Abstract
Soybean is an economically very important crop throughout the word and particularly in Argentina. Soybean yield may be affected by many factors such as the lack of some essential nutrients or pathogens attack. In this work we demonstrated that the co-inoculation of the native biocontrol bacterium Bacillus sp. CHEP5 which induces resistance against Cercospora sojina in soybean and the nitrogen fixing strain Bradyrhizobium japonicum E109, was more effective in reducing frog leaf spot severity than the inoculation of the biocontrol agent alone. Probably, this is related with the increase in the ability to form biofilm when both bacteria are growing together. Furthermore, Bacillus sp. CHEP5 inoculation did not affect Bradyrhizobium japonicum E109 symbiotic behavior and flavonoids composition of root exudates in pathogen challenged plants. These results suggest that co-inoculation of plants with rhizobia and biocontrol agents could be a strategy to improve soybean production in a sustainable system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rhizosphere is inhabited by a great amount of beneficial and pathogenic microorganisms. Some of them establish a close interaction with plants, colonizing their roots or aerial tissues and triggering a specific response. Hence, plants have developed an efficient recognition machinery to discriminate and perform an accurate and fast response towards their presence or contact. The recognition between plants and microorganisms is mediated by a molecular dialog that finally induces plant defense responses or stimulates the establishment of mutualistic relationships (Yamazaki and Hayashi 2015).
PGPB (plant-growth-promoting bacteria) stimulate plant growth by direct or indirect mechanisms. The main mechanisms for plant growth promotion include suppression of disease (biocontrol), enhancement of nutrient availability (biofertilization), and production of plant hormones (phytostimulation) (Bhattacharyya and Jha 2012). Among PGPB, microorganisms called rhizobia induce nodule formation on root of legumes where bacteria provide fixed nitrogen for the host (Podile and Kishore 2006; Lugtenberg and Kamilova 2009; Fabra et al. 2010). Other PGPB are able to induce systemic resistance (ISR) in their host, triggering plant defense state only upon pathogen attack (Kloepper et al. 1992).
Glycine max (soybean) is an economically very important crop throughout the word and particularly in Argentina. Legume crops such as soybean provide proteins and oil for human and animals feeding. However, soybean growth may be affected by many factors causing great yield losses, such as the lack of some essential nutrients or pathogens attack. The application of chemical pesticides and fertilizers is a widely used strategy to prevent such growth losses, but their cost is high and could cause severe environmental damage.
Frogeye leaf spot (FLS) caused by the necrotrophic fungi Cercospora sojina Hara can dramatically affect soybean production (Hershman 2013). FLS symptoms usually appear at V3–V4 phenological stages and lead to a premature defoliation (Fehr and Caviness 1977; Mian et al. 2008). Effective control of FLS disease can be reached by supplying fungicides or by using resistant cultivars. A major problem with genetically resistant plants is that host-differentiated pathogenic races can be selected; therefore, many breeding programs become continuous processes to develop disease resistant plant lines. Biological control of phytopathogenic fungi is an environmental friendly strategy and an alternative for integrated disease management. In previous studies, we have demonstrated that the native biocontrol bacterium Bacillus sp. CHEP5 reduced the severity of FLS in soybean by inducing systemic resistance (Tonelli and Fabra 2014).
On the other hand, it has been extensively demonstrated that Bradyrhizobium japonicum E109 is the most suitable strain for soybean inoculant formulation in Argentina. This microorganism is able to establish a symbiotic association with soybean roots that culminates in the formation of nitrogen-fixing root nodules leading to a significant increase in soybean production (Ressia et al. 2003; Cassán et al. 2009).
Many studies on the interaction between soybean and one microorganism or PGPB, especially with its rhizobial microsymbiont, have been conducted (Lodeiro et al. 2000; López-García et al. 2001; Yaryura et al. 2008). However, links between inter-organism signaling under distress conditions, especially between above- and below-ground tissues, are poorly understood. Fundamental knowledge of how plants interact with their attackers and beneficial microorganisms provides the basis of modern agriculture (Pineda et al. 2015).
Since both the induction of systemic resistance against phytopathogens such as C. sojina and an improvement in nitrogen availability are important features for plant growth, it becomes relevant to evaluate Bacillus sp. CHEP5 and B. japonicum E109 co-inoculation in soybean plants. Therefore, the aim of this research work was to evaluate the PGPB co-inoculation effect on plant growth promotion compared to single bacterium inoculation.
Materials and methods
PGPB strains, phytopathogen and culture conditions
The native biocontrol agent Bacillus sp. CHEP5 (Tonelli et al. 2010) and the soybean symbiont B. japonicum E109, recommended by the Instituto Nacional de Tecnología Agropecuaria (INTA) as the most suitable rhizobial strain for inoculant formulation in Argentina, were used in this study.
Bacillus sp. CHEP5was grown at 28 °C in Luria–Bertani broth (LB) or agarized medium and B. japonicum E109 was cultured at 28 °C in yeast extract mannitol broth (YEM) or YEM-agar (YEMA) (Vincent 1970).
The strains were kept in 20% glycerol at −80 °C for long-term storage and in 40% glycerol at −20 °C for short-term storage.
The fungal pathogen C. sojina CCC 172-09 (Carmona et al. 2009) was obtained from Centro de Referencia en Micología (CEREMIC), Universidad Nacional de Rosario, Argentina. It was grown at room temperature for 10 days on potato dextrose agar (PDA) (Kong et al. 2010) supplemented with streptomycin 100 µg ml−1. The phytopathogen was kept in 15% glycerol at −20 °C for long-term storage or in tubes containing PDA or V8 (Stevens 1974) media covered with vaseline.
Plant material and growth conditions
Soybean seeds cv. DM 4676 susceptible to C. sojina, were surface sterilized. Briefly, the seeds were soaked in 96% ethanol for 20 s followed by 20% bleach for 20 min, and then washed six times with sterile distilled water (Buensateai et al. 2009). The surface sterilized seeds were germinated at 28 °C in sterilized Petri dishes with one layer of Whatman #1 filter paper and moist cotton until the radicle reached approximately 2 cm. Seedlings were sown in plastic cups filled with sterilized vermiculite. They were watered regularly and supplied once a week with Hoagland medium (Hoagland and Arnon 1950). Plants were grown under controlled environment (light intensity of 200 mmol m−2 s−1, 16-h day/8-h night cycle, at a constant temperature of 28 °C and a relative humidity of 50%).
Bacterial inoculum preparation
Bacillus sp. CHEP5 was cultured on LB broth and B. japonicum E109 on YEM broth at 28 °C with agitation, until each culture reached an OD620nm = 1 (108–109 cfu ml−1) (24 h for Bacillus sp. CHEP5 and 5 days for B. japonicum E109). The cultures were separately centrifuged at 6000 rpm for 12 min at room temperature and the cells were suspended in 10 mM MgSO4 sterile solution. The number of viable cells was determined following the methodology described by Somasegaran and Hoben (1994).
Phytopathogen inoculum preparation
Cercospora sojina was grown on PDA supplemented with streptomycin 100 µg ml−1 at room temperature for 10 days. Fungal spores were suspended in a sterile aqueous solution containing 0.015% of the surfactant Tween 20 to a final concentration of 106 spores ml−1.
Bioassays to evaluate the effect of the co-inoculation of the PGPB on their biocontrol activity and symbiotic performance
Soybean seedlings were obtained as described above. Their roots were inoculated with 4 ml of a Bacillus sp. CHEP5 or a B. japonicum E109 cell suspension in 10 mM MgSO4 sterile solution or with a mix of both bacterial suspensions in a 1:1 ratio to obtain a final concentration of 107 cfu g−1 of vermiculite. At V3–V4 phenological growth stage, trifoliate leaves were challenged with C. sojina (106 spores ml−1) (Carmona et al. 2009). Non-pathogenized and non-bacterized control plants were also included. The plants were watered regularly and supplied once a week with Hoagland medium (Hoagland and Arnon 1950) without nitrogen addition, with the exception of plants uninoculated with BradyrhizobiumjaponicumE109 which were watered with complete Hoagland medium. Plants were grown under controlled environment (light intensity of 200 mmol m−2 s−1, 16-h day/8-h night cycle, at a constant temperature of 28 °C and a relative humidity of 50%).
Twenty-one days after pathogen challenge, disease severity was assessed by determining the disease level and the percentage of damaged leaf area (Carmona et al. 2009). Disease rating was expressed on the basis of symptom severity and damaged leaf area measured in challenged trifoliate leaves: 1 healthy leaves without spots, 2 spots covering 11–25% of leaves area approximately, 3 spots covering 26–50% of leaves area approximately, 4 spots covering 51–75% of leaves area approximately, 5 75% or more of their surfaces affected by the FLS. The disease data recorded based on this scoring scale were converted to percentage severity index (PSI) according to Wheeler (1969):
The number and dry weight of nodules, red nodules percentage (red color is a consequence of nodule leghemoglobin presence), and root and shoot plant dry weights were determined.
The experiment was repeated two times with six replicates for each treatment.
Biofilm formation assay
Bacillus sp. CHEP5 and B. japonicum E109 were separately cultured on YEM medium. They were incubated at 28 °C, until cultures reached an OD620nm = 0.1. For co-culture treatments, the inoculum was obtained by mixing these axenic cultures in a 1:1 ratio.
Bacillus sp. CHEP5 and B. japonicum E109 were cultured on YEM medium. They were incubated at 28 °C, until cultures reached an OD620nm = 0.1. For co-inoculation treatments, a mixed inoculum was prepared in a 1:1 ratio.
Biofilm formation was determined using the method described by O’Toole and Kolter (1998). Each well of a 96-well microtiter plate was filled with 150 µl of individual or mixed bacterial suspension. The negative control consisted of sterile YEM medium. The plate was incubated at 28 °C without shaking for 72 h. At this time, cell turbidity was measured using a microtiter plate reader at an optical density of 620 nm. The cultures were removed and the wells carefully washed with sterile distilled water to remove loosely associated bacteria. Then, every well was stained with 150 µl of 0.1% (w/v) Crystal Violet solution for 20 min. After staining, the plate was washed with sterile distilled water and 150 µl of 95% ethanol were added to each well, resuspending carefully the solution. Turbidity of every well content was measured using a microtiter plate reader at an optical density of 570 nm.
The average of optical densities obtained at 620 and 570 nm from the control wells was subtracted from the absorbance values obtained from all test wells.
Biofilm formation was normalized with respect to bacterial growth to obtain the biofilm formation index (BFI), which was calculated by the following equation:
The experiment was repeated three times with seven replicates for each treatment.
Collection and chemical analysis of root flavonoids from soybean plants
Growth of seedlings and preparation of root exudates
Soybean seeds were surface disinfected and germinated as described previously. Seedlings were individually transferred to 100 ml tubes containing 20 ml of sterile distilled water. Roots were passed through a tube hold and ensured that only this organ was submersed in the water. After 5 days, the seedlings were simultaneously inoculated and/or challenged with the bacteria and the pathogen respectively, as described above. Plants were grown under controlled environment (light intensity of 200 mmol m−2 s−1, 16-h day/8-h night cycle, at a constant temperature of 28 °C and a relative humidity of 50%).
Seven days after soybean seedlings inoculation and/or challenge, root exudates were centrifuged at 6200 rpm for 15 min and filtered through 0.45 and 0.2 µm filters. Then, they were concentrated to dryness by lyophilization and store at −20 °C in dark until use.
Detection of flavonoids from root exudates
The lyophilized soybean exudates were resuspended in 1 ml methanol: acetic acid 1% (38: 62) filtered through a 0.25 μm nylon filter to remove cell debris. HPLC analysis was carried out in a Waters 1525 HPLC system with a binary pump and Waters 2998 UV photodiode array detector (PDA)UV detector at 250, 280 and 325 nm by injecting 10 μl of samples onto a Waters Chrompax C18 analytical column (4.6 mm internal diameter × 150 mm length). The mobile phase consisted of methanol: acetic acid 1% (38:62) for 10 min and then methanol: acetic acid 1% (65:35) for 15 min delivered at 1 ml min−1. The column was held at 25 °C. The chromatographic run time was 30 min. Solvents used were HPLC grade and commercial standards of flavonoids narigenin (4ʹ,5,7 trihydroxyflavanone), apigenin (4ʹ,5,7 tetrahydroxyflavone), chrysin (5,7 dihydroxyflavone), luteolin (3ʹ,4ʹ,5,7 tetrahydroxyflavone), genistein (4ʹ,5,7 trihydroxyisoflavone) and daidzein (4ʹ,7 dihydroxyisoflavone) were purchased from Sigma Aldrich. Standard solutions were prepared as 1 mg ml−1 in methanol and used to detect flavonoids by co-elution.
Statistical analysis
Data were subjected to analysis of variance (ANOVA) and analyzed by LSD tests, using Infostat software (1.0, FCA, UNC, Argentina). A p < 0.05 significance level was used.
Results
Bradyrhizobium japonicum E109 inoculation enhances Bacillus sp. CHEP5 biocontrol activity
In order to determinate if B. japonicum E109 affects Bacillus sp. CHEP5 ability to protect soybean plants against C. sojina, the percentage severity index (PSI), percentage of damaged leaf area, number of spots on trifoliate leaves and shoot dry weight were determined.
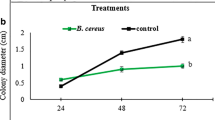
These FLS symptoms were registered at 21 days after pathogen challenge. As expected, PSI was reduced by 8% in soybean plants inoculated with the biocontrol agent Bacillus sp. CHEP5 compared to plants only challenged with the pathogen. Interestingly, identical PSI diminution was registered in plants inoculated with B. japonicum E109. Considering that in the system used in this study, B. japonicum E109 and C. sojina are physically separated, it is proposed that the interaction between soybean plants and their microsymbionts induces systemic resistance against this fungal phytopatogen. However, the major PSI reduction, 38%, was obtained in plants co-inoculated with both the microsymbiont and the biocontrol bacterium (Table 1). Likewise, the percentage of damaged leaf area and the number of foliar spots registered confirmed the beneficial effect of the co-inoculation over the single biocontrol strain inoculation (Table 1). These results suggest that the beneficial effect showed by each bacterium was synergistically increased by their co-inoculation. Moreover, shoot dry weight from C. sojina challenged soybean plants inoculated with the biocontrol bacterium or the mixture of both PGPB, was higher than in plants challenged only with the phytopathogen (Fig. 1).
Bacillus sp. CHEP5 inoculation does not affect B. japonicum E109 symbiotic performance
With the aim to evaluate if Bacillus sp. CHEP5 affects the symbiotic behavior of B. japonicum E109 in plants challenged with C. sojina, plant shoot dry weight and symbiotic parameters (number of nodules, their dry weight and the percentage of red nodules formed) were determined.
Evaluation of the symbiotic parameters indicated that Bacillus sp. CHEP5 did not affect B. japonicum E109 symbiotic performance and, therefore, no differences were observed between the shoot dry weight of plants co-inoculated and inoculated only with the microsymbiont (Fig. 2). However, it was evident that the phytopathogen C. sojina affects the plant-microsymbiont symbiosis, reducing drastically the total number and weight of nodules formed and also the number of red nodules(capable of fixing nitrogen).Interestingly, Bacillus sp. CHEP5 inoculation reverted the deleterious effects of C. sojina on the number of nodules formed, reaching this parameter in co-inoculated plants values that did not differed from those obtained in unchallenged plants inoculated only with B. japonicum E109 (Table 2). Nevertheless, the number of red nodules and the shoot dry weights of these challenged and co-inoculated plants were similar to that from challenged and B. japonicum E109 inoculated plants, indicating that the increase in the total nodule number was not enough to promote plant biomass production, at least at the time that it was evaluated.
Therefore, it seems that even when PGPB co-inoculation controlled synergistically FLS disease, it was not enough to prevent the deleterious effect of C. sojina on the plant-rhizobia symbiosis.
Biofilm formation ability by Bradyrhizobium japonicum E109 and Bacillus sp. CHEP5 mixed cultures is improved compared with pure cultures
Soil microorganisms multiply in response to plant environment and often form multicellular complexes that range from small aggregates to expansive highly structured biofilms. Biofilms are defined as the well-organized cooperating communities of surface-associated microorganisms enclosed an extracellular matrix produced by themselves (Rafique et al. 2015). In this research work, the BFI from mixed bacterial suspension with was compared with those from each bacterial suspension.
Bacillus sp. CHEP5 seems to be a stronger biofilm forming strain than B. japonicum E109, since it reached the highest BFI (Table 3). Interestingly, the biofilm forming ability of mixed culture was higher than that showed by the microsymbiont or the biocontrol bacteria growing in pure culture. It is known that the microbial diversity harbored within biofilms influence the plant interaction to varying degrees, dependent on plant type, growth stage and environmental conditions. Considering the symbiotic performance of plants co-inoculated (Table 2), the higher biofilm forming ability of the co-cultures determined in in vitro assay seems not to be associated with an improved behavior of B. japonicum E109. However, it is possible to speculate that the enhanced biocontrol efficacy against C. sojina showed by co-inoculated plants may be correlated with more successful bacterial root colonization.
Root flavonoid profiles of soybean plants co-inoculated with Bradyrhizobium japonicum E109 and Bacillus sp. CHEP5, and challenged with C. sojina
Plants are forced to develop efficient recognition machinery to discriminate beneficial microbes from pathogens. Chemical components of root exudates have been shown to play a role in the plant defense response against microbes or in the attraction of beneficial ones (Walker et al. 2003).Therefore, we attempted to evaluate whether the root flavonoid profile of B. japonicum E109 inoculated plants differs in presence or absence of C. sojina and Bacillus sp CHEP5.
A single chromatographic condition, showing high signal-to-noise ratio, was optimized in order to detect the presence of flavonoids. HPLC analysis showed that chrysin is the major flavonoid in exudates from both plants inoculated only with the microsymbiont or co-inoculated and pathogen challenged. Interestingly, in the exudates of plants challenged only with the phytopathogen the main component was luteolin while chrysin was not detected (Fig. 3).
Discussion
Until recently, ecological and mechanistic studies have mostly focused on exploring plant–microbe interactions using simplified systems involving a single microorganism, which is far from the naturally occurring interactions (Bhattacharyya and Jha 2012). In this study we explore how the interaction among soybean roots and two below ground different species from beneficial bacteria affects their performance as nitrogen fixers and systemic resistance inducers against above ground pathogens. Many species of rhizobia were found to promote plant growth and also to inhibit the growth of various soil-borne pathogens (Deshwal et al. 2003). However, to our knowledge, this is the first report showing that the interaction between a rhizobial bacterium and its plant host reduces the foliar symptoms of a fungal disease and also show a mutualistic behavior with a biological control agent.
Plant–microbe interactions utilize similar chemical signatures to mediate processes leading to symbiotic or pathogenic relationships (Liang et al. 2013; Evangelisti et al. 2014; Miyata et al. 2014; Wang et al. 2014; Zhang et al. 2015; Lagunas et al. 2015). It has been hypothesized that development of mutualistic interactions among microbes and plants requires prevention of host defense programs for microbe survival (Pel and Pieterse 2013). In fact, many reports indicate that at early steps of the symbiotic interaction between legumes and rhizobia, a decrease in the level of reactive oxygen species is required. Considering results from this work, it is possible to speculate that the enhanced defense response in C. sojina challenged plants is disturbing the symbiotic interaction with B. japonicum E109.
Microbes form biofilms in response to many factors, such as cellular recognition of specific or non-specific attachment sites on surface, nutritional cues and some cases, by exposure to sub-inhibitory concentrations of antibiotics (Karatan and Watnick 2009). Development of biofilms by rhizobia is crucial to overcoming environmental stresses and, in certain species, is an important feature of symbiotic ability. Gram-positive microbes such as the biocontrol agent Bacillus subtilis also develops biofilm, and its biocontrol capacity is related to the ability to form this aggregate on plant surface (Pal Bais et al. 2004). Thus, effective colonization of plant roots by PGPB plays an important role in growth promotion, irrespective of the mechanism of action. In this sense, it is interesting that the biofilm forming ability of Bacillus sp CHEP5 and B. japonicum E109 mixed culture was higher than that showed by each bacterium growing in pure culture. BurmØlle et al. (2006) also reported changes in the biofilm biomass formed by several strains compared to single strains.
On the other hand, the knowledge of mechanisms allowing plants to discriminate the innumerable signals they receive from root and shoot microbes is not deep enough (Bais et al. 2006). Numerous molecules (phenylpropanoids, organic acids, amino acids, etc.) have been involved in many plant–microbe interactions. Phenylpropanoids, such as flavonoids, are ubiquitous plant phenolics that are exuded by the plant roots (Baetz and Martinoia 2014). They are well known since are involved in the molecular dialogue between rhizobia and their legume host. However, they also play a role in the establishment of arbuscular mycorrhizal symbiosis,in plant defense response and in allelopathic interactions (Bais et al. 2006, Hassan and Mathesius 2012). The presence of microorganisms in the rhizosphere undoubtedly influences the quality and quantity of root exuded flavonoids (Cooper 2004). This may either be through modification of root exudation patterns, or, via microbial catabolism of exuded flavonoids.
It has been hypothesize that microbial alteration and attenuation of flavonoid signals may have ecological consequences for plant–microbe interactions. In soybean and Medicago truncatula, it has been informed a fast up-regulation of genes related to isoflavones and isoflavanones synthesis in presence of the bacterial pathogen Pseudomonas syringae pv. Glycinea (Samac and Graham 2007). Up-regulation of genes involved in the phenylpropanoid pathway was also determined in a soybean line resistant to Fusarium solani, suggesting that the products of this pathway participate in the resistance to the phytopathogen (Iqbal et al. 2005). Similarly, genes involved in the isoflavone and isoflavonoid synthesis was reported to be up-regulated in the response of M. truncatula to the biotrophic pathogen Erysiphe pisi (Foster-Hartnett et al. 2007).
Antioxidant functions in plant tissues exposed to different abiotic and biotic stresses have been attributed to flavonoids (William et al. 2004).
Webster et al. (1998) speculated about the possibility that flavonoids could induce the expression of bacterial genes involved in root colonization. Considering the findings from this work, we hypothesize that luteolin may acts as a chemical attractant of biocontrol agents. Further studies are needed to prove this hypothesis. Increase in our knowledge of flavonoids role as signal molecules in plant-beneficial microbes others than rhizobia and as defense against pathogens is vital for better rhizosphere fertility and pest control.
In summary, there is strong evidence that shifts in microbial community structure and the resulting microbial equilibrium influences plant growth and health (Barea et al. 2005; Bais et al. 2006).
In this research work, we demonstrated that the co-inoculation of the biocontrol agent Bacillus sp. CHEP5 and the microsymbiont B. japonicum E109 was more effective in reducing FLS severity than the inoculation of the biocontrol agent alone. Even when the mechanisms behind this effect are not understood, it is possible that the enhanced nutritional status of plants inoculated with B. japonicum E109 would help the pathogen biocontrol. On the other hand, the PGPB co-inoculation, apparently did not disturb the B. japonicum E109 symbiotic behavior in pathogen challenged and unchallenged plants.
This study helps to define that co-inoculation of soybean plants with rhizobia and biocontrol agents is an efficient strategy to control FLS disease in a sustainable crops production system. This hypothesis must however be tested under field conditions.
References
Baetz U, Martinoia E (2014) Root exudates: the hidden part of plant defense. Trends Plant Sci 19:90–98
Bais HP, Fall R, Vivanco JM (2004) Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin Production. Plant Physiol 134:307–319
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266
Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C (2005) Microbial co-operation in the rhizosphere. J Exp Bot 56:1761–1778
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327–1350
Buensateai N, Yuen G, Prathuangwong S (2009) Priming, signaling, and protein production associated with induced resistance by Bacillus amyloliquefaciens KPS46. World J Microbiol Biotechnol 25:1275–1286
BurmØlle M, Webb JS, Rao D, Hansen LH, SØrensen SJ, Kielleberg S (2006) Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl Environ Microbiol 72:3916–3923
Carmona MA, Scandiani M, Luque A (2009) Severe outbreaks of soybean frogeye leaf spot caused by Cercospora sojinain the pampean region, Argentina. Plant Dis 93:966
Cassán F, Perrig D, Sgroy V, Masciarelli O, Penna C, Luna V (2009) Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea mays L.) and soybean (Glycine max L.). Eur J Soil Biol 45:28–35
Cooper JE (2004) Multiple responses of rhizobia to flavonoids during legume root infection. Inc Adv Plant Pathol 41:1–62
Deshwal VK, Pandey P, Kang SC, Maheshwari DK (2003) Rhizobia as a biological control agent against soil borne plant pathogenic fungi. Indian J Exp Biol 41:1160–1164
Evangelisti E, Rey T, Schornack S (2014) Cross-interference of plant development and plant–microbe interactions. Curr Opin Plant Biol 20:118–126
Fabra A, Castro S, Taurian T, Angelini J, Ibañez F, Dardanelli M, Tonelli ML, Bianucci E, Valetti L (2010) Interaction among Arachis hypogaea L. (peanut) and beneficial soil microorganisms: how much is it known? Crit Rev Microbiol 36:179–194
Fehr WR, Caviness CE (1977) Stages of soybean development. Iowa St Univ Spec Rep 80:11p
Foster-Hartnett D, Danesh D, Peñuela S, Sharopova N, Endre G, VandenBosch KA, Young ND, Samac DA (2007) Molecular and cytological responses of Medicago truncatula to Erysiphepisi. Mol Plant Pathol 8:307–319
Hassan S, Mathesius U (2012) The role of flavonoids in root–rhizosphere signalling: opportunities and challenges for improving plant–microbe interactions. J Exp Bot 63:3429–3444
Hershman DE (2013) Soybean foliar spots and blights. Plant pathology fact sheet. Cooperative extension service. University of Kentucky, College of Agriculture, USA
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:1–39
Iqbal MJ, Yaegashi S, Ahsan R, Shopinski K, Lightfoot DA (2005) Root response to F. solani f. sp. glycines, temporal accumulation of transcripts in partially resistant and susceptible soybean. Theor App Genet 110:1429–1438
Kataran E, Watnick P (2009) Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol R73:310–347
Kloepper J, Tuzun S, Kúc J (1992) Proposed definitions related to induced disease resistance. Biocontrol Sci Technol 2:349–351
Kong Q, Shan S, Liu Q, Wang X, Yu F (2010) Biocontrol of Aspergillus flavus on peanut kernels by use of a strain of marine Bacillus megaterium. Int J Food Microbiol 139:31–35
Lagunas B, Schäfer P, Gifford ML (2015) Housing helpful invaders: the evolutionary and molecular architecture underlying plant root-mutualist microbe interactions. J Exp Bot 66:2177–2186
Liang Y, Cao Y, Tanaka K, Thibivilliers S, Wan J, Choi J, Ho Kang C, Qiu J, Stacey G (2013) Nonlegumes respond to rhizobial Nod factors by suppressing the innate immune response. Science 341:1384–1387
Lodeiro AR, López-García SL, Vázquez TEE, Favelukes G (2000) Stimulation of adhesiveness, infectivity, and competitiveness for nodulation of Bradyrhizobium japonicum by its pretreatment with soybean seed lectin. FEMS Microbiol Lett 188:177–184
López-García S, Vázquez TEE, Favelukes G, Lodeiro A (2001) Improved soybean root association of N-starved Bradyrhizobium japonicum. J Bacteriol 183:7241–7252
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting Rhizobacteria. Annu Rev Microbiol 63:541–556
Mian MAR, Missaoui AM, Walker DR, Phillips DV, Boerma HR (2008) Frogeye leaf spot of soybean: a review and proposed race designations for Isolates of Cercospora sojina Hara. Crop Sci 48:14–24
Miyata K, Kozaki T, Kouzai Y, Ozawa K, Ishii K, Asamizu E, Yoshihiro O, Yosuke U, Ayano M, Yoshihiro K, Kohki A, Hanae K, Yoko N, Naoto S, Tomomi N (2014) The bifunctional plant receptor, OsCERK1, regulates both chitin-triggered immunity and arbuscular mycorrhizal symbiosis in rice. Plant Cell Physiol 55:1864–1872
O’Toole GA, Kolter R (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30:295–304
Pel MJC, Pieterse CMJ (2013) Microbial recognition and evasion of host immunity. J Exp Bot. doi:10.1093/jxb/err313
Pineda A, Soler R, Pozo MJ, Rasmann S, Turlings TCJ (2015) Editorial: above-belowground interactions involving plants, microbes and insects. Front Plant Sci 6:1–3
Podile AR, Kishore K (2006) Plant growth-promoting rhizabacteria. In: Gnanamanickam SS (ed) Plant-associated bacteria. Springer, New York, pp 195–230
Rafique M, Hayat K, Mukhtar T, Khan AA, Afridi MS, Hussain T, Sultan T, Munis MFH, Imran M, Chaudhary HJ (2015) Bacterial biofilm formation and its role against agricultural pathogens. In: Méndez-Vilas A (ed.) The battle against microbial pathogens: basic science, Technological Advances and Educational Programs, pp 373–382
Ressia JL, Lázaro L, Lett G, Mendivil G, Portela R, Balbuena RH (2003) Sistemas de labranza e inoculación en soja. Ef Sobre Crecim Rend Cultivo Agrocienc 37:167–176
Samac DA, Graham MA (2007) Recent advances in legume-microbe interactions: recognition, defense response, and symbiosis from a genomic perspective. Microb Interact Plant Defin 144:582–587
Somasegaran P, Hoben H (1994) Quantifying the growth of rhizobia. Hand book for rhizobia: methods in legume rhizobiatechnology. Springer-Verlag Inc, New York, pp 382–390 (Section 3)
Stevens RB (1974) Mycology guidebook. University of Washington, Seattle, p 703
Tonelli ML, Fabra A (2014) The biocontrol agent Bacillus sp. CHEP5 primes the defense response against Cercospora sojina. World J Microbiol Biot 30:2503–2509
Tonelli ML, Taurian T, Ibáñez F, Angelini J, Fabra A (2010) Selection and in vitro characterization of biocontrol agents to protect peanut plants against fungal pathogens. J, Plant Pathol 92:73–82
Vincent JM (1970) A manual for the practical study of root nodule bacteria. International biological programme handbook no. 15. Blackwell Scientific Publications Ltd, Oxford, pp 73–97
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003) Root exudation and rhizosphere biology. Plant Physiol 132:44–51
Wang W, Xie Z-P, Staehelin C (2014) Functional analysis of chimeric lysin motif domain receptors mediating Nod factor-induced defense signaling in Arabidopsis thaliana and chitin-induced nodulation signaling in Lotus japonicus. Plant J78:56–69
Webster G, Jain V, Davey MR, GsugC VasseJ, Dénarié J, Cocking EC (1998) The flavonoid naringenin stimulates he intercellular colonization of wheat roots by Azorhizobium caulinodans. Plant Cell Environ 21:373–383
Wheeler JB (1969) An introduction to plant diseases. Wiley, London, p 347
Williams RJ, Spencer JP, Rice-Evans C (2004) Flavonoids: antioxidants or signalling molecules? Free Radic Bio Med 36:838–849
Yamazaki A, Hayashi M (2015) Building the interaction interfaces: host responses upon infection with microorganisms. Curr Opin Plant Biol 23:132–139
Yaryura PM, León M, Correa OS, Kerber NL, Pucheu NL, García AF (2008) Assessment of the role of chemotaxis and biofilm formation as requirements for colonization of roots and seeds of soybean plants by Bacillus amyloliquefaciens BNM339. Curr Microbiol 56:625–632
Zhang X, Dong W, Sun J, Feng F, Deng Y, He Z et al (2015) The receptor kinase CERK1 has dual functions in symbiosis and immunity signalling. Plant J81:258–267
Acknowledgements
This study was financially supported by the SECyT-UNRC, CONICET, Ministerio de Ciencia y Tecnología de Cordoba, ANPCyT. M. L. Tonelli, C. Magallanes-Noguera and A. Fabra are members of the Research Career from CONICET.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jorge Membrillo-Hernández.
Rights and permissions
About this article
Cite this article
Tonelli, M.L., Magallanes-Noguera, C. & Fabra, A. Symbiotic performance and induction of systemic resistance against Cercospora sojina in soybean plants co-inoculated with Bacillus sp. CHEP5 and Bradyrhizobium japonicum E109. Arch Microbiol 199, 1283–1291 (2017). https://doi.org/10.1007/s00203-017-1401-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-017-1401-2