Abstract
Summary
This paper describes age-specific BMD and TBS data of Sri Lankan women aged 20–70 years. No significant change of TBS and BMDs were seen between 20 and 50 years but a rapid decline was seen between 50 and 70 years. Prevalence of osteoporosis showed a marked difference when local reference data were used instead of manufacture provided data.
Introduction
It is recommended that country-specific reference data are used when estimating diagnostic and therapeutic thresholds in osteoporosis. This study estimated normative BMD and TBS reference data for women aged 20–70 in Sri Lanka and the effect of local reference data on the diagnosis of osteoporosis among postmenopausal women.
Methodology
A group of healthy community-dwelling women (n = 355) aged 20–70 was recruited from Galle district in the Southern province in Sri Lanka using stratified random sampling method. They underwent DXA adhering to the manufacturer’s protocol and regional BMDs and TBS of the lumbar spine were measured.
Results
The highest mean BMD in the spine (0.928 g/cm2) was seen in 20–29 age group while there was a delay in achieving the peak BMD in the femoral neck (0.818 g/cm2) and total hip (0.962 g/cm2) regions(40–49 years). BMDs showed only a mild change between 20 and 49 years but a rapid decline was seen after 50 years (spine 0.013, femoral neck 0.012, and total hip 0.011 g/cm2 per year). The highest TBS was seen in 20–29 age group (1.371) and TBS trend with age was parallel to spine BMD. When the reference data provided by the manufacturer was used, 37% of postmenopausal women were found to have osteoporosis but this value changed to 17.6% when the local reference data were used.
Conclusion
We found a significant difference in the prevalence of osteoporosis when the local reference values were used instead of data provided by the manufacturer. However, representative data from more centers and fracture data are required before a recommendation to use local instead of international reference data can be stated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone mineral density (BMD), the most quantifiable risk factor of fragility fracture, is widely used in the diagnosis, risk stratification, and monitoring treatment in osteoporosis [1]. BMD in a given population is determined by many factors including ethnicity and environment. BMD varies between countries [2, 3] and different ethnicities within the same country [4]. While Kaptoge et al. demonstrated a significant variation of BMD in the European region, separate reference values are given for non-Hispanic black, non-Hispanic whites, and African Americans in the USA [4]. Hence, there are no universal BMD reference values available [5].
Prevalence of osteoporosis in a given population is determined by the reference data used to analyze BMD. In a study in Southern England, when manufacturer’s reference data were used, 5.8% had osteoporosis and this figure changed to 14.8% when the local reference data were used [6]. In Korea, among women of 80s, 17% difference in the prevalence of osteoporosis was seen when local reference data was used instead of the manufacturer’s reference data [7]. Some consider that the use of local reference data instead of the reference data provided by the manufacturer gives an accurate estimate of osteoporosis prevalence [5]. Although many developed countries have made country-specific BMD reference data, this is a daunting task for countries with limited resources. Some Asian counties such as Bangladesh [7], Hong Kong [8], and Korea [9] have followed this recommendation and developed their own reference data. Sri Lanka, however, despite the availability of central DXA technology almost for two decades still lacks country-specific reference data.
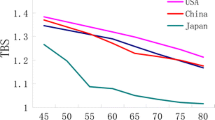
Trabecular bone score (TBS) is a measure of bone trabecular microarchitecture assessed by textural analysis of DXA images using variogram principle. It is used to adjust the conventional FRAX® output further by incorporating the textural quality of trabecular bone tissue [10]. Low TBS is linked with increase in both prevalent and incident fractures, independent of clinical risk factors and areal BMD [11]. Further, TBS is predictive of fracture, independent of fracture probabilities estimated using FRAX® algorithm [12]. TBS is not widely used especially in Asian countries mainly due to cost constrains. Studies show that, especially in women, age-related trends in TBS are parallel to those of spine BMD reflecting deterioration of bone microarchitecture with age and menopause [13]. Studies on TBS in the South Asian region are sparse as the technology is relatively new and expensive. We were unable to find TBS reference data from South Asian populations published previously.
The aim of this study was to develop TBS and regional BMD reference data using a representative sample of women aged 20–70 years. The study was conducted in the Southern province since the region has socio-economic indices, ethnic composition, and disease pattern comparable with the entire country.
Methods
Study design and participants
This was a cross-sectional study conducted in 2017–2018. The research protocol was approved by the Ethical Review Committee of the Faculty of Medicine, University of Ruhuna, Sri Lanka (Ref No 09.03.2016: 3.17). All participants were provided with full information about the study purpose and written informed consent was obtained prior to data collection.
We used stratified random sampling technique for identifying potential participants. The latest electoral registers were used to identify women who were in the age range of 20 to 70 in the region. We approached Grama Niladhari divisions (the smallest administrative unit of the country) to obtain the lists of members, and then individuals were randomly selected and included in five subgroups; 20–29 years, 30–39 years, 40–49years, 50–59 years, and 60–70 years (a minimum of 50 subjects in each category).
Measurements and data collection
The data collection included an interviewer-administered questionnaire and a brief clinical examination. Apart from clinical data, reproductive history (i.e., parity, age of menarche, and age of menopause), medical history (i.e., previous fracture, previous and current major diseases), and drug history were obtained.
Participants were excluded from the study if they had diseases which could affect bone metabolism such as hyperthyroidism, hyperparathyroidism, renal failure, malabsorption, alcohol dependence, chronic inflammatory diseases, or active malignancy or were on medications that could affect bone metabolism (glucocorticoids, hormonal contraceptives, thyroxine, thiazide diuretics, pharmacological doses of vitamin D or A). However, women with non-communicable diseases such as hypertension, diabetes, hyperlipidaemia, or myocardial ischemia were not excluded. BMDs of the lumbar spine (L1–L4) and proximal femur (non-dominant side) were measured with Dual Energy X-ray Absorptiometry (DXA) scanner (Hologic Discovery, Bedford, MA, USA) adhering to the manufacturer’s protocols. Daily in vitro calibration of the DXA machine, quality control of data, and data analyses were performed by a trained technical officer. In vivo precision error of the machine has been published previously [14]. Body weight was measured on an electronic scale and standing height was measured on a portable stadiometer with mandible plane parallel to the floor.
We measured the BMDs of the total spine (L1to L4), femoral neck, and total hip in all subjects and in addition, TBS values of the spine (L1 to L4) were measured adhering to the manufacturer’s protocols (TBS iNsight®). One technician performed all DXA scans and analyzed all scans to avoid inter-personal variability.
Statistics
To estimate reference data, BMD and TBS were expressed as mean (SD) for 10-year age categories after checking for the distribution of data. To observe age-related trends in the BMDs and TBS values, scatter plots with Locally Weighted Scatterplot Smoothing lines (Loess) were fitted. To assess the effect of height, weight, and age on BMD and TBS, Pearson correlations (r) and linear regression analyses were used. The reference data provided by the DXA manufacturer and those observed in this study were compared and the prevalence of osteoporosis among the postmenopausal women based on the two reference datasets was also determined.
Results
Mean weight, height, and BMI of study subjects were 55.9 (10.6) kg, 1.52 (0.05) m, and 24.4(4.5) kg/m2. None of them had ever smoked and none were current alcohol users.
All regional BMDs showed positive correlations with weight (r = 0.28 to 0.48, p < 0.001 for all) and height (r = 0.17 to 0.33, p < 0.001 for all). TBS showed positive correlations with spine BMD (r = 0.62, p < 0.001) and height (r = 0.38, p < 0.01) but not with weight. The highest mean spine BMD and TBS were seen in women aged 20–29 and both measures gradually declined afterwards (Table 1 and Figs. 1, 2, 3, and 4). The highest mean BMDs in the femoral neck and total hip, however, were seen in women aged 40–49. Women aged 20–29 had reached 93% of the maximum BMD both at the femoral neck and the total hip regions.
Spine BMD did not change significantly between 20 and 49 years but there was a rapid decline (0.013 g/cm2 or 1.4% per year) after 50 years. Femoral neck BMD declined at the rate of 0.012 g/cm2 or 1.6% per year after 50 years and the corresponding figures for the total hip BMD were 0.011 g/cm2 or 1.2% per year. Mean femoral neck BMDs of women aged 30–39 was higher compared with those aged 20–29 (difference of 0.055 g/cm2, p = 0.006). Similarly mean total hip BMDs of women aged 30–39 was higher compared with those aged 20–29 (difference of 0.064 g/cm2, p = 0.003).
We considered BMDs and TBS values observed in women aged 20–29 as the reference values. A significant difference was found in the comparison of Asian reference data provided by the Hologic manufacturer and the reference data found in this study (Table 2). Furthermore, a significant variation was found in the prevalence of osteoporosis among postmenopausal women in the study group when the two reference datasets were used. While 37% of postmenopausal women were detected to have osteoporosis (T score equal or lower than − 2.5 in the spine, femoral neck, or total hip) based on the manufacturer’s reference data, only 17.6% qualified for the diagnosis of osteoporosis when the local reference data were used.
Discussion
In this study, we report age-specific TBS and regional BMD data for women aged 20–70 years in Sri Lanka. We were unable to find previous local data; hence, we consider these are the first age-specific BMD and TBS data published for women aged 20–70 years in Sri Lanka. The age trends of spine BMD and TBS were somewhat parallel and both remained unchanged until 49 years and started declining after 50 years. BMDs of proximal femur sites, however, showed a significant increase between 20 and 49 years (7% from 20–29 age category). BMDs at all three sites started declining after 50 years almost at the same rate. Asian BMD reference values provided by the manufacturer were lower than values we observed in this analysis. This led to a marked difference in osteoporosis prevalence among postmenopausal women in our study group.
Our observations are broadly concordant with observations made in previous studies on this subject. Age-related decline of BMD, especially after menopause, is a universal phenomenon and considered to be the main reason for the increased fracture risk seen in women in old age. Low estrogen in postmenopausal period unbalances bone remodeling cycle leading to a more bone resorption than formation resulting a net BMD loss [1]. Studies have shown similar changes in TBS indicating deterioration of bone microarchitecture with advancing age especially following menopause in women [13].
In previous studies, the use of local reference data instead of data provided by the manufacturer led to a wide discrepancy in the prevalence of osteoporosis [6, 9, 15]. We found nearly 2-fold difference (37% vs 17.8%) in the prevalence of osteoporosis and our observations are concordant with previous studies such as Kudlacek et al. [16] who reported 4–9-fold increase in osteoporosis prevalence among women and Lee et al. who reported change of osteoporosis prevalence from 12.2 to 78% [9].
We observed that BMDs in different skeletal sites do not peak together and some are delayed. Although spine BMD reached the peak value around 20–29 years, there was a continuous increase of BMDs in the proximal femur until 40–49 years. The variation between 20–29 years and 40–49 years was only 7% and women in 20–29 age category had gained 93% of peak bone mass (PBM). According to previous data, there is a discrepancy in timing of PBM in different skeletal sites and ethnic groups. Although studies from the USA have consistently shown that PBM is achieved between 20 and 30 years in the spine and hip region [4], studies from some countries have shown a delay in achieving PBM in certain skeletal sites. A previous study in Sri Lanka demonstrated that phalangeal PBM was delayed and achieved only between 30 and 40 years [17]. Furthermore, Ghannam et al. showed that Saudi women reach spine PBM around 35 years but earlier in the proximal femur [18]. In Turkish women, peak spine BMD was seen between 30 and 35 years [19] and in Chinese, peak BMD in the forearm bones was delayed until 40–44 years. [20]. We observed 7% higher proximal femur BMDs in women aged 30–39 compared with women aged 20–29. This observation is congruent with 6.8% median gain of spine BMD and 12.5% total body BMD seen among women in 3rd decade reported by Recker et al. [21].
The exact reasons for the disparity in the timing of PBM are unclear. PBM is influenced by endogenous factors (genetic composition and hormones) and exogenous factors (physical activity, nutrition, and muscle action). It is believed that change in skeletal morphology is a continuous process but slower after puberty. Changes in bone morphology after puberty are largely due to bone remodeling where bone slowly expands due to periosteal bone apposition which exceeds endosteal bone resorption leading to accumulation of more bone material. This process is influenced by many factors which can vary, regionally and individually [22, 23].
Due to the inconsistency in age of achieving the PBM, it is recommended that age group 20–30 should be considered the young normal reference population in calculating BMD T-scores [24]. Many studies [6, 9, 15] have followed this recommendation and we also considered BMD in this age group in calculating T-scores of postmenopausal women in our study.
The International Society of Clinical Densitometrists (ISCD) while recommending Caucasian (non-race adjusted) female normative reference data for the calculation of T-scores for all ethnicities advocates the use of local reference data when appropriate [https://www.iscd.org/official-positions/2015-iscd-official-positions-adult/]. Local reference data, however, should be concordant with fracture data of the same community to ensure accurate estimation of fracture risk. Using the reference values observed in this study, especially in the total spine, would lead to a lower prevalence of osteoporosis and whether this is an accurate reflection of fracture risk in the community needs to be confirmed with fragility fracture data. This is currently not possible due to the lack of fracture data in the country. An ongoing study in the Southern province in Sri Lanka indicates a low incidence of hip fracture when compared with most parts of the world (unpublished data) but more studies are needed to ensure that local reference data provide an accurate estimation of fracture risk in the community without causing over-treatment or under-treatment. Furthermore, as per the recommendations made by the ISCD, our data would be more suitable for the calculation of Z-scores in this community [25].
This study has a few strengths and weaknesses. The study sample was selected from community-dwelling women in random manner. We observed that participants had never smoked and were not current users of alcohol. Smoking and consumption of alcohol among women in Sri Lanka are negligible. Only those with diseases or on medications that could have affected BMD were excluded. Women with other diseases were not excluded and 95% participants initially invited participated in the study. The small proportion of non-participants was not systematically different from participants with regard to age, ethnicity, and area of residence. This makes the participants representative of the general population. Furthermore, all subjects were long-term residents of Galle district. According to the data from the Department of Census and Statistics, Sri Lanka (www.statistics.gov.lk), the study area has socio-economic indices comparable to the entire Sri Lanka. Poverty (proportion of people below the national poverty line), crude mortality, infant mortality, literacy, life expectancy at birth, and ethnic composition of the area are comparable with national values and hence findings can be generalized to the rest of the country. We used central-type DXA in measuring BMD and TBS with daily in vitro calibrations. All measurements were done by a single technician with nearly 10 years in DXA measurements. We, however, encourage more studies with larger samples from other regions of the country to verify our findings.
Conclusions
We found a significant difference in the prevalence of osteoporosis when the local reference values were used instead of data provided by the manufacturer. However, representative data from more centers are required before a recommendation to use local instead of international reference data can be stated. Furthermore, age-specific BMD values we observed should be concordant with fracture data in the same community to ensure their validity as local reference data.
References
Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 1994, 843:1–129
Kaptoge S, da Silva JA, Brixen K, Reid DM, Kroger H, Nielsen TL, Andersen M, Hagen C, Lorenc R, Boonen S et al (2008) Geographical variation in DXA bone mineral density in young European men and women. Results from the Network in Europe on Male Osteoporosis (NEMO) study. Bone 43:332–339
Nam HS, Kweon SS, Choi JS, Zmuda JM, Leung PC, Lui LY, Hill DD, Patrick AL, Cauley JA (2013) Racial/ethnic differences in bone mineral density among older women. J Bone Miner Metab 31:190–198
Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC Jr, Lindsay R (1998) Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8:468–489
Majumdar SR, Leslie WD (2013) Of fracture thresholds and bone mineral density reference data: does one size really fit all? J Korean Med Sci 16:543–548
Petley GW, Cotton AM, Murrills AJ, Taylor PA, Cooper C, Cawley MI, Wilkin TJ (1996) Reference ranges of bone mineral density for women in southern England: the impact of local data on the diagnosis of osteoporosis. Br J Radiol 69:655–660
Begum RA, Ali L, Takahashi O, Fukui T, Rahman M (2015) Bone mineral density: reference values and correlates for Bangladeshi women aged 16-65 years. J Orthop Sci 20:522–528
Leung KS, Lee KM, Cheung WH, Ng ES, Qin L (2004) Characteristics of long bone DXA reference data in Hong Kong Chinese. J Clin Densitom 7:192–200
Lee KS, Bae SH: New reference data on bone mineral density and the prevalence of osteoporosis in Korean adults aged 50 years or older: the Korea National Health and Nutrition Examination Survey 2008–2010. 2014, 29:1514–1522
Harvey NC, Gluer CC, Binkley N, McCloskey EV, Brandi ML, Cooper C, Kendler D, Lamy O, Laslop A, Camargos BM et al (2015) Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 78:216–224
Iki M, Tamaki J, Kadowaki E, Sato Y, Dongmei N, Winzenrieth R, Kagamimori S, Kagawa Y, Yoneshima H (2014) Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese Population-Based Osteoporosis (JPOS) cohort study. J Bone Miner Res 29:399–407
Tamaki J, Iki M, Sato Y, Winzenrieth R, Kajita E, Kagamimori S, Group JS (2018) Does Trabecular Bone Score (TBS) improve the predictive ability of FRAX((R)) for major osteoporotic fractures according to the Japanese Population-Based Osteoporosis (JPOS) cohort study? J Bone Miner Metab
Iki M, Tamaki J, Sato Y, Winzenrieth R, Kagamimori S, Kagawa Y, Yoneshima H (2015) Age-related normative values of trabecular bone score (TBS) for Japanese women: the Japanese Population-based Osteoporosis (JPOS) study. Osteoporos Int 26:245–252
Lekamwasam S, Rodrigo M, Arachchi WK, Munidasa D (2007) Measurement of spinal bone mineral density on a Hologic Discovery DXA scanner with and without leg elevation. J Clin Densitom 10:170–173
Lee S, Choi MG, Yu J, Ryu OH, Yoo HJ, Ihm SH, Kim DM, Hong EG, Park K, Choi M, Choi H (2015) The effects of the Korean reference value on the prevalence of osteoporosis and the prediction of fracture risk. BMC Musculoskelet Disord 16:69
Kudlacek S, Schneider B, Peterlik M, Leb G, Klaushofer K, Weber K, Woloszczuk W, Willvonseder R (2003) Normative data of bone mineral density in an unselected adult Austrian population. Eur J Clin Investig 33:332–339
Lekamwasam S, Wijerathne L, Rodrigo M, Hewage U (2009) Age-related trends in phalangeal bone mineral density in Sri Lankan men and women aged 20 years or more. J Clin Densitom 12:58–62
Ghannam NN, Hammami MM, Bakheet SM, Khan BA (1999) Bone mineral density of the spine and femur in healthy Saudi females: relation to vitamin D status, pregnancy, and lactation. Calcif Tissue Int 65:23–28
Hadjidakis D, Kokkinakis E, Giannopoulos G, Merakos G, Raptis SA (1997) Bone mineral density of vertebrae, proximal femur and os calcis in normal Greek subjects as assessed by dual-energy X-ray absorptiometry: comparison with other populations. Eur J Clin Investig 27:219–227
Liao EY, Wu XP, Deng XG, Huang G, Zhu XP, Long ZF, Wang WB, Tang WL, Zhang H (2002) Age-related bone mineral density, accumulated bone loss rate and prevalence of osteoporosis at multiple skeletal sites in chinese women. Osteoporos Int 13:669–676
Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB (1992) Bone gain in young adult women. JAMA 268:2403–2408
Jepsen KJ, Andarawis-Puri N (2012) The amount of periosteal apposition required to maintain bone strength during aging depends on adult bone morphology and tissue-modulus degradation rate. J Bone Miner Res 27:1916–1926
Lazenby RA (1990) Continuing periosteal apposition. II: the significance of peak bone mass, strain equilibrium, and age-related activity differentials for mechanical compensation in human tubular bones. Am J Phys Anthropol 82:473–484
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42:467–475
Funding
This study was funded by a research grant from the University of Ruhuna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The research protocol was approved by the Ethical Review Committee of the Faculty of Medicine, University of Ruhuna, Sri Lanka (Ref No 09.03.2016:3.17). All participants were provided with full information about the study purpose and written informed consent was obtained prior to data collection.
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rathnayake, H., Lekamwasam, S., Wickramatilake, C. et al. Trabecular bone score and bone mineral density reference data for women aged 20–70 years and the effect of local reference data on the prevalence of postmenopausal osteoporosis: a cross-sectional study from Sri Lanka. Arch Osteoporos 14, 91 (2019). https://doi.org/10.1007/s11657-019-0640-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-019-0640-z