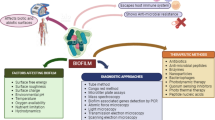
Abstract
Biofilms contain group(s) of microorganisms that are found to be associated with the biotic and abiotic surfaces. Biofilms contain either homogenous or heterogeneous populations of bacteria which remain in the matrix made up of extracellular polymeric substances secreted by constituent population of the biofilm. Biofilms can be either single or multilayered. Biofilms are an increasing issue of concern that is gaining importance with each passing day. Due to the ubiquitous nature of biofilms, it is difficult to eradicate them. It has been seen that many infectious diseases harbour biofilms of bacterial pathogens as the reservoir of persisting infections which can prove fatal at times. The presence of biofilms can be seen in diseases like endocarditis, cystic fibrosis, periodontitis, rhinosinusitis and osteomyelitis. The presence of biofilms has been mostly seen in medical implants and urinary catheters. Various signalling events including two-component signalling, extra cytoplasmic function and quorum sensing are involved in the formation of biofilms. The presence of an extracellular polymeric matrix in biofilms makes it difficult for the antimicrobials to act on them and make the bacteria tolerant to antibiotics and other drugs. The aim of this review was to discuss about the basic formation of a biofilm, various signalling cascades involved in biofilm formation, possible mechanisms of drug resistance in biofilms and recent therapeutic approaches involved in successful eradication of biofilms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biofilms can be defined as an organized group of microorganisms living within a self-produced matrix of polymeric substances which gets attached to several surfaces (Hurlow et al. 2015). These microbial collectives are found to be ubiquitous in almost every environment (Parsek and Singh 2003). Biofilms can be found in both biotic and abiotic surfaces (Cortes et al. 2011). Biofilms have been seen to be present on liquid surfaces as a floating mat and in submerged state also (Vasudevan 2014). Biofilms contain either homogenous or heterogeneous communities of bacteria, embedded on a matrix of extracellular polymeric substances (EPS). EPS mainly consist of polysaccharides, but other biomolecules like proteins, lipids and nucleic acids are also present in EPS (Cortes et al. 2011). Polymers like glycopeptides, lipids and lipopolysaccharides form a scaffold and hold the biofilm together (Flemming and Wingender 2010). Analysis of the EPS coat present in the biofilm has lead to the discovery that biofilms are technically hydrogels which exhibit viscoelastic behaviour (Stoodley et al. 2002a; Hall-Stoodley et al. 2004). Such properties allow the biofilms to withstand mechanical stress. The nutrients that are present in the matrix of EPS are trapped for the use of bacteria. The water present in the matrix is also efficiently trapped by hydrogen bonding with the hydrophilic polysaccharides in EPS (Kostakioti et al. 2013). There is a continuing debate about determining factors that contribute to the formation of biofilms. Existing literature showed that both genetic and environmental factors contribute towards the microbial biofilm formation (Maric and Vranes 2007). Bacteria can adapt to different environmental conditions by modulating their biofilm structure (Maric and Vranes 2007).
Microbial communities of the biofilms usually take part in the production and degradation of organic matter, the remediation of many environmental recalcitrant pollutants, the cycling of nitrogen, sulphur and many metals. Existing literature revealed that microbial biofilms are involved in the purification of sewage (Davey and O’toole 2000). It has been reported that in the treatment of groundwater contaminated with petroleum (Massol-Deya et al. 1995), and in the process of nitrification (De Boer et al. 1991), microbial biofilms play a major role. Microbial biofilm in rhizospheric soil has also been found to increase the soil fertility and plant growth (Qurashi and Sabri 2012). In extreme acidic environment, such as in acid mine drainage (at a pH of 1), microbial biofilm plays an important role in the cycling of sulphur (Edwards et al. 2000). It was reported that microbial biofilm on polymer enhances the degradation of polymer efficiently (Balasubramanian et al. 2010; Tribedi et al. 2015). Tribedi et al. (2015) showed that microorganisms in biofilm on polymer surface achieved higher fitness in terms of reproductive competency. Tribedi et al. (2015) also explained that microorganisms in biofilm exhibited higher metabolic functional diversity and metabolic cooperation than microorganisms in planktonic form. Thus, the enhanced metabolic functional diversity and phenotypic plasticity in biofilm residing microorganisms facilitate improved degradation of the polymer. Marine snow, a special type of macromolecular structure, containing bacterial biofilm associated with suspended particles of organic and inorganic material that is frequently observed in marine ecosystem, was found to transform particulate organic carbon (Paerl and Pinckney 1996) in marine environment. Microbial biofilm has long been used for the remediation of heavy metals (Pal and Paul 2008). Being poly anionic in nature, EPS forms complexes with positively charged metal cations resulting in metal immobilization within the exopolymeric network (Pal and Paul 2008). Moreover, extracellular enzymatic activities in EPS assist detoxification of heavy metals by transformation and subsequent precipitation in the exopolymeric mass (Pal and Paul 2008).
Apart from these beneficial effects, biofilms have also severe harmful pathogenic manifestations. Biofilm may also exist in a variety of microbial infections as well as on the surface of the medical implants including catheters (Maric and Vranes 2007; Vasudevan 2014). Some populations of biofilm-associated bacteria exhibit antibiotic resistance (Vasudevan 2014), reduced growth rate, secretion of different surface molecules and virulence factors (Hall-Stoodley and Stoodley 2009). Biofilm also facilitates gene transfer among bacteria which can lead to increase in the number of virulent strains (Lewis 2001). Moreover, these sessile cells can evade the host immune response as well as can remain unaffected by antibiotics (Crossley et al. 2009). This biofilm population almost contributes to around 80 % of the total microbial infection (Cortes et al. 2011). As mentioned before, biofilm matrix is made up of EPS, which retards the diffusion of antibiotics through the biofilm (Crossley et al. 2009), and thus biofilm increases drug resistance among microbial population noticeably. Another possible mechanism of drug resistance of microbial biofilm can be mediated by the differential gene expression of biofilm cells compared to its planktonic form. Besides this drug resistance, biofilms also exhibit resistance against bacteriophages, chemical biocides, amoebae, etc., (Costerton et al. 1999). Moreover, host immune responses exhibit futile responses against microbial biofilms as these biofilm cells regularly change the surface antigens through rapid alteration in gene expression. However, host immune responses against biofilm prove harmful for body tissues as the secretion of inflammatory cytokines at the site of biofilm formation is unable to destroy the biofilm structure but rather can cause wounds at the surrounding tissues (Wilson 2001). Thus, biofilm environment protects bacteria from external assaults, predator attack and chemical treatment like antibiotics. In this current review, we have tried to put light upon the biofilms, its structure and formation, pathogenesis and therapeutic approaches against biofilm.
Steps of microbial biofilm formation
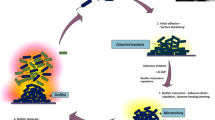
Biofilm represents the complex association of bacteria that are closely clustered within a matrix. Biofilm growth is guided by a series of physical, chemical and biological processes. Microbial biofilm develops through five consecutive stages such as initial reversible attachment, irreversible attachment, maturation stage I, maturation stage II and dispersion (Sauer et al. 2002; Stoodley et al. 2002b).
In the first stage, planktonic microbial cells adhere to the surface either by physical forces or by bacterial appendages such as Pilli or flagella (Maric and Vranes 2007). Different factors like surface functionality, temperature and pressure can modulate the bacterial adhesion greatly. Attachment of a microbial cell to a surface is known as adhesion, whereas the attachment among microbial cells is termed as cohesion. Physical forces related to bacterial adhesion to surfaces include the van der Waals forces, steric interactions and electrostatic (double layer) interactions (Garrett et al. 2008).
In the second stage, some of the reversibly attached cells remain immobilized and become irreversibly adhered when the attractive forces are greater than repulsive forces (Garrett et al. 2008). It has been reported that the physical appendages of bacteria like flagella, fimbriae and pili overcome the physical repulsive forces of the electrical double layer of the cell and the surface and consolidate the interactions between bacteria and the surface (Kumar and Anand 1998). Cell surface hydrophobicity also plays a crucial role in biofilm formation when the bacteria adhere to a hydrophobic nonpolar surface because the hydrophobic interaction between the surface and the bacteria reduces the repulsive force between them (Tribedi and Sil 2014). Therefore, in brief, in the first and second stages of biofilm development, microbial cells initially loosely associate with the concerned surface, which is then succeeded by specific and strong adhesion (Hall-Stoodley et al. 2004).
The third phase in biofilm formation is the maturation I. In this phase, microbial cells start communicating among each other by the production of autoinducer signals (Davies et al. 1998; Vasudevan 2014) that resulted in the expression of biofilm-specific genes. In this stage, microorganism secretes a matrix of extracellular polysaccharide substances (EPS) to stabilize the biofilm network. In this context, it was reported that P. aeruginosa makes and releases three polysaccharides, namely alginate, Pel and Psl which provide the stability to the biofilm. Alginate interacts with nutrients and water and supplies nutrients to the biofilm (Rasamiravaka et al. 2015). Pel (glucose rich polysaccharide) and Psl (pentasaccharide) act as a scaffold for the structure of the biofilm (Colvin et al. 2011; Franklin et al. 2011). In addition to EPS, it has been reported that e DNA is also responsible for cellular communication and stabilization of P. aeruginosa biofilm (Gloag et al. 2013). Young Pseudomonas biofilms are more susceptible to DNase treatment compared to mature biofilm, suggesting the stabilizing role for e DNA during the initial biofilm stages when EPS components are less (Whitchurch et al. 2002). In this stage, the biofilm becomes multi-layered and their thickness is increased up to 10 µm.
In the next stage, the size of the microcolony increases and its thickness reaches to about 100 µm. Microcolonies in biofilm quiet often consist of diverse microbial communities. These multispecies micro-consortia function in a relatively complex and coordinated manner. Their close proximity enhances substrate exchange, distribution of metabolic products and removal of toxic end products (Davey and O’toole 2000). For example, degradation of complex organic matter into methane and carbon di oxide during anaerobic digestion requires the involvement of at least three types of bacteria. Fermentative bacteria initiate the catabolism of complex organic compounds and produces acids and alcohols from the organic compounds. These substrates are then consumed by acetogenic bacteria as their substrates. Methanogen gains energy by converting acetate, carbon di oxide and hydrogen to methane. Biofilm offers a perfect environment for the establishment of syntrophic association (Davey and O’toole 2000). Syntrophism is a type of symbiosis in which two metabolically distinct bacteria depend on each other in order to utilize certain substrates for their energy source (Davey and O’toole 2000). A schematic diagram of syntrophism is explained in Fig. 1. Thus, the third and fourth stages include the aggregation of the cells, forming microcolonies followed by the growth and maturation of the adhered cells. In this maturation stage, biofilm becomes adopted with the external condition by manipulating its structure, physiology and metabolism.
Microbial interactions in biofilm. Sugar fermenters utilize different sugars producing organic acids, which in turn can be utilized by another set of microbes capable of using acids and producing hydrogen. The hydrogen producers attract the hydrogen-utilizing bacteria. This entire event is based on intercellular and intracellular interactions giving rise to a complex biofilm which consists of different types of microbes staying together
The fifth stage is the dispersion which marks the shedding of the biofilm and return of sessile cells to the motile form (Hall-Stoodley et al. 2004). Finally, in this stage, biofilm spreads and colonizes to the new surfaces. In this phase, the microbial community inside the biofilm produces different saccharolytic enzymes which break the biofilm stabilizing polysaccharides and thereby releases surface bacteria residing on the top of biofilm structure for colonization to a new surface. For example, Pseudomonas fluorescens and Pseudomonas aeruginosa release alginate lyase, Escherichia coli releases N-acetyl-heparosan lyase and Streptococcus equi produce hyaluronidase enzymes for the breakdown of the biofilm matrix (Sutherland 1999). At this stage, microorganisms upregulate the expression of the flagella proteins so that the organisms become motile and bacteria can translocate to a new site. Disruptive forces are also important in biofilm cycle as detachment of cells from the biofilm helps in spreading the infection from the biofilms to other sites (Otto 2013). A schematic diagram of microbial biofilm development is shown in Fig. 2.
Formation of a biofilm. Planktonic cells attach first reversibly to the surface and then become associated irreversibly which leads to the formation of a colony of bacterial cells on the surface. With the aid of quorum sensing and other signalling events, maturation and stabilization of biofilms occur. Thereafter, microbes inside the biofilm disperse by the release of surface bacteria residing on the top of biofilm structure for colonization to a new surface
Types of biofilm
Biofilms can be of monolayer or multilayer depending on the interaction between the surface and constituent cells (Karatan and Watnick 2009).
For a single-layered biofilm, interactions between cell and surface are more prominent rather than interaction between constituent cells (Karatan and Watnick 2009). Different classes of adhesive structures have been reported in the formation of the monolayer microbial biofilm (Karatan and Watnick 2009). In one type, the preformed adhesion structures, flagellum or pilus increase the transient attachments with the surface and thus accelerate the formation of the monolayer biofilm. In another type, the microbial adhesin is synthesized with the simultaneous transition to permanent attachment (Karatan and Watnick 2009).
Microorganisms often develop multilayer biofilm when they are able to adhere to a surface and also to each other. In many cases, it has been noted that the surface characteristics of bacteria lead to repulsion (Karatan and Watnick 2009). For instance, the chemical properties of the cell wall of gram-negative bacteria are generally determined by the O antigen, which is generally negatively charged in nature. For the formation of multilayer biofilm, this repulsive force due to similar charge among microorganisms should be neutralized. This negative charge may be masked by the mutation or downregulation or silencing of the O antigen synthesizing genes, addition of divalent cations, synthesis of extracellular polymeric substances (EPS), etc., (Feldman et al. 2005).
Signalling events in biofilm formation
Formation of a biofilm is dependent on the interaction between the environmental stimuli and the reciprocation of the corresponding signalling events by the microorganisms. There are many sensing systems that can integrate the environmental stimuli into signalling pathways. These sensing systems can induce responses from two-component systems (TCS), extra cytoplasmic function (ECF) signalling pathway and quorum sensing (QS) events. Secondary messengers like c-di-GMP (cyclic guanosine monophosphate) are also involved in triggering biofilm formation (Jonas et al. 2009). For the development of biofilm, a coordinated network of gene expression is required in a stepwise manner. Thus, these signalling events play a very important role for microbial biofilm formation by developing adaptive responses against external and internal stimuli (Bordi and de Bentzmann 2011).
Two-component signalling system consists of histidine kinase (HK) and response regulator (RR) protein. HK is a sensor protein usually has an N-terminal ligand-binding domain and a C-terminal kinase domain. Signal transduction occurs through the transfer of phosphoryl groups from adenosine triphosphate (ATP) to a specific conserved histidine residue in HK (Fig. 3). Subsequently, HK transfers the phosphoryl group from histidine residue to the aspartate residue of RR (Stock et al. 2000). This phosphate activates RR which acts as a transcriptional regulator (Fig. 3). Two-component systems of GacS (HK)/GacA (RR) are generally involved in the formation of Pseudomonas aeruginosa biofilm (Rasamiravaka et al. 2015). This system induces the expression of rsm genes which code for RsmY and RsmZ which control the transition between planktonic and sedentary forms (Brencic et al. 2009). The two additional histidine kinases have been reported to be associated with the Gac system, namely RetS and LadS (Rasamiravaka et al. 2015). RetS suppresses the genes needed for biofilm formation (Kong et al. 2013), whereas LadS activates the genes that help in biofilm formation (Fig. 4). Gac system confers resistance against aminoglycosides like amikacin and gentamycin (Brinkman et al. 2001). Biofilm formation in gram-positive bacteria is regulated by modified oligopeptides which act as autoinducers. They do not enter the cell but can be detected by a sensor kinase. The kinase transfers its phosphoryl group to the response regulator (RR) which then activates the target genes for biofilm formation (Fig. 5). Two-component system of GraS (HK)/GraR (RR) has been found to be active in biofilms formed by Staphylococcus aureus (Boles et al. 2010), which also confer resistance against antibiotics like vancomycin (Fridman et al. 2013). Staphylococcus aureus produces a multilayered biofilm which can be either PIA dependent or PIA independent. PIA is polysaccharide intercellular adhesin that helps in biofilm formation and is encoded by Ica operon (Cramton et al. 1999; Archer et al. 2011). The IcaR (regulatory) and Ica ADBC (biosynthetic) genes are important for the formation of biofilms and impart virulence to the bacteria (Archer et al. 2011). The expression of PIA is suppressed by IcaR which regulates the biofilm formation (Fig. 6). Spx gene has also been reported to modulate IcaR regulation which in turn regulates biofilm formation (Archer et al. 2011). PIA independent biofilm formation takes place by the accumulation of associated protein (Aap), biofilm-associated proteins (Bap) and related proteins which help in the formation of biofilm structure (Archer et al. 2011) (Fig. 7).
Two-component signalling system of Pseudomonas aeruginosa for biofilm formation. GacS (HK)/GacA (RR) system. In this system in response to extracellular signal, GacS is activated which in turn phosphorylates and activates GacA. GacA activation induces the expression of rsm genes which code for RsmY and RsmZ which control the transition between planktonic and sedentary forms of Pseudomonas aeruginosa
Two-component signalling system (GraS and GraR) of Staphylococcus aureus for the formation of biofilm. Oligopeptides first interact with GraS (sensor kinase) and GraS and then become phosphorylated and activated. GraS in its activated form thereafter activates GraR (response regulator protein) which then activates the expression of genes needed for biofilm formation
Polysaccharide intercellular adhesion (PIA)-dependent microbial biofilm formation. PIA is required to develop the biofilm formation in microorganisms and it has been synthesized from the expression of ica operon. IcaR acts like an inhibitor for the expression of this ica operon and ultimately inhibits the microbial biofilm formation
ECF signalling pathway is also a significant signalling event in biofilm formation. In this signalling event, an alternative sigma factor along with antisigma factor present in the cell membrane is involved in biofilm formation. In addition to that, few outer membrane and periplasmic proteins are also engaged in this process (Helmann 2002). The extracellular signals are perceived by the periplasmic proteins which are followed by the degrading of the antisigma factor that can release the sigma factor which then leads to transcription of few target genes that are necessary for biofilm formation. In Pseudomonas aeruginosa biofilms, AlgU factor is the sigma factor that can control EPS alginate production which has an impact on biofilm structure (Hay et al. 2009). The sigma factor AlgU works in association with antisigma factor Muc A whose C-terminal periplasmic domains are cleaved by a protease AlgW in response to some unknown stimuli. The sigma factor AlgU is then released after the cleavage of AlgW. Therefore, the active sigma factor AlgU activates algUmucABCD operon which in turn leads to alginate production and type IVA pili assembly that ultimately leads to biofilm formation (Bordi and de Bentzmann 2011).
Quorum sensing is a multicellular response in a biofilm population that works in a density-dependent way (Schauder and Bassler 2001). It is a process of bacterial communication that makes use of autoinducers or pheromones. For gram-negative bacteria, the autoinducer is N-acyl homoserine lactones, whereas for gram-positive bacteria the autoinducer is oligopeptides. These molecules gather on the outside of the cell, and when the microbial population reaches a certain threshold level, these autoinducers can regulate the expression of genes related to virulence and biofilm formation (Bordi and de Bentzmann 2011). The transition from planktonic to biofilm forms in Staphylococcus aureus is regulated by QS. Quorum sensing in S. aureus is regulated by agr operon in which Agr D encodes for an autoinducer peptide AIP (Bordi and de Bentzmann 2011). In QS signalling, AIP activates the QS cascade. AIP peptides can then activate the two-component system of Agr C (sensor kinase which is membrane bound) and Agr A (response regulator RR). On activation, Agr C transfers the phosphoryl group to Agr A which activates P2 and P3 operon. The P2 operon amplifies the QS cascade and P3 operon activates for RNAIII expression which leads to biofilm maturation and dispersal (Karatan and Watnick 2009). Thus, autoinducers can trigger a genetic response either by entering the cells actively/passively or by the use of histidine kinases (Podbielski and Kreikemeyer 2004).
In addition to these pathways, a secondary messenger c-di-GMP in high concentration also acts as a stimulus for the formation of biofilms in bacteria (Bordi and de Bentzmann 2011). The high amount of c-di-GMP is generally regarded as the stimuli for the formation of microbial biofilm by the synthesis of EPS or alginate polymer formation or adhesive surface organelles (pili) (Rasamiravaka et al. 2015).
Biofilm-mediated infections and pathogenesis
The presence of biofilms in bacterial infections can increase the pathogenicity of the bacteria and protects the bacteria from being destroyed by external treatment. Biofilm formation is an ancient mode of survival for bacteria in hostile environments. Biofilms protect the cells from assaults like UV radiation, pH stress, chemical exposure, phagocytosis, dehydration and antibiotics.
Device-related infections
One of the first clinical infections associated with biofilm formation was medical device-related infections. Pacemakers, electrical dialysers, joint prosthetics, intravenous catheters, urinary catheter are indispensible for the patients as there has not been any other alternative against these devices. These devices also come up with a heightened risk of biofilm-associated infection. Mostly, Staphylococci and Pseudomonas species opportunistically infect a medically intervening device and get entry to the host. Such infections are nowadays referred to as chronic polymer-associated infection (Gotz 2002; von Eiff et al. 1999). In this regard, it has been observed that Staphylococci can infect both open wounds and implants (Akiyama et al. 2002). S. epidermis has also been reported to colonize the medical devices efficiently (Otto 2009).
Central venous catheter infection
Central venous catheters are used for fluid administration, medication, administering nutrients and monitoring haemodynamic activities (Kokare et al. 2009). Biofilm-forming organisms have been reported to be found dwelling on the surface of these catheters. The colonizing microorganisms in such cases are S. epidermis, S. aureus, C. albicans, P. aeruginosa, K. pneumoniae, etc., (Kokare et al. 2009). These biofilms may be present either on the lumen or on the outer surface of the catheter. It has also been reported that microbial colonization on catheters may occur within 10 days of catheterization. In cases where the catheter is administered for long time period, biofilms occur in the lumen of the catheter (Kokare et al. 2009).
Prosthetic heart valves
Nowadays, mechanical valves along with bioprostheses are used as prosthetic heart valves. The implantation of such prosthetics is susceptible to microbial colonization and subsequent biofilm formation. During surgical procedure, tissue damage may occur that leads to platelet and fibrin accumulation at the site of suture and also on the device (Kokare et al. 2009). Microbes colonize to these surfaces with higher affinity as a result of which biofilms develop on the surrounding tissues of the prosthesis. The common microbes forming biofilms in such cases are S. aureus, Streptococcus spp, gram-negative bacilli, Candida spp, Enterococci and diptheroids (Kokare et al. 2009).
Urinary catheters
Urinary catheters are made of either latex or silicone. These catheters are inserted through the urethra to the bladder where the device measures urine during surgical procedures. Catheters can have either open or closed systems. In case of an open system catheter, the urine is drained in an open collection centre. This type of system is susceptible to contamination and may also lead to the development of urinary tract infection (UTI) within a matter of few days (Kokare et al. 2009). In a closed system, the catheter is emptied in a tightly fastened plastic bag. This type of a closed system is less susceptible to opportunistic infections in comparison with the open system ones. Prolonged use of catheters leads to a higher chance of acquiring UTI (Kokare et al. 2009). The organisms contaminating such devices are S. epidermis, E. coli, Proteus mirabilis, P. aeruginosa, K. pneumoniae, Enterococcus faecalis and some gram-negative bacteria (Kokare et al. 2009).
Other device-related infections
Intra-uterine devices (IUD) and contact lenses also harbour biofilm causing infections. The tail of the IUDs is very susceptible to contamination by Lactobacilli plantarum, S. epidermis, Candida albicans, S. aureus and few species of Cornybacterium, Enterococci, etc., (Kokare et al. 2009). Bacteria also colonize the surfaces of contact lenses. The attachment affinity of the microbes depends on the nature of the lenses, electrolyte concentration, composition of the polymer of the lenses and the strain of colonizing bacteria. Most common bacteria that are found to adhere to the surfaces of the lenses and cause biofilm-associated infections are S. epidermis, E. coli, few species of Proteus, Serratia, Candida, P. aeruginosa and S. aureus (Kokare et al. 2009).
Cystic fibrosis
Cystic fibrosis is a genetic disorder (autosomal recessive) that impairs the normal functioning of the lungs. In this disease, the patients generally have a defect in cystic fibrosis transmembrane conductance regulator protein (CFTR) which leads to defective secretions in the respiratory epithelium. This results in the production of viscous mucus on the epithelium that causes difficulty in the breathing. The presence of such mucus layer is the crucial player for harbouring bacterial infections in patients. The bacteria found to be associated with lung infections in cystic fibrosis patients are S. aureus, H. influenzae and P. aeruginosa. The region of the lung, infected with H. influenzae, also becomes predisposed for further infection with P. aeruginosa subsequently. The mutation in CFTR gene also houses pulmonary colonies of S. aureus or H. influenzae in the lower respiratory tract of young patients which is replaced by colonies of P. aeruginosa in adults (Lyczak et al. 2002; Koch and Hoiby 1993). The presence of P. aeruginosa biofilms in cystic fibrosis affects lungs badly, and their presence is tested by the detection of homoserine lactone (HSL) secreted by the bacteria in the sputum of patients (Singh et al. 2000).
Endocarditis
Endocarditis is the interaction between the surfaces of the endothelium and bacteria. Though the early association is weak, but with the advent of any wound, the microbes turn opportunistic and form a strong biofilm-aided association which can damage heart valves (Kokare et al. 2009). A condition namely native valve endocarditis (NVE) arises due to the interaction among the mitral, aortic, tricuspid and the pulmonic valves of the heart and microbes that are present in the blood stream (Kokare et al. 2009). The microorganisms associated with this condition are species of Staphylococcus, Candida, Pneumococci, Streptococcus and few other gram-negative bacteria. The organisms can primarily enter into the blood stream through oropharynx, genitourinary tract and gastrointestinal tract. Generally, the adherence of microbes to the intact endothelium is very poor, but in case of a wounded or damaged epithelium, a condition namely nonbacterial thrombotic endocarditis (NBTE) develops (Kokare et al. 2009). In this condition, the red blood cells, platelets and fibrin accumulate at the site of injury. Endothelial cells secrete fibronectin which have the ability to bind to collagen, fibrin, human cell as well as bacteria. Microbes like Staphylococcus and Streptococcus sp. have fibronectin receptors which can form biofilms on the site of injury as well as damage the tissue of the valves (Kokare et al. 2009).
Periodontitis
Periodontitis is a gum infection which causes damage to the soft tissues as well as the bones that support the teeth. Periodontitis can also cause tooth loss. It usually results from poor oral and dental hygiene. The microbes responsible for periodontitis are Fusobacterium nucleatum and Pseudomonas aerobicus which can colonize on a variety of surfaces including mucosal surfaces in oral cavity (Lamont and Jenkinson 1998; Kokare et al. 2007). Colonization helps the microbes to alter the calcium flux, invade mucosal cells and release toxins. Plaque, which is also a biofilm community, is seen within 2–3 weeks of bacterial infection. With increase in the amount of plaque, saliva (which has bactericidal properties) cannot penetrate or reach the whole biofilm and so the dental carries develop in teeth (Overman 2007).
Osteomyelitis
Osteomyelitis is an infection of the bone which can be caused by bacteria or fungi. Bacterial entry into the bone can be facilitated by either direct route (bloodstream) or trauma or an earlier infection (Ziran 2007). When contracted via bloodstream, the metaphysic of the bone gets infected, after which the leucocytes enter the region. These leucocytes try to engulf the pathogen by secreting enzymes which in turn lyse the bone. This leads to the formation of pus which spreads on the blood vessels of the bone, thus stopping the proper flow of blood and making the infected areas of the bone devitalized (Kumar et al. 2007). Interestingly, S. aureus is found to be predominantly present in such cases as a causative agent (Lew and Waldvogel 2004). S. aureus has fibrin receptors and thus can bind to fibrinogen present in the bone matrix and can start biofilm formation. This affinity of S. aureus to bind to fibronectin, collagen and laminin makes it easy for the pathogen to colonize the bone by forming a biofilm (Ciampolini and Harding 2000).
Infection in chronic wound
Recent literature has documented the presence of biofilm-associated bacteria in chronic wounds which leads to their persistence (Alhede and Alhede 2014). It has been observed that S. aureus biofilms are related to chronic wounds like diabetic foot ulcer, pressure sores and venous ulcers. It has been reported that the dermal tissues of chronic wounds house many bacteria which can cause persisting infections in wounded tissues (Bjarnsholt 2013). Almost 88–98 % of wound infections have been found to be S. aureus positive (Hansson et al. 1995; Gjodsbol et al. 2006). Patients having S. aureus biofilm infections in diabetic ulcers need more healing time (Bowling et al. 2009) due to delay in re-epithelialization of the infected tissue. It has been seen that wounds infected with P. aeruginosa are larger in size in comparison with wounds having no bacteria. The presence of P. aeruginosa also delayed the healing process (Bjarnsholt 2013). Analysis of 22 patient samples by using specific peptide nucleic acid (PNA) and fluorescence in situ hybridization (FISH) revealed that the wounds that were colonized by P. aeruginosa remained in the wound bed (Bjarnsholt 2013) and S. aureus microcolonies were seen on the surface of the wound (Bjarnsholt 2013).
Rhinosinusitis
Generally, rhinosinusitis can be described as an inflammation of sinuses. Symptoms of sinusitis include thick nasal mucus, nasal irritation, plugged nose and pain in face. Generally, rhinoviruses are known to cause nasal infections like common cold. Apart from that, few bacteria like S. pneumoniae and H. influenza are also known to cause inflammation of sinuses. Chronic rhinosinusitis can be described as an inflammatory disorder, where patients harbour bacterial biofilms of S. aureus, Streptococcus pneumoniae, Haemophilus influenza and Moraxella catarrhalis. S. aureus biofilms have also been reported on the nasal mucosal surface of the 50 % of patients (Foreman and Wormald 2010; Stephenson et al. 2010).
Biofilm and drug resistance
It is known that host immune system responds to bacterial infections by activating several signalling cascades, cytokines and expressing genes associated with stress management (Hartmann and Schikora 2012; Hartmann et al. 2014). However, host immune responses are not much effective against bacterial biofilms in comparison with their planktonic counterpart (Schultz et al. 2010). Many bacterial pathogens that are initially considered as strictly extracellular can persist inside the host by the formation of biofilm through the process of adaptation (De la Fuente-Nunez et al. 2013) that results in the evasion of the bacteria from innate immunity of the host. The evasion of biofilms from host innate response proves harmful to the host, as the inflammatory influx released by the body in response to the bacterial infection may damage the host tissues (Archer et al. 2011). Three hypotheses have been proposed to explain the possible underlying mechanism of antibiotic resistance of biofilm-associated bacteria.
The first hypothesis suggests that the antibiotic may not be able to penetrate completely into the biofilm (Stewart and Costerton 2001). Sometimes, if the antibiotic gets degraded while penetrating the biofilm, the antibiotic action declines rapidly. Antibiotics may get adsorbed on the extracellular polymeric surfaces of the biofilm which can reduce the penetration of the antibiotic (aminoglycosides) (Kumon et al. 1994; Shigeta et al. 1997). Sometimes, antibiotics which are positively charged in nature can bind to the negatively charged molecules of the biofilm matrix. This interaction thereby hampers the passage of the antibiotic to the biofilm depth (Gordon et al. 1988; Nichols et al. 1988).
Secondly, the microenvironment of the biofilm changes rapidly that resulted in the malfunction of the antibiotics. In deep layers of the biofilm, there is no consumable oxygen left and the niche becomes anaerobic (de Beer et al. 1994). It has been reported that a class of antibiotics namely aminoglycosides are not effective in anaerobic environmental condition (Tack and Sabath 1985). It has also been reported that the amount of acidic waste accumulation inside a biofilm increases which changes the pH of the environment that may reduce the action of some antibiotics (Stewart and Costerton 2001). The accumulation of toxic waste or limitation of necessary substrate can lead the bacterial population to remain in a dormant, nongrowing form which can then protect the bacteria from certain antibiotics like cell wall inhibiting agents and penicillin (Tuomanen et al. 1986). There are zones within a biofilm which are metabolically inactive and this also advocates for this hypothesis (Stewart and Costerton 2001). Under osmotic stress, biofilm population reduces the abundance of porins in the bacterial membrane that resulted in the considerable reduction in the transport of some antibiotics inside the cell (Stewart and Costerton 2001).
The third hypothesis is still under some speculation. It has been hypothesized that a small population of the bacteria residing in a biofilm may adapt a protective phenotype (which is in parity with spore formation phenotype) that resulted in the development of drug resistance in biofilm population.
Therapeutic approaches
The biofilm mode of bacterial survival and growth is now being seen as a serious threat to public health and awareness about such cases draws importance among the scientific as well as social communities. The bacteria residing in biofilms have enhanced extent of virulence and pathogenicity. For example, P. aeruginosa can form biofilms in a wide variety of environmental conditions which can lead to chronic persisting infections (Bjarnsholt 2013). So to control the formation and development of biofilm becomes mandatory. But the increase in virulence and resistance against known antibiotic also poses a problem in therapeutic designing of drugs. So new strategies for the prevention of biofilm formation are necessary which can be done by coating the implants with antibacterial agents, use of broad spectrum antibiotics and newer drug combinations that are effective against sessile bacteria. Few strategies that are being adopted for removal of biofilm-associated bacteria are: avoidance of attachment of the bacteria to the surface, use of compounds that can disrupt the biofilm formation, induction of dispersion or degradation of the formed biofilm (Yang et al. 2012; Blackledge et al. 2013; Masak et al. 2014). Thus, microbial biofilm inhibition can be managed either by preventing the attachment of the organism to the surface or by breaking the structure of the biofilm if they formed (Fig. 8). Therapeutic approaches are one of the most important disciplines that need to be focused for proper treatment and prevention of such biofilm-mediated infections. Nowadays, techniques which do not involve microbicidal approaches are also being explored for biofilm inhibition (Sharma et al. 2014).
Strategies for prevention of biofilm formation on implant surfaces by use of three different approaches. Use of nonadhesive coatings over surfaces to inhibit the microbial attachment to the surface. Use of nanoparticles and antibiotics to disrupt the survival of attached bacteria. Use of compounds like dispersin and DNase to disrupt preformed biofilm
S. aureus biofilms are the most persistent infections that sometimes have to be surgically removed. Some therapeutic approaches like administration of vancomycin need to be used in some cases after surgical removal of the biofilm, while some patients have to be administered with oral antibiotics (Osmon and Berbari 2002). A technique named antimicrobial lock technique (ALT) can be used to inhibit biofilm formation in catheters (Bordi and de Bentzmann 2011). For this technique, antibacterial drugs have to be instilled in high amount on the catheter surface. But the flipside for this technique is the occurrence of secondary infections and toxicity. Compounds that interfere with the QS (NO, dispersin) can be used to dismantle the biofilm formation (Bordi and de Bentzmann 2011; Donlan 2011). Compounds that would interfere with the di-c-GMP biosynthesis can be of huge help (Antoniani et al. 2010) for the attenuation in biofilm formation. A compound sulphathiazole (sulphonamide) has been reported to work against E. coli biofilms by inhibiting c-di-GMP biosynthesis. Iron-chelating compounds can be used to disrupt Pseudomonas aeruginosa biofilms if used along with aminoglycosides (Moreau-Marquis et al. 2009; Reid et al. 2009; Bordi and de Bentzmann 2011). Beta lactam agents can be used for treatment of biofilm infections which exhibit time-dependent action against microorganism. For diseases like osteomyelitis, penicillin G and toxacillin can be used as a first line of antibiotics against S. aureus biofilms (Archer et al. 2011). Vancomycin is used for treating of methicillin-resistant S. aureus biofilm infection as the resistance against this antibiotic is rare (only 15 resistant cases have been found worldwide; Perlroth et al. 2008). But too much usage of vancomycin can lead to infections of Clostridium difficile in patients (Archer et al. 2011). But to be on the safer side, linezolid and daptomycin can also be used for effective treatment of S. aureus infections (Fraimow 2009). Other antibiotics like rifampin can kill sessile bacteria which can be of help in removal of biofilm-associated bacteria. Rifampin has negligible side effects but can be used only with vancomycin as a drug combination (Perlroth et al. 2008). Biofilm infection can also be eradicated with the use of an antimicrobial agent on the implanted device. Bone cement containing antibiotics, calcium sulphate beads coupled with antibiotics (Wahlig and Dingeldein 1980) show high efficacy against infections on open fractures followed by prophylaxis (Archer et al. 2011). The beads can also dissolve in the body fluids without any side effects and so there is no need for any other procedure for removal of the beads. The type of causative agent, the availability of the antibiotic in the powdered form and the retention of activity of the antibiotic in host play crucial role during the selection of drug against a particular microbial infection (Rao et al. 2011). Antibiotics like gentamycin and tobramycin are being used in countries of Europe, USA and UK for biofilm eradication (Archer et al. 2011). Other antimicrobial agents like nanosilver particles (Alt et al. 2004), inflammatory mediators like IL 12 (Boyce et al. 2012), secondary messengers like nitric oxide (Archer et al. 2011) and phages (anti-Staphylococcal) are being tested for their efficacy against the infectious biofilms of S. aureus (Archer et al. 2011). There are other therapeutic approaches for combating biofilm-related infections like the use of titanium prosthesis having silver coating which have been effectively reducing infection in bone sarcoma patients up to 5.9 % (Bruellhoff et al. 2010). Silicon elastomers along with triclosan have been reported to prevent 99 % of E. coli biofilms. Silicone coated with triclosan (> 0.1 %w/w) has been found to inhibit biofilm formation of S. epidermis. Mangainin I is a well-known antimicrobial peptide (23 amino acids long) extracted from Xenopus laevis which shows activity against both gram-negative and gram-positive bacteria. It has been observed that Mangainin I when covalently linked to II-mercapto undecanoic acid and 6-mercaptohexanol in 1:3 ratios reduces the bacterial adhesion (Humblot et al. 2009). Recently, non-leaching, permanent, sterile-surface materials have been developed where the antimicrobial compounds are covalently attached to the surface of a material. The antimicrobials can work on the membrane of the bacteria instead of any intracellular organelle and kill the cells. These sterile-surface materials kill both air and waterborne drug-resistant pathogens (Lewis and Klibanov 2005). It has been observed that a peptide named nisin in combination with lipid II can make pores on the membrane of gram-positive bacteria (Humblot et al. 2009) that results in the killing of bacterial population in biofilm. Another way of avoiding bacterial biofilms on medical devices is creating antimicrobial surfaces with silicone rubber, covalently coupled with quaternary ammonium silane (QAS). This reduces 90 % of the staphylococci growth, and the infection by other microbes can also be reduced (Gottenbos et al. 2002). Resin beads coated with PEO (polyethylene oxide) spacers and antimicrobial proteins can decrease the microbial infection as well as biofilm formation (Bruellhoff et al. 2010). It was reported that there was a decrease of 88–98 % in the infection rate of S. epidermis, S. mutans, P. aeruginosa by the use of RGD (arginyl-glycyl-aspartic acid) peptides (Bruellhoff et al. 2010). Bioactive RGD peptides also refute the adhesion of S. aureus on any substratum or surface (Maddikeri et al. 2008). It has also been reported that a biopolymer namely collage can also reduce the biofilm formation of E. coli by reducing the adhesion of the bacteria (Bruellhoff et al. 2010). A compound named cis-2-decanoic acid released from P. aeruginosa can disperse biofilms of E. coli, K. pneumoniae, P. mirabilis, S. pyogenes, B. subtilis, S. aureus and C. albicans (Davies and Marques 2009). D-amino acids secreted by certain bacteria can also inhibit the biofilm formation of P. aeruginosa (Kolodkin-Gal et al. 2010). N-acetyl cysteine, a derivative comes from the amino acid l-cysteine, inhibits biofilm formation of S. epidermis (Perez-Giraldo et al. 1997). Enzymes that target matrix of the biofilm can also be used as a disrupting agent against bacterial biofilms. Among the gram-negative bacteria, Actinobacillus actinomycetemcomitans can disrupt biofilms of other bacteria like S. epidermis by the production of a compound dispersin B (Kaplan et al. 2004). Proteases like trypsin and proteinase K can disrupt biofilms produced by S. aureus (Chaignon et al. 2007). It has been reported that bacteria use extracellular DNA to form biofilms. To disrupt such kind of biofilms, DNase I can be used to degrade the e DNA released by S. aureus (Izano, et al. 2008). But the in vivo efficacy of such enzymatic treatment has not yet been established properly. Another class of antimicrobials namely furanone that is secreted by red alga Delisea pulchra has been reported to inhibit the biofilm formed by S. epidermis (Baveja et al. 2004). In case of P. aeruginosa, alginate lyase which is produced by the bacterium itself has been reported to act in combination with antibiotics and thus helps in the clearance of P. aeruginosa biofilms (Rasamiravaka et al. 2015). But another study has also reported that alginate lyase does not have any catalytic activity but acts a source of nutrition and it can modulate the metabolism of the cells which lead to detachment of the cells which in turn enhance the activity of the antibiotics (Lamppa and Griswold 2013).
Nano-plasma trimethyl silane (TMS) coating can be used on stainless steel or hydrophilic surfaces to prevent S. epidermis biofilms (Ma et al. 2012). Glass surfaces grafted with poly carboxybetaine methacrylate have been used to prevent S. epidermis and P. aeruginosa attachment (Cheng et al. 2007). Silane xerogel coatings can provide super hydrophobic coating and act as antiadhesion agent against biofilm-forming bacteria (Privett et al. 2011). Cationic surfactant CTAB (cetrimonium bromide) can be used to control biofilm formed by Pseudomonas fluorescens by reducing the respiration of bacterial cells (Simoes et al. 2005). Rosmarinic acid is a compound (phenol) that is naturally produced from the root of Ocimum basilicum L. can act against LasR and RhlR receptors proteins, thereby inhibiting QS mechanism in P. aeruginosa (Annapoorani et al. 2012). Another compound ellagic acid (Terminalia chebula Retz) and its derivatives have also been reported to downregulate genes associated with las IR and rh1 IR which leads to reduction in virulence and increases sensitivity towards antibiotics (Sarabhai et al. 2013). A compound (allyl sulphide) namely ajoene which has been isolated from garlic can affect the genes which regulate QS in P. aeruginosa. This compound (ajoene) also acts synergistically with tobramycin in order to kill the bacteria in pulmonary infection (Jakobsen et al. 2012). Another compound namely S-phenyl-l-cysteine sulphoxide and the derivative compound diphenyl disulphide have also shown to disrupt biofilm of P. aeruginosa (Cady et al. 2012). Ginger (Zingiber officinale Rosc.) extracts have also been reported to inhibit biofilm formation of P. aeruginosa PA14 by reducing the production of c-di-GMP and total polysaccharide (Kim and Park 2013). Some Chinese traditional medicinal plants have also shown promise as antibiofilm agents. An anthraquinones compound from Rheum palmatum L. has shown to inhibit biofilm formation of P. aeruginosa when administered at a concentration of 20 µM and also increases the activity of antibiotic ampicillin (Ding et al. 2011). Another compound named flavan-3-ol catechin extracted from the bark of a plant Combretum albilorum (Tul.) Jongkind has been reported to interfere with QS mechanisms of a strain PAO1 of P. aeruginosa (Rasamiravaka et al. 2013).
Future area of study
The treatment of microbial infections involving biofilm becomes quite challenging because of the difficulty to understand the complexity of the microbial interactions within the biofilm along with their increasing antibiotic resistance properties and ability to persist in harsh environments. For the inhibition of microbial biofilm formation, efforts have to be given to prevent the microbial colonization to the surface since colonization is the first step in microbial biofilm formation. The identification of potential targets for the inhibition of intercellular communications may also provide the means to inhibit biofilm development since intercellular communications are certainly required for biofilm development and persistence. In order to break the existing biofilms, serious attempts have to be given to break the extracellular polymeric matrix as this matrix holds the biofilm firmly. The future of biofilm research relies upon various concerted efforts from scientists of different disciplines to understand the complexity of biofilm formation and device efficient strategy for biofilm inhibition.
Conclusion
Biofilm formation by bacteria and their subsequent resistance to antibiotic and bactericidal is a slow but serious threat to public as well as domestic health. Biofilm formation has become a ubiquitous phenomenon not only for human infections, but also on nonbiological aspects. Biofilms are formed on food items and water which are considered as the basic necessities of daily life. Current therapeutic approaches for prevention of biofilms is limited to use of antimicrobial agents and postinfection remedy lies in surgical removal of the biofilm followed by continued antibiotic administration. But nonetheless novel strategies are also being used to combat the problem. Option of vaccination against specific biofilm-associated bacteria is also being explored and one can hope that prevention and inhibition of biofilms by bacteria can be achieved in near future.
References
Akiyama H, Huh WK, Yamasaki O, Oono T, Iwatsuki K (2002) Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in mouse skin: does S. aureus generally produce a biofilm on damaged skin? Br J Dermatol 147(5):879–885
Alhede M, Alhede M (2014) The biofilm challenge. EWMA J 14:1
Alt V, Bechert T, Steinrucke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R (2004) An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 25(18):4383–4391
Annapoorani A, Umamageswaran V, Parameswari R, Pandian SK, Ravi AV (2012) Computational discovery of putative quorum sensing inhibitors against LasR and RhlR receptor proteins of Pseudomonas aeruginosa. J Comput Aided Mol Des 26(9):1067–1077
Antoniani D, Bocci P, Maciag A, Raffaelli N, Landini P (2010) Monitoring of diguanylate cyclase activity and of cyclic-di-GMP biosynthesis by whole-cell assays suitable for high-throughput screening of biofilm inhibitors. Appl Microbiol Biotechnol 85(4):1095–1104
Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME (2011) Staphylococcus aureus biofilms properties, regulation and roles in human disease. Virulence 2(5):445–459
Balasubramanian V, Natarajan K, Hemambika B, Ramesh N, Sumathi CS, Kottaimuthu R, Rajash KV (2010) High-density polyethylene(HDPE)-degrading potential bacteria from marine ecosystem of Gulf of Mannar, India. Lett Appl Microbiol 51(2):205–211
Baveja JK, Willcox MDP, Hume EBH, Kumar N, Odell R, Poole-Warren LA (2004) Furanones as potential anti-bacterial coatings on biomaterials. Biomaterials 25(20):5003–5012
Bjarnsholt T (2013) The role of bacterial biofilms in chronic infections. APMIS 121(s136):1–58
Blackledge MS, Worthington RJ, Melander C (2013) Biologically inspired strategies for combating bacterial biofilms. Curr Opin Pharmacol 13(5):699–706
Boles BR, Thoendel M, Roth AJ, Horswill AR (2010) Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One 5(4):e10146
Bordi C, de Bentzmann S (2011) Hacking into bacterial biofilms: a new therapeutic challenge. Ann Intensive Care 1(1):19
Bowling FL, Jude EB, Boulton AJ (2009) MRSA and diabetic foot wounds: contaminating or infecting organisms? Curr Diabetes Rep 9(6):440–444
Boyce BM, Lindsey BA, Clovis NB, Smith ES, Hobbs GR, Hubbard DF, Emery SE, Barnett JB, Li B (2012) Additive effects of exogenous IL-12 supplementation and antibiotic treatment in infection prophylaxis. J Orthop Res 30(2):196–202
Brencic A, McFarland KA, McManus HR, Castang S, Mogno I, Dove SL, Lory S (2009) The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol Microbiol 73(3):434–445
Brinkman FS, Macfarlane EL, Warrener P, Hancock RE (2001) Evolutionary relationships among virulence-associated histidine kinases. Infect Immun 69(8):5207–5211
Bruellhoff K, Fiedler J, Moller M, Groll J, Brenner RE (2010) Surface coating strategies to prevent biofilm formation on implant surfaces. Int J Artif Organs 33(9):646–653
Cady NC, McKean KA, Behnke J, Kubec R, Mosier AP, Kasper SH, Burz DS, Musah RA (2012) Inhibition of biofilm formation, quorum sensing and infection in Pseudomonas aeruginosa by natural products-inspired organosulfur compounds. PLoS One 7(6):e38492
Chaignon P, Sadovskaya I, Ragunah C, Ramasubbu N, Kaplan JB, Jabbouri S (2007) Susceptibility of Staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Appl Microbiol Biotechnol 75(1):125–132
Cheng G, Zhang Z, Chen S, Bryers JD, Jiang S (2007) Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 28(29):4192–4199
Ciampolini J, Harding KG (2000) Pathophysiology of chronic bacterial osteomyelitis. Why do antibiotics fail so often? Postgrad Med J 76(898):479–483
Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, Wong GC, Parsek MR (2011) The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog 7(1):e1001264
Cortes ME, Consuegra J, Sinisterra RD (2011) Biofilm formation, control and novel strategies for eradication. Sci Against Microbial Pathog Commun Curr Res Technol Adv 2:896–905
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284(5418):1318–1322
Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F (1999) The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 67(10):5427–5433
Crossley KB, Jefferson KK, Archer GL, Fowler VG (2009) Staphylococci in human disease, 2nd illustrated edn. Blackwell, West Sussex
Davey ME, O’toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64(4):847–867
Davies DG, Marques CN (2009) A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol 191(5):1393–1403
Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP (1998) The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280(5361):295–298
De Beer D, Stoodley P, Roe F, Lewandowski Z (1994) Effects of biofilm structure on oxygen distribution and mass transport. Biotechnol Bioeng 43(11):1131–1138
De Boer W, Gunnewiek PK, Veenhuis M, Bock E, Laanbroek HJ (1991) Nitrification at low pH by aggregated chemolithotrophic bacteria. Appl Environ Microbiol 57(12):3600–3604
De la Fuente-Nunez C, Reffuveille F, Fernandez L, Hancock RE (2013) Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol 16(5):580–589
Ding X, Yin B, Qian L, Zeng Z, Yang Z, Li H, Lu Y, Zhou S (2011) Screening for novel quorum-sensing inhibitors to interfere with the formation of Pseudomonas aeruginosa biofilm. J Med Microbiol 60(12):1827–1834
Donlan RM (2011) Biofilm elimination on intravascular catheters: important considerations for the infectious disease practitioner. Clin Infect Dis 52(8):1038–1045
Edwards KJ, Bond PL, Gihring TM, Banfield JF (2000) An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 287(5459):1796–1799
Feldman MF, Wacker M, Hernandez M, Hitchen PG, Marolda CL, Kowarik M, Morris HR, Dell A, Valvano MA, Aebi M (2005) Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc Natl Acad Sci USA 102(8):3016–3021
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623–633
Foreman A, Wormald PJ (2010) Different biofilms, different disease? A clinical outcomes study. Laryngoscope 120(8):1701–1706
Fraimow HS (2009) Systemic antimicrobial therapy in osteomyelitis. Semin Plast Surg 23(2):90
Franklin MJ, Nivens DE, Weadge JT, Howell PL (2011) Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front Microbiol 22(2):167
Fridman M, Williams GD, Muzamal U, Hunter H, Siu KW, Golemi-Kotra D (2013) Two unique phosphorylation-driven signaling pathways crosstalk in Staphylococcus aureus to modulate the cell-wall charge: Stk1/Stp1 meets GraSR. Biochemistry 52(45):7975–7986
Garrett TR, Bhakoo M, Zhang Z (2008) Bacterial adhesion and biofilms on surfaces. Prog Nat Sci 18(9):1049–1056
Gjodsbol K, Christensen JJ, Karlsmark T, Jørgensen B, Klein BM, Krogfelt KA (2006) Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 3(3):225–231
Gloag ES, Turnbull L, Huang A, Vallotton P, Wang H, Nolan LM, Mililli L, Hunt C, Lu J, Osvath SR, Monahan LG, Cavaliere R, Charles IG, Wand MP, Gee ML, Prabhakar R, Whitchurch CB (2013) Self-organization of bacterial biofilms is facilitated by extracellular DNA. Proc Natl Acad Sci 110(28):11541–11546
Gordon CA, Hodges NA, Marriott C (1988) Antibiotic interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis-derived Pseudomonas aeruginosa. J Antimicrob Chemother 22(5):667–674
Gottenbos B, van der Mei HC, Klatter F, Nieuwenhuis P, Busscher HJ (2002) In vitro and in vivo antimicrobial activity of covalently coupled quarternary ammonium silane coatings on silicone rubber. Biomaterials 23(6):1417–1423
Gotz F (2002) Staphylococcus and biofilms. Mol Microbiol 43(6):1367–1378
Hall-Stoodley L, Stoodley P (2009) Evolving concepts in biofilm infections. Cell Microbiol 11(7):1034–1043
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2(2):95–108
Hansson C, Hoborn J, Moller A, Swanbeck G (1995) The microbial flora in venous leg ulcers without clinical signs of infection. Repeated culture using a validated standardised microbiological technique. Acta Derm Venereol 75(1):24–30
Hartmann A, Schikora A (2012) Quorum sensing of bacteria and trans-kingdom interactions of N-acyl homoserine lactones with eukaryotes. J Chem Ecol 38(6):704–713
Hartmann A, Rothballer M, Hense BA, Schroder P (2014) Bacterial quorum sensing compounds are important modulators of microbe-plant interactions. Front Plant Sci Front Plant Sci 5:131
Hay ID, Gatland K, Campisano A, Jordens JZ, Rehm BH (2009) Impact of alginate overproduction on attachment and biofilm architecture of a supermucoid Pseudomonas aeruginosa strain. Appl Environ Microbiol 75(18):6022–6025
Helmann JD (2002) The extracytoplasmic function (ECF) sigma factors. Adv Microb Physiol 46:47–110
Humblot V, Yala JF, Thebault P, Boukerma K, Hequet A, Berjeaud JM, Pradier CM (2009) The antibacterial activity of Mangainin I immobilized onto mixed thiols self-assembled monolayers. Biomaterials 30(21):3503–3512
Hurlow J, Couch K, Laforet K, Bolton L, Metcalf D, Bowler P (2015) Clinical biofilms: a challenging frontier in wound care. Adv Wound Care 4(5):295–301
Izano EA, Amarante MA, Kher WB, Kaplan JB (2008) Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol 74(2):470–476
Jakobsen TH, van Gennip M, Phipps RK, Shanmugham MS, Christensen LD, Alhede M, Skindersoe ME, Rasmussen TB, Friedrich K, Uthe F, Jensen PO, Moser C, Nielsen KF, Eberl L, Larsen TO, Tanner D, Hoiby N, Bjarnsholt T, Givskov M (2012) Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob Agents Chemother 56(5):2314–2325
Jonas K, Melefors O, Romling U (2009) Regulation of c-di-GMP metabolism in biofilms. Future Microbiol 4(3):341–358
Kaplan JB, Ragunath C, Velliyagounder K, Fine DH, Ramasubbu N (2004) Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 48(7):2633–2636
Karatan E, Watnick P (2009) Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev 73(2):310–347
Kim HS, Park HD (2013) Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14. PLoS One 8(9):e76106
Koch C, Hoiby N (1993) Pathogenesis of cystic fibrosis. Lancet 341(8852):1065–1069
Kokare CR, Kadam SS, Mahadik KR, Chopade BA (2007) Studies on bioemulsifier production from marine Streptomyces sp. S1. Indian J Biotechnol 6(1):78–84
Kokare CR, Chakraborty S, Khopade AN, Mahadik KR (2009) Biofilm: importance and applications. Indian J Biotechnol 8(2):159–168
Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R (2010) D-amino acids trigger biofilm disassembly. Science 328(5978):627–629
Kong W, Chen L, Zhao J, Shen T, Surette MG, Shen L, Duan K (2013) Hybrid sensor kinase PA1611 in Pseudomonas aeruginosa regulates transitions between acute and chronic infection through direct interaction with RetS. Mol Microbiol 88(4):784–797
Kostakioti M, Hadjifrangiskou M, Hultgren SJ (2013) Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the post antibiotic era. Cold Spring Harb Perspect Med 3(4):a010306:1–23
Kumar CG, Anand SK (1998) Significance of microbial biofilms in food industry: a review. Int J Food Microbiol 42(1):9–27
Kumar V, Abbas AK, Fausto N, Mitchell R (2007) Robbins basic pathology, 8th edn. Elsevier. pp 810–811. ISBN 978-1-4160-2973-1
Kumon H, Tomochika KI, Matunaga T, Ogawa M, Ohmori H (1994) A sandwich cup method for the penetration assay of antimicrobial agents through Pseudomonas exopolysaccharides. Microbiol Immunol 38(8):615–619
Lamont RJ, Jenkinson HF (1998) Life below gum line: pathogenetic mechanisms of Porphromonas gingivalis. Microbiol Mol Biol Rev 62(4):1244–1263
Lamppa JW, Griswold KE (2013) Alginate lyase exhibits catalysis-independent biofilm dispersion and antibiotic synergy. Antimicrob Agents Chemother 57(1):137–145
Lew DP, Waldvogel FA (2004) Osteomyelitis. Lancet 364(9431):369–379
Lewis K (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45(4):999–1007
Lewis K, Klibanov AM (2005) Surpassing nature: rational design of sterile-surface materials. Trends Biotechnol 23(7):343–348
Lyczak JB, Cannon CL, Pier GB (2002) Lung infections associated with cystic fibrosis. Clin Microbiol Rev 15(2):194–222
Ma Y, Chen M, Jones JE, Ritts AC, Yu Q, Sun H (2012) Inhibition of Staphylococcus epidermidis biofilm by trimethylsilane plasma coating. Antimicrob Agents Chemother 56(11):5923–5937
Maddikeri RR, Tosatti S, Schuler M, Chessari S, Textor M, Richards RG, Harris LG (2008) Reduced medical infection related bacterial strains adhesion on bioactive RGD modified titanium surfaces: a first step toward cell selective surfaces. J Biomed Mater Res Part A 84(2):425–435
Maric S, Vranes J (2007) Characteristics and significance of microbial biofilm formation. Period Bilogor 109:115–121
Masak J, Cejkova A, Schreiberova O, Rezanka T (2014) Pseudomonas biofilms: possibilities of their control. FEMS Microbiol Ecol 89(1):1–14
Massol-Deya AA, Whallon J, Hickey RF, Tiedje JM (1995) Channel structures in aerobic biofilms of fixed-film reactors treating contaminated groundwater. Appl Environ Microbiol 61(2):769–777
Moreau-Marquis S, O’Toole GA, Stanton BA (2009) Tobramycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells. Am J Respir Cell Mol Biol 41(3):305–313
Nichols WW, Dorrington SM, Slack MP, Walmsley HL (1988) Inhibition of tobramycin diffusion by binding to alginate. Antimicrob Agents Chemother 32(4):518–523
Osmon DR, Berbari EF (2002) Outpatient intravenous antimicrobial therapy for the practicing orthopaedic surgeon. Clin Orthop Relat Res 403:80–86
Otto M (2009) Staphylococcus epidermidis—the ‘accidental’ pathogen. Nat Rev Microbiol 7(8):555–567
Otto M (2013) Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 64:175–188
Overman PR (2007) Biofilm : a new view of plaque. J Contemp Dent Pract 1(3):18–29
Paerl HW, Pinckney JL (1996) A minireview of microbial consortia: their roles in aquatic production and biogeochemical cycling. Microb Ecol 31:225–247
Pal A, Paul AK (2008) Microbial extracellular polymeric substances: central elements in heavy metal bioremediation. Indian J Microbiol 48(1):49–64
Parsek MR, Singh PK (2003) Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 57(1):677–701
Perez-Giraldo C, Rodriguez-Benito A, Moran FJ, Hurtado C, Blanco MT, Gomez-Garcia AC (1997) Influence of N-acetylcysteine on the formation of biofilm by Staphylococcus epidermidis. J Antimicrob Chemother 39(5):643–646
Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG (2008) Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med 168(8):805–819
Podbielski A, Kreikemeyer B (2004) Cell density-dependent regulation: basic principles and effects on the virulence of Gram-positive cocci. Int J Infect Dis 8(2):81–95
Privett BJ, Youn J, Hong SA, Lee J, Han J, Shin JH, Schoenfisch MH (2011) Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir 27(15):9597–9601
Qurashi AW, Sabri AN (2012) Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz J Microbiol 43(3):1183–1191
Rao N, Ziran BH, Lipsky BA (2011) Treating osteomylitis: antibiotics and surgery. Plast Reconstr Surg 127(1):177S–187S
Rasamiravaka T, Jedrzejowski A, Kiendrebeogo M, Rajaonson S, Randriamampionona D, Rabemanantsoa C, Andriantsimahavandy A, Rasamindrakotroka A, Duez P, El Jaziri M, Vandeputte OM (2013) Endemic malagasy Dalbergia species inhibit quorum sensing in Pseudomonas aeruginosa PAO1. Microbiology 159(Pt 5):924–938
Rasamiravaka T, Labtani Q, Duez P, El Jaziri M (2015) The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. BioMed Res Int 2015:1–17
Reid DW, O’May C, Kirov SM, Roddam L, Lamont IL, Sanderson K (2009) Iron chelation directed against biofilms as an adjunct to conventional antibiotics. Am J Physiol Lung Cell Mol Physiol 296:L857–L858
Sarabhai S, Sharma P, Capalash N (2013) Ellagic acid derivatives from Terminalia chebula Retz. downregulate the expression of quorum sensing genes to attenuate Pseudomonas aeruginosa PAO1 virulence. PLoS One 8(1):e53441
Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG (2002) Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol 184(4):1140–1154
Schauder S, Bassler BL (2001) The languages of bacteria. Genes Dev 15(12):1468–1480
Schultz G, Phillips P, Yang Q, Stewart PS (2010) Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J Wound Care 19(8):320
Sharma G, Rao S, Bansal A, Dang S, Gupta S, Gabrani R (2014) Pseudomonas aeruginosa biofilm: potential therapeutic targets. Biologicals 42(1):1–7
Shigeta M, Tanaka G, Komatsuzawa H, Sugai M, Suginaka H, Usui T (1997) Permeation of antimicrobial agents through Pseudomonas aeruginosa biofilms: a simple method. Chemotherapy 43(5):340–345
Simoes M, Pereira MO, Vieira MJ (2005) Action of a cationic surfactant on the activity and removal of bacterial biofilms formed under different flow regimes. Water Res 39(2):478–486
Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP (2000) Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407(6805):762–764
Stephenson MF, Mfuna L, Dowd SE, Wolcott RD, Barbeau J, Poisson M, James G, Desrosiers M (2010) Molecular characterization of the polymicrobial flora in chronic rhinosinusitis. J Otolaryngol Head Neck Surg 39(2):182–187
Stewart PS, Costerton JW (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358(9276):135–138
Stock AM, Robinson VL, Goudreau PN (2000) Two-component signal transduction. Annu Rev Biochem 69(1):183–215
Stoodley P, Cargo R, Rupp CJ, Wilson S, Klapper I (2002a) Biofilm material properties as related to shear-induced deformation and detachment phenomena. J Ind Microbiol Biotechnol 29(6):361–367
Stoodley P, Sauer K, Davies DG, Costerton JW (2002b) Biofilms as complex differentiated communities. Annu Rev Microbiol 56(1):187–209
Sutherland IW (1999) Polysaccharases for microbial exopolysaccharides. Carbohydr Polym 38(4):319–328
Tack KJ, Sabath LD (1985) Increased minimum inhibitory concentrations with anaerobiasis for tobramycin, gentamicin, and amikacin, compared to latamoxef, piperacillin, chloramphenicol, and clindamycin. Chemotherapy 31(3):204–210
Tribedi P, Sil AK (2014) Cell surface hydrophobicity: a key component in the degradation of polyethylene succinate by Pseudomonas sp. AKS2. J Appl Microbiol 116(2):295–303
Tribedi P, Gupta AD, Sil AK (2015) Adaptation of Pseudomonas sp. AKS2 in biofilm on low-density polyethylene surface: an effective strategy for efficient survival and polymer degradation. Bioresour Bioprocess 2(1):1–10
Tuomanen E, Cozens R, Tosch W, Zak O, Tomasz A (1986) The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol 132(5):1297–1304
Vasudevan R (2014) Biofilms: microbial cities of scientific significance. J Microbiol Exp 1(3):00014
von Eiff C, Heilmann C, Herrmann M, Peters G (1999) Basic aspects of the pathogenesis of staphylococcal polymer associated infections. Infection 27:S7–S10
Wahlig H, Dingeldein E (1980) Antibiotics and bone cements experimental and clinical long-term observations. Acta Orthop Acta Orthop Scand 51(1):49–56
Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS (2002) Extracellular DNA required for bacterial biofilm formation. Science 295(5559):1487
Wilson M (2001) Bacterial biofilms and human disease. Sci Prog 84(3):235–254
Yang L, Liu Y, Wu H, Song Z, Hoiby N, Molin S, Givskov M (2012) Combating biofilms. FEMS Immunol Med Microbiol 65(2):146–157
Ziran BH (2007) Osteomyelitis. J Trauma 62(6):59–60
Acknowledgments
The authors would like to thank Manash Chandra Das, Trishna Mahanty, Antu Das and Maumita Saha for their valuable contributions for the improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Gupta, P., Sarkar, S., Das, B. et al. Biofilm, pathogenesis and prevention—a journey to break the wall: a review. Arch Microbiol 198, 1–15 (2016). https://doi.org/10.1007/s00203-015-1148-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-015-1148-6