Abstract
Summary
This is the first study to examine the association of adherence to the dietary approaches to stop hypertension (DASH) dietary pattern and osteoporosis risk in postmenopausal Iranian women. Findings suggest the inverse association of adherence to the DASH dietary pattern and osteoporosis risk in postmenopausal Iranian women.
Introduction
This study was aimed to examine the association of adherence to the dietary approaches to stop hypertension (DASH) dietary pattern and osteoporosis risk in postmenopausal Iranian women, hypothesizing that higher adherence to this pattern is associated with lower risk of postmenopausal osteoporosis.
Methods
One hundred fifty-one postmenopausal Iranian women aged 50–85 years participated in this cross-sectional study. Lumbar spine and femoral neck bone mineral densities (BMDs) were measured via dual-energy X-ray absorptiometry. Postmenopausal osteoporosis was then defined using the WHO criteria as a BMD T-score of ≤ − 2.5 standard deviations. The usual past-year dietary intakes were assessed by a valid and reliable 168-item food frequency questionnaire. The DASH score was then calculated based on energy-adjusted intakes of eight major dietary components usually emphasized (i.e., fruits, vegetables, nuts and legumes, low-fat dairy products, and whole grains) or minimized (i.e., sodium, sweets, and red or processed meats) in the DASH diet. The higher the DASH score of a participant, the higher the adherence to the DASH dietary pattern.
Results
After adjusting for several potential covariates in the multivariable-adjusted binary logistic regression analysis, participants in the highest tertile of DASH score had lower risk of osteoporosis at lumbar spine than those in the lowest tertile (odds ratio = 0.28; 95% confidence interval, 0.09–0.88; p = 0.029). However, no significant association was observed between adherence to the DASH dietary pattern and risk of osteoporosis at femoral neck.
Conclusion
Findings suggest the inverse association of adherence to the DASH dietary pattern and osteoporosis risk in postmenopausal Iranian women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postmenopausal osteoporosis and associated fractures are widely recognized as major public health problems worldwide, contributing to considerable morbidity, mortality, and health care cost as well as a substantial reduction in quality of life [1,2,3,4,5]. With an alarming overall prevalence of 32%, osteoporosis is also considered as one of the most common diseases in postmenopausal Iranian women [6]. Given the growing prevalence of this debilitating disease and the difficult nature of its treatment [1, 3], finding novel strategies to maintain optimal bone density during the postmenopausal period is thus an absolute necessity for osteoporosis prevention.
Bone density is significantly influenced by an array of genetic and environmental factors, with a host of interactions among these factors [2, 7]. Among the environmental determinants of bone density, dietary factors have gained the most interest in the literature because they are more amenable to modification [2, 8]. However, the relationship between diet and bone density is yet to be well understood [2, 8]. This could be largely attributed to the fact that most research in this respect have traditionally focused on the intakes of individual nutrients or food groups [2, 8]. Although studying individual nutrients or food groups could substantially contribute to the understanding of underlying biological mechanisms of a disease, their effects might be too small to detect and could be confounded by the effects of dietary patterns [9, 10]. To address the inherent limitations of this traditional approach, the dietary pattern analysis has been widely suggested as an alternative method to comprehensively assess the diet-disease associations [9, 10]. The holistic approach of dietary pattern analysis is more applicable to clinical and public health interventions than the traditional approach; appropriately accounts for potential antagonistic and synergistic interactions that might occur among individual nutrients or food groups; and provides a unique opportunity to better clarify the etiologic role of diet in the pathogenesis of diseases such as osteoporosis [2, 8,9,10].
Among dietary patterns defined using the hypothesis-driven or a priori approaches to dietary pattern analysis, the dietary approaches to stop hypertension (DASH) dietary pattern is one of the most well-studied patterns that higher adherence to which has been associated with not only a lower risk of hypertension but also a reduced risk of other health outcomes (e.g., cancer, heart disease, stroke, and diabetes mellitus) [11,12,13]. Although the major characteristics of DASH dietary pattern (i.e., higher intakes of fruits, vegetables, nuts and legumes, low-fat dairy products, and whole grains and lower intakes of sodium, sweets, and red or processed meats) are also in line with dietary recommendations to prevent osteoporosis [11, 13], the only study so far to assess the potential association of adherence to this healthy pattern and bone density/osteoporosis risk has resulted in null findings [14]. Despite its prominence, we are also unaware of any previous studies on the relationship between adherence to this dietary pattern and postmenopausal osteoporosis risk. The present study was therefore aimed to examine the potential association of adherence to the DASH dietary pattern and osteoporosis risk in a sample of postmenopausal Iranian women, hypothesizing that higher adherence to this pattern is associated with lower risk of postmenopausal osteoporosis.
Methods
Study population and sampling
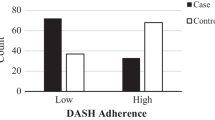
Figure 1 depicts the participant flow throughout the study. From a total of 220 postmenopausal Iranian women aged 50–85 years visiting a community-based outpatient health center for annual health check-ups in Tehran who were invited to participate in the present cross-sectional study, 213 agreed to do so (i.e., the participation rate was 96.8%) and were consecutively enrolled. Menopause was defined as self-reported amenorrhea for > 1 year. After excluding those who had a diagnosed medical condition (n = 13) or were taking medications capable of affecting bone health/nutritional status (n = 29), those following a weight management diet (n = 11) or with > 40% blank items on the food frequency questionnaire (FFQ; n = 6), and those with a daily energy intake outside the range of 800–4600 kcal (3347–19,246 kJ) (n = 3), only 151 eligible participants (mean age 61.2 years) remained for the current analysis. Notably, none of these participants reported any history of alcohol intake.
Ethics
The protocol of this study was approved by the research ethics committee of the National Nutrition and Food Technology Research Institute (WHO Collaborating Center), Shahid Beheshti University of Medical Sciences, Tehran, Iran (ethics approval number P/25/47/5421). All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments. Written informed consents were obtained from all individual participants prior to their inclusion in this study.
Measurements
Bone mineral density
Bone mineral density (BMD; g/cm2) at lumbar spine (L1–L4) and left femoral neck was measured by a trained technician via dual-energy X-ray absorptiometry (DXA), which is the gold standard technique for BMD measurement and osteoporosis diagnosis [15]. Using the widely accepted WHO criteria for osteoporosis diagnosis in postmenopausal women, osteoporosis was then defined as a BMD T-score of ≤ − 2.5 standard deviations (SDs) [15]. Although the densitometer (Hologic Discovery W QDR Series, Hologic Inc., Bedford, MA, USA) had been initially calibrated by the manufacturer, it was systematically and automatically calibrated throughout the study using an automatic internal reference system. Quality control measures were also carried out based on guidelines for standard operating procedures. The coefficient of variation for BMD measurements was 1%. All measurements were conducted during winter to control for the seasonal variations in BMD.
Dietary intake
A valid and reliable semi-quantitative Willett-format FFQ consisting of 168 food items commonly consumed by Iranians [16, 17] was used for assessing the usual past-year dietary intakes. Participants were initially asked to report their consumption frequency of a given standard serving size of each food item on a daily, weekly, or monthly basis. The daily grams of intake for each food item were then calculated according to the reported consumption frequency by using the Iranian Manual for Household Measures, Cooking Yields Factors, and Edible Portion of Foods [18]. Participants’ daily energy and nutrient intakes were computed by entering their daily grams of intake of each food item into the nutritionist software version IV (First Databank Inc., San Bruno, CA, USA), whose nutrient database was based on the USDA food composition table modified for Iranian food items. All dietary intakes were adjusted for daily energy intake using the residual method described by Willett and Stampfer [19] and then reported as daily intake/1000 kcal.
Among four major methods for constructing a composite score to represent the adherence to the DASH diet, we chose the one proposed by Fung et al. [20] as it is believed to better capture the actual characteristics of this dietary pattern [21] and also because it is the only method that considers the intake of sweets (i.e., a food group which its consumption could significantly affect BMD) [22]. According to this method [20], the DASH score is calculated based on energy-adjusted intakes of eight major dietary components usually emphasized (i.e., fruits, vegetables, nuts and legumes, low-fat dairy products, and whole grains) or minimized (i.e., sodium, sweets, and red or processed meats) in the DASH diet [11]. To do so, participants were initially classified based on the energy-adjusted quintile (Q) categories of their dietary intake of these components. For fruits, vegetables, nuts and legumes, low-fat dairy products, and whole grains, the scores of 1, 2, 3, 4, and 5 were then allocated to those in the Q1 (lowest), Q2, Q3, Q4, and Q5 (highest), respectively. The exact opposite of this scoring protocol was also used for sodium, sweets, and red or processed meats. The scores of all eight components were then summed up to construct a DASH score ranging theoretically from 8 to 40 for each participant. The higher the DASH score of a participant, the higher her adherence to the DASH dietary pattern [20].
Other variables
Anthropometric characteristics were measured according to the standard guidelines with participants standing in an upright position and wearing minimal clothing and no shoes [23]. Weight was measured to the nearest 0.1 kg using a SECA 881 digital floor scale (SECA Inc., Hamburg, Germany). Height was measured to the nearest 0.001 m by a SECA 214 portable stadiometer (SECA Inc., Hamburg, Germany). Body mass index (BMI; kg/m2) was then estimated using the following standard formula: weight (kg)/height (m2).
Physical activity was assessed using a valid and reliable Persian version of the physical activity scale (PAS) [24], which measures physical activity in 24 h of sports, work, and leisure time on an average weekday [25]. The PAS is comprised of nine different metabolic equivalent (MET) categories ranging from 0.9 (sleep/rest) to > 6 (high-intensity physical activities) and generates a physical activity summary score as MET-h/d [25]. It is notable that this simple self-report instrument had been validated for evaluation of physical activity in adult men and women against 4-day physical activity diaries (i.e., the Spearman correlation coefficient between the PAS and 4-day physical activity diaries was 0.74) [25].
Data on age (years), age at menarche (years), age at menopause (years), parity (n), duration of lactation (months), sunlight exposure (< 1 h, ≥ 1 h), smoking (yes, no), supplement intake (yes, no), and education (< 12 years, ≥ 12 years) were obtained via general questionnaires.
In the present study, a well-trained nutritionist enrolled the participants, assessed their eligibility for the study, administered all questionnaires through face-to-face interviews, and performed the anthropometric measurements on the same day as the bone densitometry.
Statistical analysis
Participants were classified into three categories based on tertiles of DASH score (n = 50 in T1 and T3, n = 51 in T2). The chi-square or Fisher’s exact tests were used for comparison of all categorical variables among tertiles of DASH score, as appropriate. For continuous variables, the normality assumption was initially examined by the Shapiro-Wilk test, and those with a non-normal distribution were normalized by standard transformation techniques prior to any further analysis. The one-way analysis of variance (ANOVA) was then used for comparison of all continuous variables among tertiles of DASH score. In case of any significant differences among tertiles of DASH score, the one-way ANOVA was followed by pairwise between group comparisons using the Bonferroni post hoc test to adequately adjust for multiple comparisons. Multivariable-adjusted means of BMD were computed for and compared among tertiles of DASH score using the one-way analysis of covariance (ANCOVA). The odds ratios (ORs) and 95% confidence intervals (CIs) for postmenopausal osteoporosis across tertiles of DASH score were computed using the binary logistic regression analysis. Three separate multivariable-adjusted models were used in both one-way ANCOVA and binary logistic regression analysis. Model 1 was adjusted for age. Model 2 was adjusted for age, BMI, physical activity, age at menarche, age at menopause, parity, duration of lactation, and energy intake. Model 3 was adjusted for covariates in model 2 and for sunlight exposure, smoking, supplement intake, and education. Pairwise differences in the means of BMD between highest (T3) and lowest (T1) tertiles of DASH score were examined by the Bonferroni post hoc test. To evaluate the overall trend of OR across increasing tertiles of DASH score, the categorized DASH score was used as a continuous predictor in every model.
The IBM SPSS Statistics version 21 (IBM Corp., Armonk, NY, USA) was used to conduct all statistical analyses, considering a two-sided p value of < 0.050 as significant. Data were presented as n (%), mean ± SD, and OR (95% CI).
Results
Table 1 shows the characteristics of study participants across tertiles of DASH score. The DASH score ranged from 10 to 35 among study participants. The prevalence of osteoporosis at lumbar spine and femoral neck was 30.5% and 15.9%, respectively. Participants in the highest tertile of DASH score were older and less likely to have osteoporosis at lumbar spine, compared with those in the lowest tertile (both p < 0.050). No other significant differences were found in terms of characteristics of study participants across tertiles of DASH score.
Table 2 demonstrates the dietary intakes of study participants across tertiles of DASH score. Compared with those in the lowest tertile, participants in the highest tertile of DASH score had higher intakes of fiber, vitamin A, β-carotene, vitamin K, vitamin B1, vitamin B2, folate, vitamin C, calcium, phosphorous, magnesium, copper, selenium, potassium, fruits, vegetables, nuts and legumes, and low-fat dairy products and lower intakes of total fat, saturated fatty acid (SFA), cholesterol, sodium, sweets, and red or processed meats (all p < 0.050). There were no other significant differences in dietary intakes of study participants across tertiles of DASH score.
The multivariable-adjusted means of BMD across tertiles of DASH score are shown in Table 3. After controlling for several potential covariates in the one-way ANCOVA (model 3), there were no significant differences in multivariable-adjusted means of lumbar spine or femoral neck BMD among tertiles of DASH score.
Table 4 presents the ORs and 95% CIs for postmenopausal osteoporosis across tertiles of DASH score. After adjusting for several potential covariates in the multivariable-adjusted binary logistic regression analysis (model 3), participants in the highest tertile of DASH score had lower risk of osteoporosis at lumbar spine than those in the lowest tertile (OR = 0.28; 95% CI, 0.09–0.88; p = 0.029). However, no significant association was observed between adherence to the DASH dietary pattern and risk of osteoporosis at femoral neck.
Discussion
To our knowledge, this is the first study on the association of adherence to the DASH dietary pattern and postmenopausal osteoporosis risk. The findings indicated that higher adherence to the DASH pattern was associated with lower risk of osteoporosis at lumbar spine in a sample of postmenopausal Iranian women. However, there was no significant association between the adherence to this dietary pattern and risk of osteoporosis at femoral neck. In brief, these results confirm the study hypothesis that higher adherence to the DASH dietary pattern is associated with lower risk of postmenopausal osteoporosis. As mentioned earlier, the major characteristics of DASH dietary pattern are in line with dietary recommendations to prevent osteoporosis. If confirmed in prospective studies of high methodological quality, the results of this study thus implicate that this dietary pattern could be promoted as a novel non-pharmacological strategy for maintaining optimal bone density, especially during the postmenopausal period.
As far as we are aware, the only study so far to assess the potential association of adherence to the DASH dietary pattern with bone density/osteoporosis risk is the one conducted by Monjardino et al., in which they failed to find any significant associations with BMD at distal radius in a large cohort of Portuguese adolescents [14]. In contrast, an inverse association between adherence to the DASH dietary pattern and postmenopausal osteoporosis risk at lumbar spine was observed in the present study. Although there was a major difference in terms of study population (i.e., postmenopausal women versus adolescents) between the two studies, we believe that choosing distal radius as the only BMD measurement site in the Monjardino et al. study [14] could partially explain why they were not able to find any significant associations between adherence to the DASH dietary pattern and bone density. Like femoral neck, distal radius is predominantly composed of cortical bone which its surface area and rate of remodeling is significantly lower than that of trabecular bone abundantly found in lumbar spine [26, 27]. This particular characteristic of distal radius and femoral neck makes them relatively less sensitive than lumbar spine to environmental changes (e.g., dietary changes) over time [26,27,28] and therefore might justify the lack of any significant associations between adherence to the DASH dietary pattern and bone density/osteoporosis risk at these two skeletal sites.
The inverse association of adherence to the DASH dietary pattern and postmenopausal osteoporosis risk in our study is supported by findings of a recent meta-analysis of 17 observational studies, in which higher adherence to a healthy dietary pattern with similar characteristics to those of DASH diet (i.e., higher intakes of fruits, vegetables, whole grains, and white meats) was found to be associated with an 18% reduction in risk of low BMD [2]. Our findings are also in line with those reported in a recent scoping review of 49 human studies, suggesting that higher adherence to a similar healthy dietary pattern (i.e., characterized by emphasizing the intakes of fruits, vegetables, nuts and legumes, low-fat dairy products, whole grains, and white meat and minimizing the intakes of sweets, red or processed meats, soft drinks, fried foods, and refined grains) can improve bone density and decrease osteoporosis risk [8].
In an experimental study by Doyle and Cashman, feeding a DASH-type diet to hypertensive rats for 8 weeks led to a 16–33% reduction in markers of bone formation (i.e., serum osteocalcin) and resorption (i.e., urinary pyridinoline and deoxypyridinoline) compared with a control diet [29]. Similarly, in a randomized controlled trial (RCT) by Lin et al., feeding DASH diets with three levels of sodium reduction to hypertensive adults for 30 days resulted in an 8–18% decrease in serum levels of osteocalcin and cross-linked C-terminal telopeptide of type-I collagen (i.e., a marker of bone resorption) as well as a dose-dependent reduction of urinary calcium [30]. The fall in urinary calcium by a DASH-type diet was also reported in a 14-week RCT of hypertensive adult women by Nowson et al. [31]. All these findings support the results of the present study and indicate that higher adherence to the DASH dietary pattern could lower the risk of osteoporosis mainly by improving the markers of bone turnover and calcium metabolism.
Although the exact mechanisms by which the DASH dietary pattern exerts its protective effects against osteoporosis are unclear [13], the inverse relationship between adherence to this pattern and postmenopausal osteoporosis risk in our study could be largely explained by its major characteristics. The DASH diet is characterized by higher intakes of food items rich in potassium, magnesium, calcium, vitamin C, β-carotene, vitamin K, protein, and fiber, all of which are of great importance to bone health [11, 13]. In fact, a growing evidence suggests that even in the context of adequate calcium intake, a greater dietary alkali load resulting from higher intakes of potassium and magnesium (i.e., the major determinants of alkali load in a diet) reduces bone calcium efflux via suppression of osteoclasts and stimulation of osteoblasts and thereby decreases osteoporosis risk [8, 13, 28, 32, 33]. In addition, higher dietary intakes of potent antioxidants such as vitamin C and β-carotene have been found to play pivotal roles in combating the oxidative stress-induced bone loss (i.e., one of the major underlying mechanisms of postmenopausal osteoporosis) [8, 34]. Furthermore, since inflammation is implicated as a primary mediator of accelerated postmenopausal bone loss [35], it is possible that greater intakes of nutrients with significant antiinflammatory properties such as vitamin C, β-carotene, magnesium, and fiber through adherence to the DASH dietary pattern could lead to a decrease in postmenopausal osteoporosis risk [36, 37]. Finally, calcium, magnesium, vitamin C, vitamin K, and protein are among the most essential nutrients for bone formation and/or maintenance, and thus, their abundance in the DASH diet could greatly contribute to the bone-protective properties of this dietary pattern [8, 13, 38,39,40].
Another major characteristic of DASH diet is its emphasis on lowering the intakes of food items rich in sodium, total fat, SFA, and cholesterol, all of which are detrimental to bone health [11, 13]. In fact, a high sodium intake is widely known to increase bone turnover and osteoporosis risk via elevation of urinary calcium excretion [8, 13, 41]. In addition, existing evidence indicates that high intakes of total fat and SFA can have direct and indirect effects that may reduce intestinal calcium absorption, increase bone resorption, and decrease bone formation [8, 42, 43]. Furthermore, a high-cholesterol diet has been shown to inhibit the differentiation and proliferation of rat osteoblasts, thereby limiting bone formation and elevating osteoporosis risk [44].
Even though every one of these mechanisms could be responsible for the inverse association of adherence to the DASH dietary pattern and postmenopausal osteoporosis risk in the present study, it is most likely that this pattern exerts a protective effect against osteoporosis beyond that of individual nutrients. Thus, our findings could be more appropriately explained by cumulative or synergistic effects of all bone-beneficial characteristics of DASH diet mentioned above.
The results of this study should be interpreted with caution considering the following limitations. First, due to the cross-sectional design of the study, causality cannot be inferred between adherence to the DASH dietary pattern and postmenopausal osteoporosis risk. Second, our findings can only be generalized to postmenopausal women. Third, the sample size was relatively small and thus might have attenuated our ability to detect other potential significant associations (e.g., between adherence to the DASH pattern and risk of osteoporosis at femoral neck). Fourth, although BMD measurements at femoral neck and lumbar spine by DXA are of the most clinical significance to osteoporosis diagnosis and management [15], measurement of BMD at other important skeletal sites such as distal radius could have also provided us with valuable information. Fifth, even though our FFQ was a valid and reliable tool for dietary intake assessment of Iranians [16, 17], it is a well-established fact that errors are associated with FFQ [45]. For instance, despite being in accordance with the recommendations made in the validation studies of our FFQ [16, 17], the inclusion of FFQs with up to 40% blank items in the present study might be considered as a potential source of selection bias. Sixth, there is no clear consensus on how to construct an ideal DASH score (i.e., which dietary components to include, whether to consider food groups and/or nutrients, and how to allocate a weight to each dietary component). Seventh, although the analyses were adjusted for the majority of known confounding variables, the possibility of residual confounding bias due to unknown or unmeasured covariates (e.g., genetic determinants of bone density) [7] cannot be entirely ruled out.
In conclusion, findings of the present study suggest the inverse association of adherence to the DASH dietary pattern and osteoporosis risk in postmenopausal Iranian women. It will be interesting to see if our findings could be replicated by prospective studies of high methodological quality in different study populations around the world and also to find out if adherence to the DASH dietary pattern is associated with other markers of bone health such as bone turnover or bone fractures.
References
Black DM, Rosen CJ (2016) Clinical practice: postmenopausal osteoporosis. N Engl J Med 374:254–262
Fabiani R, Naldini G, Chiavarini M (2019) Dietary patterns in relation to low bone mineral density and fracture risk: a systematic review and meta-analysis. Adv Nutr 10:219–236
Dempster DW (2011) Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care 17(Suppl 6):S164–S169
Rathnayake H, Lekamwasam S, Wickramatilake C, Lenora J (2019) Trabecular bone score and bone mineral density reference data for women aged 20-70 years and the effect of local reference data on the prevalence of postmenopausal osteoporosis: a cross-sectional study from Sri Lanka. Arch Osteoporos 14:91. https://doi.org/10.1007/s11657-019-0640-z
Lima RM, de Oliveira RJ, Raposo R, Neri SGR, Gadelha AB (2019) Stages of sarcopenia, bone mineral density, and the prevalence of osteoporosis in older women. Arch Osteoporos 14:38. https://doi.org/10.1007/s11657-019-0591-4
Hemmati F, Sarokhani D, Sayehmiri K, Motadayen M (2018) Prevalence of osteoporosis in postmenopausal women in Iran: a systematic review and meta-analysis. Iran J Obstet Gynecol Infertil 21:90–102
McGuigan FE, Murray L, Gallagher A, Davey-Smith G, Neville CE, Van’t Hof R, Boreham C, Ralston SH (2002) Genetic and environmental determinants of peak bone mass in young men and women. J Bone Miner Res 17:1273–1279
Movassagh EZ, Vatanparast H (2017) Current evidence on the association of dietary patterns and bone health: a scoping review. Adv Nutr 8:1–16
Hu FB (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 13:3–9
Tucker KL (2010) Dietary patterns, approaches, and multicultural perspective. Appl Physiol Nutr Metab 35:211–218
Mayo Clinic Staff (2016) DASH diet: healthy eating to lower your blood pressure. https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/dash-diet/art-20048456. Accessed 10 Nov 2018
Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N (1997) A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 336:1117–1124
Doyle L, Cashman KD (2004) The DASH diet may have beneficial effects on bone health. Nutr Rev 62:215–220
Monjardino T, Lucas R, Ramos E, Barros H (2014) Associations between a priori-defined dietary patterns and longitudinal changes in bone mineral density in adolescents. Public Health Nutr 17:195–205
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ III, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42:467–475
Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F (2010) Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr 13:654–662
Esfahani FH, Asghari G, Mirmiran P, Azizi F (2010) Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J Epidemiol 20:150–158
Ghaffarpour M, Houshiar-Rad A, Kianfar H (1999) The manual for household measures, cooking yields factors, and edible portion of foods. Agriculture Sciences Press, Tehran
Willett W, Stampfer MJ (1986) Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 124:17–27
Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB (2008) Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 168:713–720
Perez-Cornago A, Sanchez-Villegas A, Bes-Rastrollo M, Gea A, Molero P, Lahortiga-Ramos F, Martinez-Gonzalez MÁ (2017) Relationship between adherence to dietary approaches to stop hypertension (DASH) diet indices and incidence of depression during up to 8 years of follow-up. Public Health Nutr 20:2383–2392
Tian L, Yu X (2017) Fat, sugar, and bone health: a complex relationship. Nutrients 9:506. https://doi.org/10.3390/nu9050506
Centers for Disease Control and Prevention (2007) National Health and Nutrition Examination Survey (NHANES) Anthropometry Procedures Manual. CDC, Atlanta
Kelishadi R, Rabiee K, Khosravi A, Famouri F, Sadeghi M, Roohafza H, Sh S (2001) Assessment of physical activity in adolescents of Isfahan. J Shahrekord Univ Med Sci 3:55–66
Aadahl M, Jorgensen T (2003) Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc 35:1196–1202
Osterhoff G, Morgan EF, Shefelbine SJ, Karim L, McNamara LM, Augat P (2016) Bone mechanical properties and changes with osteoporosis. Injury 47(Suppl 2):S11–S20
Ott SM (2018) Cortical or trabecular bone: what’s the difference? Am J Nephrol 47:373–375
Shariati-Bafghi SE, Nosrat-Mirshekarlou E, Karamati M, Rashidkhani B (2014) Higher dietary acidity is associated with lower bone mineral density in postmenopausal Iranian women, independent of dietary calcium intake. Int J Vitam Nutr Res 84:206–217
Doyle L, Cashman KD (2003) The effect of nutrient profiles of the dietary approaches to stop hypertension (DASH) diets on blood pressure and bone metabolism and composition in normotensive and hypertensive rats. Br J Nutr 89:713–724
Lin PH, Ginty F, Appel LJ, Aickin M, Bohannon A, Garnero P, Barclay D, Svetkey LP (2003) The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr 133:3130–3136
Nowson CA, Patchett A, Wattanapenpaiboon N (2009) The effects of a low-sodium base-producing diet including red meat compared with a high-carbohydrate, low-fat diet on bone turnover markers in women aged 45-75 years. Br J Nutr 102:1161–1170
Tucker KL, Hannan MT, Kiel DP (2001) The acid-base hypothesis: diet and bone in the Framingham Osteoporosis Study. Eur J Nutr 40:231–237
Bushinsky DA (1996) Metabolic alkalosis decreases bone calcium efflux by suppressing osteoclasts and stimulating osteoblasts. Am J Phys 271:F216–F222
Zhou Q, Zhu L, Zhang D, Li N, Li Q, Dai P, Mao Y, Li X, Ma J, Huang S (2016) Oxidative stress-related biomarkers in postmenopausal osteoporosis: a systematic review and meta-analyses. Dis Markers 2016:7067984–7067912. https://doi.org/10.1155/2016/7067984
Mundy GR (2007) Osteoporosis and inflammation. Nutr Rev 65:S147–S151
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR (2014) Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 17:1689–1696
Shivappa N, Hebert JR, Karamati M, Shariati-Bafghi SE, Rashidkhani B (2016) Increased inflammatory potential of diet is associated with bone mineral density among postmenopausal women in Iran. Eur J Nutr 55:561–568
Sahni S, Mangano KM, McLean RR, Hannan MT, Kiel DP (2015) Dietary approaches for bone health: lessons from the Framingham Osteoporosis Study. Curr Osteoporos Rep 13:245–255
Shi Y, Zhan Y, Chen Y, Jiang Y (2020) Effects of dairy products on bone mineral density in healthy postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Arch Osteoporos 15:48. https://doi.org/10.1007/s11657-020-0694-y
Johansson J, Hult A, Morseth B, Nordstrom A, Nordstrom P (2018) Self-reported protein intake and properties of bone in community-dwelling older individuals. Arch Osteoporos 13:10. https://doi.org/10.1007/s11657-018-0421-0
Heaney RP (2006) Role of dietary sodium in osteoporosis. J Am Coll Nutr 25:271S–276S
Corwin RL, Hartman TJ, Maczuga SA, Graubard BI (2006) Dietary saturated fat intake is inversely associated with bone density in humans: analysis of NHANES III. J Nutr 136:159–165
Cao JJ (2011) Effects of obesity on bone metabolism. J Orthop Surg Res 6:30. https://doi.org/10.1186/1749-799X-6-30
You L, Z-y S, C-l T, Chen L, Pan L, J-y C (2011) High cholesterol diet increases osteoporosis risk via inhibiting bone formation in rats. Acta Pharmacol Sin 32:1498–1504
Livingstone M (1995) Assessment of food intakes: are we measuring what people eat? Br J Biomed Sci 52:58–67
Funding
This study was funded by the National Nutrition and Food Technology Research Institute (WHO Collaborating Center), Shahid Beheshti University of Medical Sciences, Tehran, Iran (grant number P/25/47/5421).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
The protocol of this study was approved by the research ethics committee of the National Nutrition and Food Technology Research Institute (WHO Collaborating Center), Shahid Beheshti University of Medical Sciences, Tehran, Iran (ethics approval number P/25/47/5421). All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Written informed consents were obtained from all individual participants prior to their inclusion in this study.
Consent for publication
The authors certify that the consent for publication of this work has been obtained by all co-authors, as well as by the responsible authorities at the institute where the work has been carried out.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahriarpour, Z., Nasrabadi, B., Shariati-Bafghi, SE. et al. Adherence to the dietary approaches to stop hypertension (DASH) dietary pattern and osteoporosis risk in postmenopausal Iranian women. Osteoporos Int 31, 2179–2188 (2020). https://doi.org/10.1007/s00198-020-05450-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05450-9