinant protein by using the plasma membrane fraction of induced cells. Amounts of a protein of approximately 78.9-kDa, the molecular weight that was expected for M. tuberculosis CtpA, were detected on the cell membrane fraction of cells that were transformed with pALT4 but not in the cells containing the pMV261 vector (Fig. S1). The fact that increased amounts of the recombinant protein were more easily detected in the plasma membrane fraction of the recombinant M. smegmatis cells, suggests that CtpA was embedded in the mycobacterial cell membrane.
To evaluate the possible influence of M. tuberculosis CtpA in the tolerance of mycobacterial cells to divalent metal cations, M. smegmatis overexpressing CtpA was grown in the presence of toxic levels of different divalent metal cations that were previously demonstrated as relevant for the mycobacterial infection process (Soldati and Neyrolles 2012; Wagner et al. 2005). The cell viability of both M. smegmatis that was individually transformed with the control vector pMV261 (control cells) and that transformed with the recombinant plasmid pALT4 diminished by approximately 80 % in presence of toxic concentrations of Co2+ (2 mM), Mn2+ (50 mM), Ni2+ (4 mM), and Zn2+ (1 mM) (Fig. 1). In contrast, the viability of M. smegmatis cells overexpressing CtpA was approximately twofold higher in the presence of toxic levels of Cu2+ (from 1 to 4 mM) compared to that of the control cells; however, the cell viability diminished by approximately 20 % in the presence of Cu2+ greater than 2 mM.
Mycobacterial viability in the presence of heavy-metal cations. The OD595 of mycobacteria growing without heavy-metal cations was considered 100 % growth. This assay was performed on three independent experiments, each performed in duplicate. The presented data have statistically significant differences compared to the values that were obtained for mycobacteria growing without cations (P < 0.05)
ATPase activity is stimulated by Cu+ ions in M. smegmatis plasma membrane vesicles expressing M. tuberculosis CtpA
To estimate the possible influence of the M. tuberculosis CtpA on the ATPase activity of the mycobacterial plasma membrane, stimulation of this enzymatic activity with different heavy metal cations was assessed using plasma membrane vesicles of M. smegmatis overexpressing M. tuberculosis CtpA. ATPase activity was measured by supplementing reactions with 10 µM of the cations; a previous assay showed that in every case, this concentration produced the maximal enzymatic activity (data not shown).
The ATPase activity of the plasma membrane vesicles that were enriched with CtpA, was threefold higher in the presence of Cu+ compared to the enzymatic activity that was displayed by the vesicles that were obtained from the control cells: wild type or M. smegmatis mc2155 cells that were transformed with the pMV261 vector (Fig. 2). Additionally, the ATPase activity was twofold higher when enzymatic reactions were supplemented with Cu+/cysteine compared to the activity obtained using just Cu+. On the other hand, the ATPase stimulated by Ni2+ was a fifth of the ATPase activity obtained for enzymatic reactions supplemented with Cu+/cysteine. In contrast, the presence of other divalent cations, such as Co2+, Cu2+, Mn2+ and Zn2+, did not stimulate the ATPase activity of the plasma membrane vesicles of recombinant cells compared to that of the control cells.
Mycobacterial plasma membrane ATPase activity as stimulated by heavy-metal cations. The enzymatic activity of the plasma membrane vesicles that were obtained from wild type M. smegmatis cells and from those over-expressing the M. tuberculosis CtpA was measured. The specific ATPase activity was estimated as the difference between the ATPase activity as stimulated by the heavy-metal cations and the basal ATPase activity that was determined under the same experimental conditions. The enzymatic ATPase activity corresponds to nmol of Pi released/mg of protein.min. Bars represent the ATPase activity over the membrane vesicles that were extracted from M. smegmatis mc2155: wild type cells (Allen et al. 2011), pMV261 (grey) and pALT4 (Wang et al. 2014) transformant cells. The SD was calculated from two independent experiments, each performed in triplicate
Kinetic parameter of the enzymatic activity of CtpA embedded in M. smegmatis plasma membrane vesicles
An ATP excess was used to supplement reactions in which the influence on the ATPase activity of pH, temperature, and enzyme or substrate concentration was evaluated. The enzymatic activity that was attributed to M. tuberculosis CtpA was considered the difference between the ATPase activity of the plasma membrane vesicles of M. smegmatis overexpressing CtpA and the cells that were transformed with the control vector pMV261. As observed in Fig. 3a, the ATPase activity increased parallel to the amount of plasma membrane vesicles that were added to the enzymatic reaction up to a maximum of 10 µg of membrane protein. The ATPase activity that was stimulated by Cu+ peaked at pH 7.5 (Fig. 3b); this pH value was corrected using the temperature coefficient ΔpKa/ΔT corresponding to each buffer (MOPS −0.011 and TRIS −0.028). Additionally, the optimal temperature for the enzymatic activity was 37 °C; however, considerable activity was detected for 18–60 °C, indicating that the transporter is active across a broad temperature range (Fig. 3c). Furthermore, the dependence of the ATPase activity on the Cu+ concentration showed an apparent K 1/2 of 46.8 ± 1.68 nM of Cu+, a V max of 10.3 ± 0.16 U/mg and an h of 1.91 ± 0.15 (Fig. 3d). We finally tested the effect of cysteine concentration on CtpA Cu+ ATPase activity and it was observed that the maximal enzymatic activity was obtained supplementing samples with 0.3 mM cysteine (Fig. 3e).
Kinetic parameters of the CtpA overexpressed in the M. smegmatis plasma membrane. The kinetic parameters that were assessed for CtpA-enriched membrane vesicles were the dependence in Cu+ ATPase activity of: a amount of membrane protein, b pH, c temperature, d Cu+ concentration and e cysteine concentration. Using the results of the Cu+ concentration dependence and the Origin 8.5.1 program to adjust the plot to a Hill model (R2 = 0.998), the apparent kinetic constants were calculated as follows: V max of 10.3 nmol of Pi released/mg of protein.min; K 1/2 of 46.8 nM for Cu+; and h of 1.91
Cu+ pumping is possibly mediated by CtpA in the mycobacterial plasma membrane
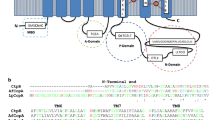
The alignment of CtpA M domain with the well-known P-type ATPases CopA from L. pneumophila and CopA and CopB from A. fulgidus (Gourdon et al. 2011; Mana-Capelli et al. 2003) showed CtpA is similar to the amino acids that are associated with Cu+ and Cu2+ transport (Fig. 4a). Most of the transport residues from CopA P-type ATPases were found in CtpA, suggesting that the enzyme substrate is Cu+; on the other hand, the amino acids from CopB that are different from CopA P-type ATPases were not found in the mycobacterial pump.
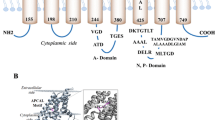
Prediction of the copper-binding sites of M. tuberculosis CtpA. a Cartoon of the membrane type I topology of CtpA. Highlighted are the residues that are involved in copper coordination in three characterised P1B ATPases; two Cu+ ATPases: CopA from L. pneumophila (CopA-Lpneu), CopA from A. fulgidus (CopA-Afulg); and a Cu2+ ATPase: CopB from A. fulgidus (CopB-Afulg). The equivalent residues were found in M. tuberculosis CtpA (CtpA-Mtb). The colours of the highlighted residues are as follows: dark blue, residues characteristic of Cu+ ATPases; green, residues described for Cu2+ ATPases; and red, residues shared by both type of pumps. b Model of the tertiary structure of CtpA (Ramachandran Z-score −0.724) was constructed using the 4 templates: c3rfuC (39 % identity) (Allen et al. 2011), c3j08A (38 % identity), c4umwA (31 % identity) and c3j09A (36 % identity). The expanded chart shows the βαββαβ ferredoxin fold in the HMBD domain located before the MA helix and near to the A domain as was determined in the templates (Gourdon et al. 2011). (Color figure online)
In general, Cu+ and Cu2+ P-type ATPases are quite similar in primary and tertiary structure, making difficult the discrimination of these type of pumps based on bioinformatics analysis; however, subtle differences indicating that Cu+ could be preferentially transported by CtpA have been identified. Recently, the amino acids of CopA (Cu+ ATPase from L. pneumophila) that are responsible for the Cu+ coordination were identified (Andersson et al. 2014). These amino acids are completely conserved in the M. tuberculosis CtpA sequence (Fig. 4a): the intracellular entry of copper at Met164 (CtpA M. tuberculosis numbering) between MB and M1, Glu224 in M2 and Asp356 in M3; internal coordination at 399-Cys-Pro-Cys-401 in M4, 701-Tyr-Asn-702 in M5, Met724 and Ser728 at M6; and extracellular exit involving Glu208 from M2. Additionally, residues that are pivotal for the opening of the release pathway of Cu+ to the exit site (Andersson et al. 2014) are fully conserved in M. tuberculosis CtpA: Pro111 and Pro717. The predicted tertiary structure of CtpA showed that the metal-binding amino acids were close together (Fig. 4b), acting as the pathway where copper coordination occurs.
Similar to other P1B-type ATPases, CtpA possesses a “heavy metal binding domain” HMBD in its N-terminus just before of the MA helix with the characteristic motif CXXC (22-Gly-Met-Ser-Cys-Ser-Ala-Cys-28 for CtpA Fig. 4a) arranged in the characteristic βαββαβ ferredoxin fold (Fig. 4b), as in other HMBDs and cytosolic copper chaperones (Gourdon et al. 2011; Zimmermann et al. 2009).
Discussion
To date, copper homeostasis in mycobacteria is not completely understood. Although, the reversible oxidation from Cu+ to Cu2+ makes copper as an essential cofactor for the activity of many enzymes (Ekici et al. 2014), this heavy metal sometimes is toxic for bacteria, including mycobacteria. In this work, the M. tuberculosis ctpA gene product, a putative P-type ATPase, was associated in the efflux of Cu+ and tolerance of mycobacterial cells to toxic levels of copper.
Copper transport across the M. tuberculosis plasma membrane has been recently correlated with the activity of P-type ATPases. For example, the deletion of M. tuberculosis CtpV, a putative P-type ATPase, reduces the tolerance of the tubercle bacilli to toxic levels of copper ions and the ability of the tubercle bacilli to grow inside lung cells during murine infection; however, CtpV deletion was not associated with defects in M. tuberculosis virulence (Ward et al. 2010). This finding suggests alternative copper transporters that may compensate for CtpV inactivation and preserve M. tuberculosis virulence. An exhaustive literature search that we previously performed (Novoa-Aponte and Soto Ospina 2014) showed that CtpA is over-transcribed when M. tuberculosis enters in a non-replicative persistent state similar to that adopted for the tubercle bacilli in tuberculous lesions (granulomes) (Muttucumaru et al. 2004). According to bioinformatics analyses that we also performed, M. tuberculosis CtpA, CtpB and CtpV display transmembrane segments that are commonly exhibited by heavy-metal cation transporters (Novoa-Aponte et al. 2012). Specifically, the M. tuberculosis ctpA gene (2286 bp) encodes a 761-amino-acid protein that displays a tertiary structure that is similar to Cu+ or Cu2+ P-type ATPases transporters and exhibits a type-I topology containing eight transmembrane segments that are similar to P1B-type ATPases (Novoa-Aponte et al. 2012). In agreement, we observed in this work that the CtpA recombinant protein is detected in the plasma membrane of M. smegmatis cells, reinforcing its transmembrane features. Additional bioinformatic analysis that was performed in this work shows that CtpA shares motifs with both Cu+ and Cu2+ ATPases; nevertheless, the presence of the conserved CPC motif in M4 and the MXXSS motif in M6 strongly suggest that CtpA is able to transport preferentially Cu+ over Cu2+ across the lipid bilayer (Meloni et al. 2014). This prediction was expected because Cu+ ATPases are the most widespread among P1B-type ATPases and have attracted attention due to malfunctions in the human ATP7A and ATP7B Cu+ ATPases causing the Menkes and Wilson diseases, respectively (Gourdon et al. 2011).
Cu+-transport-associated residues are essential for the function of the CtpA transporter, and their highly exposed position in the extracellular side of the protein points provides a possible site for the interaction of inhibitors for the control of infections that are caused by pathogens, such as M. tuberculosis (Andersson et al. 2014). This role is similar to that of cardiotonic steroids and omeprazole, which are used in medicine as inhibitors that bind the extracellular side of Na+/K+ and H+/K+ ATPases (Andersson et al. 2014; Yatime et al. 2009).
The mycobacteria viability assays indicate that M. tuberculosis CtpA induces the tolerance of M. smegmatis cells to high levels of Cu2+, suggesting that CtpA is possibly involved in the extrusion of copper from the inner mycobacterial cell, similar to that observed when the P1B-type ATPases from Pyrococcus furiosus (Q8TH11), Rhizobium radiobacter (A9CJE3, A9CJP7, and A9CIZ1), or Pseudomonas aeruginosa (Q9T3G8) (Lewinson et al. 2009) are heterologously overexpressed in E. coli cells. However, viability assays are not able to provide entire information about the ion specificity of CtpA. In this sense and considering the reported substrates for P1B-type ATPases (Axelsen and Palmgren 1998), we estimated the ATPase activity stimulated with different heavy-metal cations of M. smegmatis plasma membrane vesicles overexpressing CtpA.
The quantified ATPase activity includes the activity of phosphatase enzymes that are different from P-type ATPases that are also stimulated with the metal cations; however, the ATPase activity in the plasma membrane of the control cells (M. smegmatis wild type or transformed with the pMV261 vector) was always lower than that exhibited in the membranes that were enriched with CtpA. Thus, the obtained results, which agree with the in silico predictions, indicate that CtpA is preferentially stimulated for Cu+ in both, absence and presence of cysteine, an amino acid that is proposed to act as a chaperon that delivers Cu+ to the enzyme (Yang et al. 2007). The low CtpA stimulation by Ni2+ agrees with the lower tolerance of mycobacterial cells to toxic levels of this divalent cation compared to Cu2+; the less ATPase activity of CtpA stimulated by Ni2+ is a similar behaviour to that previously reported for M. smegmatis CtpD, which can be simultaneously activated by Co2+, Ni2+ or Zn2+ to a lesser extent (Raimunda et al. 2012). As previously suggested by Zimmermann and collaborators, metal selectivity in biology is complex, which is why different mechanisms in the cells ensure the occlusion of the correct cation at the appropriate location (Zimmermann et al. 2009). On the other hand, the activation of the ATPase activity of the plasma membrane vesicles from wild type cells by Co2+, Cu2+ and Mn2+ ions was also observed. This behaviour could be partially explained by the presence of other P1B-type ATPases, such as MSMEG2 (Probable copper P-type ATPase), CtpD (Co2+ P-type ATPase) and CtpC (Mn2+ P-type ATPase) in the plasma membrane of the environmental surrogate strain M. smegmatis mc2155 (Botella et al. 2011; Padilla-Benavides et al. 2013; Raimunda et al. 2012) and producing fluctuations in the ATPase activity.
P-type ATPases bind cytoplasmic ion substrates, producing changes in their tridimensional conformation that allow enzymes to release the bound substrates to outside the cells (Kuhlbrandt 2004; Palmgren and Nissen 2011). We previously established that most of plasma membrane vesicles that were isolated by the method that was used in this work display a right-side-out configuration (more than 70 % approximately) that exposes the ATPase binding site to Mg2+ (basal ATPase activity) and to the supplemented divalent cations (specific ATPase activity) in the enzymatic reaction (Santos et al. 2012). Thus, Cu+ is translocated inside the plasma membrane vesicles, in contrast to the natural phenomenon in whole mycobacterial cells in which metal ions are extruded from the inner cells to the external environment (Lewinson et al. 2009). The fact that whole M. smegmatis cells can tolerate toxic levels of Cu2+ but that CtpA can be activated by Cu+ means that the excessive amounts of divalent copper that enter the cells are reduced to the monovalent form due to the reducing environment of the mycobacterial cytoplasm to be then extruded to the external environment (Lewinson et al. 2009).
The kinetic parameters of CtpA when overexpressed in the M. smegmatis plasma membrane vesicles showed that the optimal temperature and V max are comparable to those of other P1B-type ATPases of mycobacteria (Raimunda et al. 2012). M. smegmatis plasma membrane vesicles were able to translocate copper across an approximate pH range between 5 and 8, in agreement with the pH that mycobacteria experience in intra-phagosomal infections (Soldati and Neyrolles 2012). In addition, the apparent K 1/2 value (46.8 nM) for Cu+ is comparable to that reported for copper pumps, such as the human ATP7A, which plays an important role in the increased intra-phagosomal copper concentration (Hung et al. 2007; Wolschendorf et al. 2011). This affinity constant value suggests that CtpA transports Cu+ under low concentration, ensuring an appropriate cation amount in the mycobacterial cytoplasm. Heavy metals are essential cofactors in cells; their active transport contributes to the fine regulation of the intracellular levels of these types of cations that cause oxidative damage when in excess in the cell. These levels are pretty low, as reflected by the high apparent affinity of P1B-type ATPases, whose dissociation constants are in the femtomolar range (Gonzalez-Guerrero et al. 2008).
Although Cu+ transport associated with M. tuberculosis CtpA can be deduced in the experimental model design in this study, further experiments using for example the recombinant transmembrane protein reconstituted in liposome models could be useful for having a deeper insight of the ion specificity of the CtpA transporter.
References
Agranoff DD, Krishna S (1998) Metal ion homeostasis and intracellular parasitism. Mol Microbiol 28:403–412
Allen GS, Wu CC, Cardozo T, Stokes DL (2011) The architecture of CopA from Archaeoglobus fulgidus studied by cryo-electron microscopy and computational docking. Structure 19:1219–1232
Andersson M et al (2014) Copper-transporting P-type ATPases use a unique ion-release pathway. Nat Struct Mol Biol 21:43–48
Axelsen KB, Palmgren MG (1998) Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol 46:84–101
Basu J, Chattopadhyay R, Kundu M, Chakrabarti P (1992) Purification and partial characterization of a penicillin-binding protein from Mycobacterium smegmatis. J Bacteriol 174:4829–4832
Botella H et al (2011) Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10:248–259
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bublitz M, Poulsen H, Morth JP, Nissen P (2010) In and out of the cation pumps: P-type ATPase structure revisited. Curr Opin Struct Biol 20:431–439
Cariani L, Thomas L, Brito J, del Castillo JR (2004) Bismuth citrate in the quantification of inorganic phosphate and its utility in the determination of membrane-bound phosphatases. Anal Biochem 324:79–83
Chan H et al (2010) The p-type ATPase superfamily. J Mol Microbiol Biotechnol 19:5–104
Ekici S, Turkarslan S, Pawlik G, Dancis A, Baliga NS, Koch HG, Daldal F (2014) Intracytoplasmic copper homeostasis controls cytochrome c oxidase production. mBio 5:e01055–e01113
Fiske SHaS Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Gonzalez-Guerrero M, Eren E, Rawat S, Stemmler TL, Arguello JM (2008) Structure of the two transmembrane Cu+ transport sites of the Cu+-ATPases. J Biol Chem 283:29753–29759
Gourdon P, Liu XY, Skjorringe T, Morth JP, Moller LB, Pedersen BP, Nissen P (2011) Crystal structure of a copper-transporting PIB-type ATPase. Nature 475:59–64
Hung YH, Layton MJ, Voskoboinik I, Mercer JF, Camakaris J (2007) Purification and membrane reconstitution of catalytically active Menkes copper-transporting P-type ATPase (MNK; ATP7A). Biochem J 401:569–579
Kall L, Krogh A, Sonnhammer EL (2007) Advantages of combined transmembrane topology and signal peptide prediction–the Phobius web server. Nucleic Acids Res 35:W429–W432
Kelley LA, Sternberg MJE (2009) Protein structure prediction on the web: a case study using the Phyre server. Nat Protoc 4:363–371
Krogh A, Larsson B, von Heijne G, Sonnhammer EL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580
Kuhlbrandt W (2004) Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol 5:282–295
Kumar M et al (2011) Identification of Mycobacterium tuberculosis genes preferentially expressed during human infection. Microb Pathog 50:31–38
Larkin MA et al (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Lewinson O, Lee AT, Rees DC (2009) A P-type ATPase importer that discriminates between essential and toxic transition metals. Proc Natl Acad Sci U S A 106:4677–4682
Lomize AL, Pogozheva ID, Mosberg HI (2011) Anisotropic solvent model of the lipid bilayer. 2. Energetics of insertion of small molecules, peptides, and proteins in membranes. J Chem Inf Model 51:930–946
Mana-Capelli S, Mandal AK, Arguello JM (2003) Archaeoglobus fulgidus CopB is a thermophilic Cu2+-ATPase: functional role of its histidine-rich-N-terminal metal binding domain. J Biol Chem 278:40534–40541
Meloni G, Zhang L, Rees DC (2014) Transmembrane type-2-like Cu2+ site in the P1B-3-type ATPase CopB: implications for metal selectivity. ACS Chem Biol 9:116–121
Muttucumaru DG, Roberts G, Hinds J, Stabler RA, Parish T (2004) Gene expression profile of Mycobacterium tuberculosis in a non-replicating state. Tuberculosis (Edinb) 84:239–246
Novoa-Aponte L, Soto Ospina CY (2014) Mycobacterium tuberculosis P-type ATPases: possible targets for drug or vaccine development. BioMed Res Int 2014:296986
Novoa-Aponte L et al (2012) In silico identification and characterization of the ion transport specificity for P-type ATPases in the Mycobacterium tuberculosis complex. BMC Struct Biol 12:25
Padilla-Benavides T, Long JE, Raimunda D, Sassetti CM, Arguello JM (2013) A novel P(1B)-type Mn2+-transporting ATPase is required for secreted protein metallation in mycobacteria. J Biol Chem 288:11334–11347
Palmgren MG, Nissen P (2011) P-type ATPases. Ann Rev Biophys 40:243–266
Raimunda D, Long JE, Sassetti CM, Arguello JM (2012) Role in metal homeostasis of CtpD, a Co(2)(+) transporting P(1B4)-ATPase of Mycobacterium smegmatis. Mol Microbiol 84:1139–1149
Santos P, Gordillo A, Osses L, Salazar LM, Soto CY (2012) Effect of antimicrobial peptides on ATPase activity and proton pumping in plasma membrane vesicles obtained from mycobacteria. Peptides 36:121–128
Soldati T, Neyrolles O (2012) Mycobacteria and the intraphagosomal environment: take it with a pinch of salt(s)! Traffic 13:1042–1052
Somerville W, Thibert L, Schwartzman K, Behr MA (2005) Extraction of Mycobacterium tuberculosis DNA: a question of containment. J Clin Microbiol 43:2996–2997
Stover CK et al (1991) New use of BCG for recombinant vaccines. Nature 351:456–460
Thever MD, Saier MH Jr (2009) Bioinformatic characterization of p-type ATPases encoded within the fully sequenced genomes of 26 eukaryotes. J Membr Biol 229:115–130
Vriend G (1990) WHAT IF: a molecular modeling and drug design program. J Mol Graph 8(52–56):29
Wagner D et al (2005) Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J Immunol 174:1491–1500
Wang K et al (2014) Structure and mechanism of Zn2+-transporting P-type ATPases. Nature 514:518–522
Ward SK, Abomoelak B, Hoye EA, Steinberg H, Talaat AM (2010) CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol Microbiol 77:1096–1110
WHO (2014) Global tuberculosis report 2014 World Health Organization:171
Wolschendorf F et al (2011) Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci USA 108:1621–1626
Yang Y, Mandal AK, Bredeston LM, Gonzalez-Flecha FL, Arguello JM (2007) Activation of Archaeoglobus fulgidus Cu(+)-ATPase CopA by cysteine. Biochim Biophys Acta 1768:495–501
Yatime L et al (2009) P-type ATPases as drug targets: tools for medicine and science. Biochim Biophys Acta 1787:207–220
Zhang L, Zhong Q, Bao L, Zhang Y, Gao L, Huang B, Zhang HD (2009) Rv0901 from Mycobacterium tuberculosis, a possible novel virulent gene proved through the recombinant Mycobacterium smegmatis. Japn J Infect Dis 62:26–31
Zimmermann M et al (2009) Metal binding affinities of Arabidopsis zinc and copper transporters: selectivities match the relative, but not the absolute, affinities of their amino-terminal domains. Biochemistry 48:11640–11654
Acknowledgments
This work was supported by the División de Investigación Bogotá (DIB)-Universidad Nacional de Colombia, Grants 15835, 16060, 18726 and 23667. AL-T and L-NA were Fellows of the “Dirección Académica”, Universidad Nacional de Colombia, and of the “Jóvenes Investigadores e Innovadores” Program, Colciencias, Colombia. AL-T and LN-A are fellow of Colciencias, Colombia.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
León-Torres, A., Novoa-Aponte, L. & Soto, CY. CtpA, a putative Mycobacterium tuberculosis P-type ATPase, is stimulated by copper (I) in the mycobacterial plasma membrane. Biometals 28, 713–724 (2015). https://doi.org/10.1007/s10534-015-9860-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-015-9860-x