Abstract
Summary
Bone mineral density (BMD) of offspring was significantly associated with their parents’ BMD. Parental BMD Z-score ≤−1 was a significant predictor for BMD Z-score ≤−1 in their offspring. Peak bone mass acquisition during early adulthood is more substantially influenced by genetic factors rather than lifestyle or environmental factors.
Introduction
A person’s BMD is affected by both genetic and environmental factors. Family history of osteoporosis or fragility fracture is a well-known risk factor for low bone mass or fracture. The purpose of the present study was to investigate the familial association of BMD between parents and offspring in Korean population.
Methods
This is a cross-sectional study based on the data from the Korea National Health and Nutrition Examination Surveys (KNHANES) conducted from 2008 to 2011. A total of 5947 subjects (3135 parents and 2812 offspring) were included.
Results
In age-adjusted partial correlation analyses, all BMD values acquired from the lumbar spine, femur neck, total hip, and whole body showed significant associations between parents and offspring. Among these associations, whole-body BMD showed the strongest relationship between offspring and parents. The narrow-sense heritability of BMD ranged from 0.203 to 0.542 in male offspring and from 0.396 to 0.689 in female offspring. Multiple linear regression analyses showed that offspring’s BMD was independently associated with BMD of both parents after adjusting for covariates. Lifestyle or environmental factors including dietary calcium intake, serum 25-hydroxyvitamin D level, regular exercise, current smoking, and alcohol intake showed only moderate or no associations with BMD. In multiple logistic regression analyses in offspring aged 19–25 years, the son’s risk of having BMD Z-score ≤−1 was associated with both parents’ BMD Z-score ≤−1, while the daughter’s risk was only associated with maternal BMD Z-score ≤−1.
Conclusions
Our findings confirm the strong familial association of BMD between parents and offspring in Korean population and suggest that peak bone mass acquisition during early adulthood is more substantially influenced by genetic factors rather than lifestyle or environmental factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic skeletal disorder characterized by decreased bone strength, which leads to an increased risk of fracture. Osteoporosis-related fractures can cause significant morbidity and disability, which reduces the quality of life and can even lead to death [1, 2]. Asia is expected to be seriously affected by osteoporosis-related fractures in the near future with a progressive increase in the elderly population. It is estimated that 50 % of all osteoporotic fractures will occur in Asia by 2050 [3]. The residual lifetime risk of osteoporosis-related fractures for Koreans ≥50 years of age was 23.8 % for men and 59.5 % for women [4, 5].
Osteoporosis is clinically diagnosed based on the measurement of bone mineral density (BMD). People usually reach their peak BMD, or peak bone mass, by their early 20s and subsequently lose bone mass with aging, especially after menopause in women. The risk of developing osteoporosis is in large part determined by the peak bone mass attained in early adulthood [6, 7]. Therefore, identifying factors involved in acquisition of peak bone mass is necessary for effective prevention against osteoporosis in later life. Both genetic and environmental factors likely contribute significantly to the determination of peak bone mass, although the relative contributions of these factors are incompletely understood [6]. The importance of genetic factors has been demonstrated by family studies, which showed that 50–85 % of the variance in BMD is genetically determined [8–16]. Aside from these genetic factors, lifestyle or environmental components including nutrition and physical activity have also been shown to affect the peak bone mass acquisition [17–22].
Although a number of family studies have demonstrated the heritability of BMD, most of them were conducted using small sample sizes. Also, many of those studies have been conducted in Caucasian populations. The heritability or genetic effects in BMD may vary between different ethnic groups given the varying risks of osteoporosis between different ethnic populations [23]. Therefore, more data from non-Caucasian populations will help us understand the ethnic differences in the heritability or genetic effects in BMD. Although there have been some Asian studies that demonstrated the familial association of BMD [14–16, 24, 25], only a few of them were conducted in the population of large sample size. The purposes of the present study were to investigate the familial association of BMD between parents and offspring and evaluate the relative contribution of genetic and environmental factors to the bone mass of adolescent and young adult offspring based on a large database from the Korea National Health and Nutrition Examination Surveys (KNHANES) conducted from 2008 to 2011.
Subjects and methods
Study participants
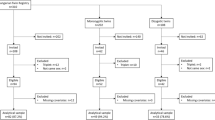
This study is based on the data acquired from the KNHANES conducted from 2008 to 2011. The KNHANES is a community-based cross-sectional survey that has been conducted by the Division of Chronic Disease Surveillance, Korea Centers for Disease Control and Prevention, to assess the health and nutritional status of the civilian non-institutionalized population of South Korea. The sampling frame was based on the 2005 population and housing census of Korea. The sampling units were randomly selected. In 2008, there were 200 randomly selected sampling units with 23 households in each unit (yielding 4600 households). In 2009–2011, there were 192 randomly selected sampling units with 20 households in each unit (yielding 3840 households). For each year, the sampling units were newly selected and did not overlap with previous samples. From 2008 to 2011, a total of 46,777 individuals were sampled, and a total of 37,753 individuals (9744 in 2008; 10,533 in 2009; 8958 in 2010; and 8518 in 2011) participated in the survey. Among those participants, BMD measurements were obtained from 21,303 individuals (3583 in 2008, 7920 in 2009, 7043 in 2010, and 2757 in 2011) aged 10 years and older (to be more exact, BMD measurements were obtained from participants aged 19 years or older from July 2008 to May 2011 and from those aged 10–18 years from July 2009 to May 2011). Among those with BMD data, offspring aged 10–25 years (2832) and their parents (3162) were included in the present study. After excluding those (and their family members) with paralysis due to stroke (eight parents and six offspring), renal failure (13 parents and eight offspring), and liver cirrhosis (six parents and six offspring), a total of 1904 families with 5947 individuals (3135 parents and 2812 offspring) were finally included in the current analyses (Supplemental Fig. 1). All the participants in this survey signed an informed consent form.
Data collection
The overall survey consists of a health interview survey, a nutrition survey, and a health examination survey. The survey collected data via household interviews and by direct standardized physical examinations conducted in specially equipped mobile examination centers. Demographic, anthropometric, and behavioral characteristics were measured. Height and weight were measured, while subjects wore light clothing using standardized techniques and equipment. Body mass index (BMI) was calculated by dividing weight by the square of height (kg/m2). Physical activity was measured by self-report using the International Physical Activity Questionnaire in subjects ≥12 years of age. Subjects were questioned about whether they exercised at an intensity that left them sweating or with slight difficulty in breathing. Subjects who exercised regularly were asked about the weekly frequency of exercise and the duration of the session. Data on smoking and alcohol drinking were collected among subjects ≥19 years of age. Smoking status was divided into three categories: current smoking, ex-smoking, and nonsmoking. Alcohol drinking was assessed by questioning the subjects about their drinking behavior during the year before the interview. Participants’ dietary intakes including total calorie and calcium were assessed through a 24-h recall interview. For biochemical analysis, blood samples were collected during the survey. Blood samples were properly processed, immediately refrigerated, and transported in cold storage to the central testing institute (Neodin Medical Institute, Seoul, Korea). Blood samples were analyzed within 24 h after transportation. Serum 25-hydroxyvitamin D [25(OH)D] levels were measured using a 1470 Wizard gamma counter (PerkinElmer, Turku, Finland) and by radioimmunoassay (DiaSorin, Stillwater, MN, USA). The intra- and inter-assay coefficient of variation was 2.9∼5.5 and 6.3∼12.9 %, respectively.
BMD measurement
BMD of total, axial, and peripheral skeleton was measured using a Discovery QDR4500W dual-energy X-ray absorptiometry device (Hologic Inc., Bedford, MA, USA) located in mobile examination centers. Before the measurement, a quality control was performed using the phantom provided by the manufacturer. To assess the precision of the device, the BMD of 30 randomly selected subjects was measured twice to determine the minimum tolerance (lumbar spine 1.9 %, femoral neck 2.5 %, total hip 1.8 %). The BMD values measured in parents and offspring aged 19–25 years were converted into the Z-score using the Asian reference provided by the manufacturer.
Statistical analyses
Statistical analyses were carried out using SPSS version 18.0 for Windows (SPSS, Chicago, IL, USA). Demographic characteristics and BMD of parents and offspring were analyzed. Offspring were divided into two groups according to their age: 10–18 and 19–25 years. Dietary calcium intake (mg/1000 kcal/day) of participants was calculated as the daily amount of dietary calcium intake adjusted by energy intake. Regular exercise was indicated as “yes” when the subject regularly did moderate exercise (>30 min per session and more than five times per week, involving activities like slow swimming, doubles tennis, volleyball, badminton, table tennis, and carrying light weights) or strenuous exercise (>20 min per session and more than three times a week, involving activities like running, climbing, fast cycling, fast swimming, football, basketball, jump rope, squash, tennis singles, and carrying heavy weights). Current smoking was indicated as yes for subjects who currently smoked cigarettes. Alcohol drinking was indicated as yes for subjects who consumed at least one glass of alcohol every month over the last year. BMD measured at the lumbar spine (L1–L4), femur neck, total hip, and whole body was used for the statistical analyses. To evaluate the association between BMDs of parents and offspring adjusted for their respective age, partial correlation analyses were performed by calculating Pearson’s correlation coefficients of residuals from two simple linear regressions of BMD on age for parents and offspring separately. Narrow-sense heritability, defined by the ratio of the additive genetic variance over the phenotypic variance, was estimated by regressing offspring’s BMD on the average BMD of their parents [26]. In addition to partial correlation analyses, multiple linear regression analyses were used to simultaneously evaluate the effects of explanatory variables on BMD in offspring with all the variables being standardized and BMD from parents as covariate being further age adjusted. Multiple logistic regression analyses were also conducted to quantify the effect of explanatory variables as risk factor on BMD, where a binary response variable took a value of one if any BMD Z-score from the lumbar spine, the femur neck, or the total hip was less than or equal to −1 and zero otherwise. Odds ratio (OR) and 95 % confidence interval (CI) were reported as a result of these analyses. All tests were two sided, and P < 0.05 was considered statistically significant.
Results
A total of 1904 families with 3135 parents and 2812 offspring were included in the final analyses. Among those families, 1228 families have both parents, 103 families have only father, and 576 families have only mother. The average age of parents was 47.9 ± 5.8 years for fathers and 44.7 ± 5.5 years for mothers. The average age of offspring was 16.5 ± 4.6 years for sons and 17.1 ± 4.7 years for daughters. Offspring were divided into two groups according to their age (10–18 and 19–25 years) for the purpose of statistical analyses. Other demographic characteristics and BMD are presented in Table 1.
Correlation of BMD between parents and offspring
Partial correlation analyses of BMD between parents and offspring adjusted for their respective age showed statistically significant associations for all four body sites (lumbar spine, femur neck, total hip, and whole body) considered in this study (Supplemental Table S1). Generally, BMD values measured at the same sites among parents and offspring showed higher partial correlation coefficients than those measured at the different sites with a few exceptions. Among these associations, whole-body BMD showed the strongest relationship between parents and offspring, with partial correlation coefficients ranging from 0.420 to 0.558. In addition, such positive correlations were also evident in estimates of the narrow-sense heritability (Table 2). For male offspring, the heritability estimates ranged from 0.203 to 0.542 over four body sites, while those for female offspring ranged from 0.396 to 0.689.
Predictors for BMD in offspring
Multiple linear regression analyses were conducted to find the predictors for BMD in offspring (Table 3). Age was a strong predictor for BMD measured at all sites in offspring aged 10–18 years, but only weak or no associations were found between age and BMD among offspring aged 19–25 years. BMI was a significant predictor for BMD in both offspring age groups. Concerning the relationship of BMD between parents and offspring, significant associations were evident for BMD measured at the same sites independent of other covariates. With regard to the lifestyle or environmental factors examined, there existed only moderate or no associations with BMD. Dietary calcium intake showed positive correlations with lumbar spine and whole-body BMD of daughters aged 10–18 years and with the lumbar spine BMD of sons aged 19–25 years. Serum 25(OH)D level was only associated with femur neck and total hip BMDs of daughters of both age groups. Among offspring aged 19–25 years, regular exercise was positively correlated with BMD measured at all sites in sons, but it was not in daughters. Moreover, current smoking and alcohol drinking showed no associations with offspring’s BMD.
Predictors for BMD Z-score ≤−1 in offspring aged 19–25 years
Among offspring aged 19–25 years (n = 453 for son, 585 for daughter) and their parents (n = 599 for father, 805 for mother), the number of participants with BMD Z-score ≤−1 at any site among the lumbar spine, the femur neck, or the total hip was 91 (15.2 %) for fathers, 168 (20.9 %) for mothers, 90 (19.9 %) for sons (19–25 years), and 162 (27.7 %) for daughters. Multiple logistic regression analyses were carried out to find the predictors for BMD Z-score ≤−1 (Fig. 1). BMI was associated with BMD Z-score ≤−1 in offspring with the OR (95 % CI) of 0.751 (0.644–0.876) and 0.768 (0.676–0.871) in sons and daughters, respectively. Among the lifestyle or environmental factors examined, however, only regular exercise was associated with low BMD with the OR (95 % CI) of 0.197 (0.064–0.610) in sons. Other lifestyle or environmental factors such as dietary calcium intake, serum 25(OH)D level, current smoking, and alcohol drinking did not show any associations with BMD Z-score ≤−1 in offspring aged 19–25 years. As for the relationship of BMD Z-score between parents and offspring, the son’s risk of having BMD Z-score ≤−1 was associated with paternal BMD Z-score ≤−1 with the OR (95 % CI) of 5.340 (2.068–13.789) and with maternal BMD Z-score ≤−1 with the OR (95 % CI) of 4.995 (2.046–12.193), while the daughter’s risk of having BMD Z-score ≤−1 was only associated with maternal BMD Z-score ≤−1 with the OR (95 % CI) of 5.289 (2.736–10.225). We also examined how much ORs of BMD Z-score ≤−1 in offspring increased when both parents had BMD Z-score ≤−1 compared with when either or neither parents had low BMD after adjusting for covariates including age, BMI, dietary calcium intake, serum 25(OH)D level, regular exercise, current smoking, and alcohol drinking (Fig. 2). When either father or mother had BMD Z-score ≤−1, the ORs (95 % CI) of BMD Z-score ≤−1 in sons increased to 8.733 (2.876–26.512) and 7.267 (2.663–19.832), respectively, while a further increase to 9.757 (1.561–60.990) was observed when both parents had BMD Z-score ≤−1 compared with when neither of their parents had BMD Z-score ≤−1. A litter different pattern was observed for daughters. The OR (95 % CI) of BMD Z-score ≤−1 in daughters increased to 4.844 (2.372–9.889) when only mother had BMD Z-score ≤−1 but showed no significant change when only father had BMD Z-score ≤−1. The OR (95 % CI) increased to 7.036 (1.581–31.307) when both parents had BMD Z-score ≤−1.
Odds ratios for BMD Z-score ≤−1 in offspring aged 19–25 years according to paternal and/or maternal BMD Z-score ≤−1. Asterisks: The lowest BMD Z-score measured at any site among the lumbar spine, the femur neck, and the total hip. BMI body mass index, 25(OH)D 25-hydroxyvitamin D, BMD bone mineral density. a P < 0.001; b P < 0.01; c P < 0.05
Discussion
The present study showed that the BMD values of offspring measured at any site among the lumbar spine, the femur neck, the total hip, or the whole body were significantly associated with parents’ BMD. After adjusting for covariates including age, BMI, and other lifestyle factors, both parental BMD remained significant factors to predict offspring’s BMD. Also, the risk of BMD Z-score ≤−1 among offspring 19–25 years of age was also associated with BMD Z-score ≤−1 of their parents. In contrast, lifestyle or environmental factors examined in this study mostly had no associations with BMD, with a few exceptions of moderate significance. These findings confirm the strong familial association of BMD between parents and offspring, and suggest that bone mass acquisition during adolescence and young adulthood is more substantially influenced by genetic factors rather than lifestyle or environmental factors.
Our results are in accordance with findings from other family studies from Western and Asian populations that showed strong familial aggregation of BMD [8–16]. This familial heritability of BMD consistently found in various ethnic groups suggests the existence of specific genes responsible for determination of BMD. For the last several years, there have been efforts to identify the genes that are associated with BMD or fragility of bone [27]. Recent genome-wide association studies have made great advances in identifying 62 genome-wide significant loci that are associated with low bone mass and osteoporotic fractures [28–30]. These findings from genetic studies suggest the highly polygenic and complex nature underlying BMD variation and the critical influences of several biologic pathways on the development of osteoporosis and fracture. In the present study, the narrow-sense heritability was estimated based on the phenotypic BMD values of offspring and parents, which is likely to be less accurate than those estimated by using genetic data from various study designs such as a twin study or a genome-wide association study. Nevertheless, the moderate-to-high heritability estimates (a range of 0.203 to 0.542 for male offspring and 0.396 to 0.689 for female offspring) also suggests that the variation in BMD can be accounted for by additive genetic components.
However, familial aggregation of BMD between parents and offspring can also be explained by factors other than genetic influences. Several lifestyle or behavioral habits known to affect bone health have been shown to aggregate in families, including milk consumption, physical activity, smoking, alcohol intake, and vitamin D status [31–33]. In the present study, statistical analyses were done to assess whether lifestyle factors, such as dietary calcium intake, serum 25(OH)D level, regular exercise, current smoking, and alcohol drinking, were correlated between parents and offspring (Supplemental Tables S2 and S3). Some parent–offspring correlations were revealed in the lifestyle factors, especially vitamin D status and alcohol drinking. This result suggests that same family members share some lifestyle or behavioral characteristics that may affect bone health. Therefore, we can speculate that common lifestyle or behavioral habits shared by family members also contribute to the strong familial association of BMD between parents and offspring observed in this study.
From the perspective of preventive strategies, identification of people at high risk for osteoporosis is an important issue. It is evident that insufficient acquisition of peak bone mass during early adulthood can lead to development of osteoporosis in later life [6]. Thus, nutritional or behavioral modifications to improve bone health need to be emphasized for young people with insufficient peak BMD. As our results suggest, insufficient acquisition of peak bone mass during early adulthood can be predicted by parents’ BMD. Therefore, young people whose parents have low BMD values should be the subject of early education for bone health in order to modify their lifestyle and behavior.
Although genetic factors primarily contribute to individual’s bone mass acquisition, lifestyle or environmental factors also play a role to some degree. Calcium intake is important in determining bone mass acquisition during the crucial years of rapid skeletal growth at younger ages [17–20]. Physical activity also positively influences the acquisition of peak bone mass [19–22]. Weight-bearing exercise, in particular, was found to be a significant positive contributor especially in males [22]. Presently, our study also showed that some associations were apparent between BMD and lifestyle factors, such as dietary calcium intake, vitamin D status, and regular exercise, although these components accounted for only a small proportion of BMD variance compared with parental BMD. Lifestyle or environmental factors may play a greater role in determining BMD with increasing age, while genetic influence on BMD may lessen in later life, particularly after menopause in women [34, 35]. Lifelong influences of lifestyle or environmental factors on bone health appear to dominate over genetic factors with age. Therefore, lifestyle modification should be emphasized to improve bone health throughout our lifetime.
The major strength of our study is its large sample size with both male and female subjects. In addition, adolescents aged 10–18 years as well as young adults aged 19–25 years were included, which allowed us to analyze the BMD of not only those with their peak bone mass but also those experiencing rapid bone growth. Furthermore, what makes this study more unique and valuable is that it revealed the relative contributions of paternal and maternal BMDs to their offspring’s BMD.
The present study also has some limitations. First, because it is a cross-sectional observation study, the association found in this study is not a proof of a causal relationship. Second, although we adjusted age, BMI, or several lifestyle factors in our statistical analyses, the familial associations found in this study might be confounded by other covariates not included in the analyses. In particular, familial aggregations of lifestyle factors such as dietary calcium intake, vitamin D status, and regular exercise might have been affected by the time of data collection. Third, the participants’ medical history was from self-reports. Also, a detailed history including the type of prescribed medications was not obtained in the survey, which made it difficult for us to clearly define the exclusion criteria. Therefore, we only excluded those who were thought to have a definite medical condition associated with abnormal bone metabolism depending on self-reports. Fourth, lifestyle or environmental factors such as smoking and alcohol drinking were not quantified or classified enough for the detailed statistical analyses.
In conclusion, our findings confirm the strong familial association of BMD between parents and offspring and showed that insufficient peak BMD in young adults can be predicted based on their parents’ BMD. Therefore, our study provides useful information about identification of young people at high risk for osteoporosis in later life, for whom we should provide early education to modify their lifestyle and behavior to improve bone health.
References
Cooper C (1997) The crippling consequences of fractures and their impact on quality of life. Am J Med 103:12S–17S
Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR (1999) Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 159:1215–1220
Cooper C, Campion G, Melton LJ 3rd (1992) Hip fractures in the elderly: a world-wide projection. Osteoporos Int 2:285–289
Lee J, Lee S, Jang S, Ryu OH (2013) Age-related changes in the prevalence of osteoporosis according to gender and skeletal site: the Korea National Health and Nutrition Examination Survey 2008-2010. Endocrinol Metab (Seoul) 28:180–191
Lee YK, Yoon BH, Koo KH (2013) Epidemiology of osteoporosis and osteoporotic fractures in South Korea. Endocrinol Metab (Seoul) 28:90–93
Kelly PJ, Eisman JA, Sambrook PN (1990) Interaction of genetic and environmental influences on peak bone density. Osteoporos Int 1:56–60
Bonjour JP, Theintz G, Buchs B, Slosman D, Rizzoli R (1991) Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab 73:555–563
Sigurdsson G, Halldorsson BV, Styrkarsdottir U, Kristjansson K, Stefansson K (2008) Impact of genetics on low bone mass in adults. J Bone Miner Res 23:1584–1590
Deng HW, Chen WM, Conway T, Zhou Y, Davies KM, Stegman MR, Deng H, Recker RR (2000) Determination of bone mineral density of the hip and spine in human pedigrees by genetic and life-style factors. Genet Epidemiol 19:160–177
Mitchell BD, Kammerer CM, Schneider JL, Perez R, Bauer RL (2003) Genetic and environmental determinants of bone mineral density in Mexican Americans: results from the San Antonio Family Osteoporosis Study. Bone 33:839–846
Lee M, Czerwinski SA, Choh AC, Demerath EW, Sun SS, Chumlea WC, Towne B, Siervogel RM (2006) Unique and common genetic effects between bone mineral density and calcaneal quantitative ultrasound measures: the Fels Longitudinal Study. Osteoporos Int 17:865–871
Peacock M, Turner CH, Econs MJ, Foroud T (2002) Genetics of osteoporosis. Endocr Rev 23:303–326
Yang TL, Zhao LJ, Liu YJ, Liu JF, Recker RR, Deng HW (2006) Genetic and environmental correlations of bone mineral density at different skeletal sites in females and males. Calcif Tissue Int 78:212–217
Ng MY, Sham PC, Paterson AD, Chan V, Kung AW (2006) Effect of environmental factors and gender on the heritability of bone mineral density and bone size. Ann Hum Genet 70:428–438
Deng FY, Lei SF, Li MX, Jiang C, Dvornyk V, Deng HW (2006) Genetic determination and correlation of body mass index and bone mineral density at the spine and hip in Chinese Han ethnicity. Osteoporos Int 17:119–124
Liu PY, Qin YJ, Zhou Q, Recker RR, Deng HW (2004) Complex segregation analyses of bone mineral density in Chinese. Ann Hum Genet 68:154–164
Bonjour JP, Carrie AL, Ferrari S, Clavien H, Slosman D, Theintz G, Rizzoli R (1997) Calcium-enriched foods and bone mass growth in prepubertal girls: a randomized, double-blind, placebo-controlled trial. J Clin Invest 99:1287–1294
Johnston CC Jr, Miller JZ, Slemenda CW, Reister TK, Hui S, Christian JC, Peacock M (1992) Calcium supplementation and increases in bone mineral density in children. N Engl J Med 327:82–87
Zhu K, Du X, Greenfield H, Zhang Q, Ma G, Hu X, Fraser DR (2004) Bone mass in Chinese premenarcheal girls: the roles of body composition, calcium intake and physical activity. Br J Nutr 92:985–993
Courteix D, Jaffre´ C, Lespessailles E, Benhamou L (2005) Cumulative effects of calcium supplementation and physical activity on bone accretion in premenarchal children: a double-blind randomised placebo-controlled trial. Int J Sports Med 26:332–338
MacKelvie KJ, Khan KM, McKay HA (2002) Is there a critical period for bone response to weight-bearing exercise in children and adolescents? A systematic review. Br J Sports Med 36:250–257
Welten DC, Kemper HC, Post GB, Van Mechelen W, Twisk J, Lips P, Teule GJ (1994) Weight-bearing activity during youth is a more important factor for peak bone mass than calcium intake. J Bone Miner Res 9:1089–1096
Pollitzer WS, Anderson JJ (1989) Ethnic and genetic differences in bone mass: a review with a hereditary vs. environmental perspective. Am J Clin Nutr 50:1244–1259
Ohta H, Kuroda T, Onoe Y, Nakano C, Yoshikata R, Ishitani K, Hashimoto K, Kume M (2010) Familial correlation of bone mineral density, birth data and lifestyle factors among adolescent daughters, mothers and grandmothers. J Bone Miner Metab 28:690–695
Kuroda T, Onoe Y, Miyabara Y, Yoshikata R, Orito S, Ishitani K, Okano H, Ohta H (2009) Influence of maternal genetic and lifestyle factors on bone mineral density in adolescent daughters: a cohort study in 387 Japanese daughter-mother pairs. J Bone Miner Metab 27:379–385
Keller LF, Grant PR, Grant BR, Petren K (2001) Heritability of morphological traits in Darwin’s finches: misidentified paternity and maternal effects. Heredity (Edinb) 87(Pt 3):325–336
Ralston SH, Uitterlinden AG (2010) Genetics of osteoporosis. Endocr Rev 31:629–662
Richards JB, Zheng HF, Spector TD (2012) Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet 13:576–588
Rivadeneira F, Styrkársdottir U, Estrada K, Halldórsson BV, Hsu YH, Richards JB, Zillikens MC, Kavvoura FK, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Grundberg E, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra B, Pastinen T, Pols HA, Sigurdsson G, Soranzo N, Thorleifsson G, Thorsteinsdottir U, Williams FM, Wilson SG, Zhou Y, Ralston SH, van Duijn CM, Spector T, Kiel DP, Stefansson K, Ioannidis JP, Uitterlinden AG, Genetic Factors for Osteoporosis (GEFOS) Consortium (2009) Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nature Genet 41:1199–1206
Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, Oei L, Albagha OM, Amin N, Kemp JP, Koller DL, Li G, Liu CT, Minster RL, Moayyeri A, Vandenput L, Willner D, Xiao SM, Yerges-Armstrong LM, Zheng HF, Alonso N, Eriksson J, Kammerer CM, Kaptoge SK, Leo PJ, Thorleifsson G, Wilson SG, Wilson JF, Aalto V, Alen M, Aragaki AK, Aspelund T, Center JR, Dailiana Z, Duggan DJ, Garcia M, Garcia-Giralt N, Giroux S, Hallmans G, Hocking LJ, Husted LB, Jameson KA, Khusainova R, Kim GS, Kooperberg C, Koromila T, Kruk M, Laaksonen M, Lacroix AZ, Lee SH, Leung PC, Lewis JR, Masi L, Mencej-Bedrac S, Nguyen TV, Nogues X, Patel MS, Prezelj J, Rose LM, Scollen S, Siggeirsdottir K, Smith AV, Svensson O, Trompet S, Trummer O, van Schoor NM, Woo J, Zhu K, Balcells S, Brandi ML, Buckley BM, Cheng S, Christiansen C, Cooper C, Dedoussis G, Ford I, Frost M, Goltzman D, González-Macías J, Kähönen M, Karlsson M, Khusnutdinova E, Koh JM, Kollia P, Langdahl BL, Leslie WD, Lips P, Ljunggren Ö, Lorenc RS, Marc J, Mellström D, Obermayer-Pietsch B, Olmos JM, Pettersson-Kymmer U, Reid DM, Riancho JA, Ridker PM, Rousseau F, Slagboom PE, Tang NL, Urreizti R, Van Hul W, Viikari J, Zarrabeitia MT, Aulchenko YS, Castano-Betancourt M, Grundberg E, Herrera L, Ingvarsson T, Johannsdottir H, Kwan T, Li R, Luben R, Medina-Gómez C, Palsson ST, Reppe S, Rotter JI, Sigurdsson G, van Meurs JB, Verlaan D, Williams FM, Wood AR, Zhou Y, Gautvik KM, Pastinen T, Raychaudhuri S, Cauley JA, Chasman DI, Clark GR, Cummings SR, Danoy P, Dennison EM, Eastell R, Eisman JA, Gudnason V, Hofman A, Jackson RD, Jones G, Jukema JW, Khaw KT, Lehtimäki T, Liu Y, Lorentzon M, McCloskey E, Mitchell BD, Nandakumar K, Nicholson GC, Oostra BA, Peacock M, Pols HA, Prince RL, Raitakari O, Reid IR, Robbins J, Sambrook PN, Sham PC, Shuldiner AR, Tylavsky FA, van Duijn CM, Wareham NJ, Cupples LA, Econs MJ, Evans DM, Harris TB, Kung AW, Psaty BM, Reeve J, Spector TD, Streeten EA, Zillikens MC, Thorsteinsdottir U, Ohlsson C, Karasik D, Richards JB, Brown MA, Stefansson K, Uitterlinden AG, Ralston SH, Ioannidis JP, Kiel DP, Rivadeneira F (2012) Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nature Genet 44:491–501
Perusse L, Leblanc C, Bouchard C (1988) Familial resemblance in lifestyle components: results from the Canada Fitness Survey. Can J Public Health 79:201–205
Gottlieb NH, Baker JA (1986) The relative influence of health beliefs, parental and peer behaviors and exercise program participation on smoking, alcohol use and physical activity. Soc Sci Med 22:915–927
Kim SH, Oh MK, Namgung R, Park MJ (2014) Prevalence of 25-hydroxyvitamin D deficiency in Korean adolescents: association with age, season and parental vitamin D status. Public Health Nutr 17:122–130
Danielson ME, Cauley JA, Baker CE, Newman AB, Dorman JS, Towers JD, Kuller LH (1999) Familial resemblance of bone mineral density (BMD) and calcaneal ultrasound attenuation: the BMD in mothers and daughters study. J Bone Miner Res 14:102–110
Michaelsson K, Melhus H, Ferm H, Ahlbom A, Pedersen NL (2005) Genetic liability to fractures in the elderly. Arch Intern Med 165:1825–1830.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A1A01054333) and by the Ministry of Education, Science and Technology (2014R1A6A3A03056928).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
Table S1
(DOCX 24 kb)
Table S2
(DOCX 21 kb)
Table S3
(DOCX 21 kb)
Supplemental Fig. 1
Selection of study data. KNHANES, Korea National Health and Nutrition Examination Survey. (PPTX 64 kb)
Rights and permissions
About this article
Cite this article
Choi, H.S., Park, J.H., Kim, S.H. et al. Strong familial association of bone mineral density between parents and offspring: KNHANES 2008–2011. Osteoporos Int 28, 955–964 (2017). https://doi.org/10.1007/s00198-016-3806-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3806-1