Abstract
Summary
We assessed the impact of weight loss strategies including calorie restriction and exercise training on BMD in adults using a systematic review of randomized controlled trials. Weight reduction results in reduced BMD at the hip, but has less effect on the spine. Both calorie restriction and a combination of calorie restriction and exercise result in a decrease in hip bone density, whereas weight loss response to exercise training without dietary restriction leads to increased hip BMD.
Introduction
Findings are not consistent on the effect of weight loss on bone mineral density (BMD). We conducted a systematic review on the randomized controlled trials to assess the effect of weight loss strategies, including calorie restriction and exercise programs on BMD in adults.
Methods
A structured and comprehensive search of MEDLINE and EMBASE databases was undertaken up to March 2016. Study-specific mean differences (MD) were pooled using a random-effects model. Subgroup analysis and meta-regression were used to find possible sources of between-study heterogeneity.
Results
Thirty-two randomized controlled trials met predetermined inclusion criteria. The meta-analysis revealed no significant difference on total BMD (MD 0.007, 95 % CI −0.020–0.034, p = 0.608). In contrast, the pooled data of studies showed a significant effect of weight loss on hip BMD (MD −0.008, 95 % CI −0.09 to −0.006 g/cm2, p < 0.001) and also lumbar spine BMD (MD −0.018 g/cm2, 95 % CI −0.019 to −0.017, p < 0.001). BMD in the hip site decreased after more than 4 months, especially in those who were obese. Moreover, calorie restriction interventions longer than 13 months showed a significant decreased in lumbar spine BMD.
Conclusion
Weight loss led to significant decreases at the hip and lumbar spine BMD but not at the total. Weight loss response following calorie restriction resulted in a decrease in hip and lumbar spine bone density especially more than 1 year; whereas an exercise-induced weight loss did not.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity contributes to risk for many chronic diseases such as hypertension, type 2 diabetes mellitus, and hyperlipidemia [1–3]. Obesity is a global public health concern, while diet and exercise are the main treatments [4]. Weight loss programs improve metabolic fitness and reduce morbidity and mortality associated with overweight and obesity. However, accumulating evidence suggests that obesity protects against bone loss due to weight-bearing effect of excess weight on the skeleton and the mechanical stress on bone, which stimulates osteoblast differentiation [5, 6]. On the other hand, obesity has been associated with low bone quality [7]. Moreover, a Japanese prospective cohort study with a 6.7-year follow-up found incidence rate of vertebral fracture in overweight and obese women was significantly higher than normal weight women [8].
Weight loss leads to systematic endocrine changes that probably influence bone mineral density (BMD) [9]. Reducing estrogen, leptin, and insulin during weight loss can decrease their anabolic effects on bone [9] while on the other hand a concomitant increase in cortisol and parathyroid hormone [10] concentration may cause detrimental impact on bone mass [9]. Increased adiponectin level during weight loss predicted greater risk of bone loss at the lumbar spine [11]. It has been suggested that adiponectin stimulates osteoblastogenesis and inhibits osteoclastogenesis [12]. However, findings of the effect of weight loss on bone are confusing because of the heterogeneous nature of the methods and subject populations. For example, studies with mixed populations including pre-, peri-, and post-menopausal women, and/or men showed a loss of total BMD, ranged 0–2.5 % [13–15]. A previous meta-analysis [16] reported weight loss interventions on overweight/obese subjects led to a significant reduction in BMD of 0.010 to 0.015 g/cm2 in the hip and 0.011 g/cm2 in total body BMD after 6 months. Moreover, clinical trials with no control groups were also included in that review [16]. Therefore we conducted an updated systematic review of randomized controlled trials to assess the impact of weight loss strategies including calorie restriction and exercise training on BMD in adults.
Methods
A comprehensive search and systematic assessment of studies and data extraction were conducted in a stepwise process in accordance with our specific question: What is the effect of weight loss strategies including calorie restriction and exercise training on BMD in adults?
The PICOS model [17], where the acronym PICOS stands for population (>18 years old), intervention (diet and exercise programs), comparison (no or other balance enhancing exercise or diet programs), outcome (weight loss and bone loss), and study design (randomized controlled trials), was applied to formulate our question.
Box 1 PICOS criteria (Patient/Population, Intervention, Comparison, Outcome, Setting)
Patient/Population: Population (>18 years old) Intervention: Weight loss program (exercise or energy restriction diet) Comparison: No or other balance enhancing exercise or diet programs Outcome: BMD of the total hip, lumbar spine (L1–L4 or L2–L4) or of the total body which was measured by dual X-ray absorptiometry and body weight Setting: Randomized control trial |
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement was used for writing this systematic review [18]. The protocol was previously published in the PROSPERO database (http://www.crd.york.ac.uk/PROSPERO), under registration no CRD42015016005.
Search strategy
We searched the English-language medical literature published up to October 2014 using the MEDLINE and EMBASE databases for studies investigating the impact of weight loss on bone mass. The electronic literature search was last updated on March 2016. Search terms, keywords, and study design in the searches are presented in Appendix 1. Review articles were also assessed to find additional eligible articles. The bibliographies of relevant articles were checked to ensure that all relevant studies were found.
Eligibility and study selection
Three reviewers (S.S, A.K, and S.S-b) independently screened the eligibility of studies according to the following pre-established criteria. Studies were included in this meta-analysis if they fulfilled the following criteria: the study design was randomized controlled trial; the intervention arm of the study had to include a weight loss program (exercise or energy restriction diet). The outcome of interest was BMD of the total hip, lumbar spine (L1–L4 or L2–L4), or of the total body which was measured by dual X-ray absorptiometry (DXA). BMD of the hip and spine was chosen based on the recommendation of the National Osteoporosis Foundation because low BMD in hip and spine sites are candidate for treatment risk fracture in post-menopausal women and men 50 years and older [19]. The exclusion criteria were reviews, conference papers, editorials, non-human studies, and non-randomized trials; investigations that participants were pregnant and lactating women or child and adolescent, studies on patients with renal, digestive tract, endocrine disorders and cancers; as well as studies employing bariatric surgery or medications for weight reduction. Studies were also excluded if the subjects had treatment with hormone replacement therapy or treatment with bisphosphonates, other medication analogues, and multi-mineral supplements. The reference lists of all included studies and previous meta-analysis were hand searched to identify studies not found by the search of electronic databases. In case of multiple publications from the same population, we considered those with the largest sample size.
Data extraction
S.S extracted quantitative data from each study included in the review using a pre-formatted spreadsheet. The extracted data was checked by two independent investigators (A.K, S.S-b) to reduce reviewer errors. If there were discrepancies, group consensus and a third reviewer was consulted to ensure accuracy of data. The data extracted included the following: year of publication, country in which conducted; study design, the participants’ age; number of allocated participants; sex and menopausal status; details of weight loss approaches (exercise; energy restriction diet) for intervention and control group; length of intervention (months); baseline BMI of participants; weight change, and BMD outcome measurement sites (BMD values with standard deviation of whole body, hip, and spine). BMD in all studies was reported as grams per square centimeter (g/cm2). We attempted to contact the authors to obtain information not furnished but needed.
Quality assessment
Jadad scale was used to assess the quality of included trials [20]. The factors contributing to study quality were randomization (described as randomized, 1 point; randomized process, additional 1 point), double-blind (described as double-blind, 1 point; use of masking such as identical placebo, additional 1 point), and follow-up (the numbers and reasons for drop out in each group; 1 point) in the report of RCTs. Studies with a score of 2 or less indicated low quality and studies with a scores of 3 or more as high quality study. As blinding is almost impossible for dietary and exercise programs, we considered the blinding as staff personnel of studies who performed scans and subsequent analysis for BMD were unaware of intervention allocation.
Statistical analysis
We calculated the mean difference between the intervention and control group for each individual study, which is equal to change in intervention group minus change in control group, where changes are the absolute differences in BMD between each follow-up and the baseline measure for the intervention and control groups. Imputing of standard deviations from other studies was used to estimate variance of calculated mean difference. If only the range was given as the measure of variation, then the SD was calculated as the range divided by 5.88 (6 SDs). If the SE was reported as variance, the SD was computed as SE × √n. For 95 % confidence intervals, the SD for each group was obtained by dividing the length of the confidence interval by 3.92, and then multiplying by the square root of the sample size.
For meta-analysis, a random-effects model was used to report heterogeneous results. Data was pooled by the generic inverse variance method by the user written “metan” command [21]. We computed the between-study variance for the random-effects model using the DerSimonian and Laird formula [22]. Statistical heterogeneity was assessed using the χ 2 test and I 2 statistic (I 2 represents the percentage of variability due to between-study variability). To investigate sources of heterogeneity, a list of predefined variables for subgroup analysis were used including the following: participants’ age (<65 vs. >65 years old), gender, initial BMI (<25 vs. >25 kg/m2 or both), follow-up period (<3 months; 4–6 months; 7–9 months, 10–12 months, and >1 year), weight loss approaches (exercise, energy restriction diet, or both) for intervention, and multi vitamin-minerals supplementation. Subgroup analyses were carried out by disaggregating results with the user written “metan” command (“by option”) [21]. We conducted an influence analysis to assess the influence of each individual trial on the overall summary estimate with the user written “metaninf” command. We also carried out formal statistical tests for funnel plot asymmetry with the user written “metabias” command [21]. All statistical analyses were conducted using STATA software version 14.0 (Stata Corp, College Station, TX, USA). A p value less than 0.05 was considered to be statistically significant.
Results
Study selection
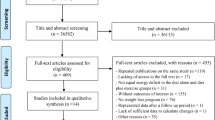
Figure 1 shows the process for the inclusion of studies in this systematic review. The systematic search identified 8319 references, of which 1244 were duplicated and 6904 were excluded at initial screening of the title and abstract. Of these, 139 studies were excluded because of lack of bone loss data following weight loss program [10, 21, 23–34]; conducted in children, adolescents, and lactating women [35–41]; repeated publications on the same study [42–46]; lack of randomization [47–52]; surgical and anti-obesity drug intervention [53–56]; lack of data for weight change [57–79]; lacking of access to the full text [80]; not RCT design [81–107]; bone density in whole body or hip or spine were incomplete [108–130]; reporting medication, supplementation, or hormone therapy as an intervention [15, 131–143]; missing control group [14, 29, 144–153]; or no weight loss program [154–157].
Study characteristics of included studies
Table 1 shows a description of the included studies. A total of 32 studies met the inclusion criteria and were assessed in the meta-analysis. Most studies were carried out in North America (22 studies) [158–179]. Four publications were conducted in European countries [180–183], four in Asia [184–187], one in Africa [188], and one Australia [189]. A total of 4471 participants were included. Study duration varied between 2 and 60 months. Sample size of studies ranged from 18 to 1274. Thirteen studies included exercise for weight loss, 6 RCTs used calorie restriction, and 13 of studies recommended both exercise and calorie restriction.
Assessment of risk of bias
The risk of bias in the studies is shown in Table 2. Nineteen from the 32 included studies based on Jadad score were classified with good quality. Eleven studies illustrated the randomization scheme and one simply reported the participants were randomized. Twelve studies reported blinding, although its process was not fully described. All of the studies adequately reported dropouts.
Weight loss and BMD
Meta-analysis
In the pooled analysis of 23 trials with 3127 participants [158, 160, 161, 164–175, 181–187, 189], no overall effect of weight loss on total BMD mean differences (MD = 0.007, 95 % CI −0.020–0.034 g/cm2, p = 0.608) was observed (Table 3). In contrast, the meta-analysis (26 studies with 3572 participants) [158, 159, 161, 163–165, 167–180, 182, 183, 185, 186, 188, 189] showed a significant overall effect of reduced body weight on lumbar spine BMD (MD = −0.018 g/cm2, 95 % CI −0.019 to −0.017 g/cm2, p < 0.001). Additionally, the pooled data of 17 studies with 3193 participants [159, 161, 162, 165–168, 170–174, 177, 179, 186, 188, 189] showed also a significant overall effect of weight loss on hip BMD (MD = −0.008, 95 % CI −0.009–0.006 g/cm2, p < 0.001). There was heterogeneity among studies for the effect of weight loss on total BMD (I 2 = 99.4 %, p < 0.001), hip BMD (I 2 = 89.3 %, p < 0.001), and spin BMD (I 2 = 86.6 %, p < 0.001). Weight loss response following combination of calorie restriction/exercise was (MD = −5.670 kg, 95 % CI −6.007 to −5.333 kg, p < 0.001). However, exercise-induced weight loss was rather modest (MD = −0.561 kg, 95 % CI −0.959 to −0.162 kg, p = 0.006) compared to diet-induced weight loss (MD = −6.883 kg, 95 % CI −7.314 to −6.453 kg, p < 0.001). The largest estimated weight reduction was for hip BMD (MD = −5.163 kg, 95 % CI −6.467 to −3.859 kg, p < 0.001). The mean of weight loss was (MD = −4.868 kg, 95 % CI −6.202 to −3.534 kg, p < 0.001) for total body and (MD = −4.017 kg, 95 % CI −5.331 to −2.702 kg, p < 0.001) for lumbar spine BMD (data not shown).
Subgroup analysis
We explored potential heterogeneity by examining effect sizes in clinical subgroups by predefined categories: participants’ age, gender, BMI, trial duration, weight loss approaches for intervention, and supplementation concomitant with intervention. Age, trial duration, baseline BMI, and concomitant supplementation with weight loss did not explain heterogeneity seen between studies for analyses of total BMD. All of the predefining factors were sources of heterogeneity for BMD of the lumbar spine. Age and concomitant supplementation were sources of heterogeneity for BMD of the hip. For studies with both sex (MD = 0.008 g/cm2, 95 % CI 0.003 to 0.012, p = 0.001) and both weight loss approaches (MD = 0.004 g/cm2, 95 % CI 0.001 to 0.006, p = 0.009), the MD for total BMD significantly increased (Table 3). For the BMD of hip, BMD decreased in both genders, in all age categories, after more than 4 months especially in those who were overweight and obese with calorie restriction (Table 3). However, exercise programs increased the BMD of hip (MD = 0.005 g/cm2, 95 % CI 0.002 to 0.009, p = 0.004). In subgroup analysis of lumbar spine, younger (MD = −0.019 g/cm2, 95 % CI −0.020 to −0.018) non-obese participants (MD = −0.019 g/cm2, 95 % CI −0.020 to −0.018) with calorie restriction (MD = −0.020 g/cm2, 95 % CI −0.021 to −0.019) after more than 13 months (MD = −0.020 g/cm2, 95 % CI −0.021 to −0.019) showed significant decrease in the BMD (Table 3).
Influence analysis
The sensitivity analysis for BMD of the lumbar spine showed that the study by Villareal et al. [179] was responsible for the heterogeneity, and its exclusion did make the pooled results in BMD of the lumbar spine non-significant (figures not shown). Therefore, the significant inverse association between weight loss and BMD of the lumbar spine should be interpreted with caution.
Publication bias
The funnel plots did not show any signs of asymmetry and the Egger’s test showed no publication of bias for total BMD (p = 0.148, Fig. 2a), BMD of hip (p = 598, Fig. 2b), and lumbar spine (p = 0.434, Fig. 2c).
Egger’s funnel plots (with pseudo 95 % CIs) of the mean differences (MDs) versus their SEs (standard errors) for studies that assessed the effect of weight loss program on whole body BMD (a), hip BMD (b), and spine BMD (c). The horizontal line shows the pooled MDs calculated with the DerSimonian and Laird random-effects model
Discussion
Previous studies have yielded little consensus concerning the effects of weight loss on BMD. For the first time, the present study evaluated randomized controlled trials to quantitatively assess the effect of all non-pharmacological weight loss programs (weight loss or diet induced) on BMD in adult populations using meta-analysis procedures. Our meta-analysis has revealed weight loss response following calorie restriction resulted in a decrease in hip and lumbar spine bone density, especially after 1 year, whereas an exercise-induced weight loss did not. Moreover, BMD of whole body showed no significant changes in response to weight reduction. However, exercise-induced weight loss was rather modest compared to diet-induced weight loss.
Our findings are in agreement with previous recent meta-analyses which have shown an association between weight reduction and BMD, which existed at the hip site [16]. Conversely, our data are in contrast to this study, reporting only minimal lumbar spine bone density loss after at least 13 months weight loss [16].
Our finding showed that the lumbar spine BMD decreased in non-obese adults. Non-obese adults have less FFM compared with obese ones which make them more susceptible to detrimental bone effects following weight loss [190]. We showed the spine BMD was decreased in younger adults. It is possible that loading of the lumbar spine is relatively lower than hip loading during locomotion, the primary loading factor in modern environments. In fact, loading of the hip may be much larger and frequent than at the spine during walking. So it is possible loss of weight will have less of an effect on BMD of the spine so will take longer to occur. So it is possible loss of weight will have less of an effect on BMD of the spine so will take longer to occur. Therefore, it is possible that decreased body weight would have less of an influence on lumbar spine loading than on hip loading thus slowing the loss of lumbar spine BMD.
Our results suggested that weight loss resulted in a decrease in the BMD of hip and lumbar spine but not in the whole body BMD. The more content of spine and hip is trabecular; sites where trabecular bone shown are more sensitive to response turn over factors could be a possible explanation [191]. Moreover, hip and lumbar spine BMD measurement by DXA is the best predictor of future hip fracture risk [192], these site-specific effects of weight reduction could have clinical implications.
The aim of weight loss in obese subjects is loss of fat mass, which may play an important role in regulating BMD [193]. Adipose tissue is a dynamic tissue that secretes a large number of anti and pro inflammatory cytokines called adipokines [194]. Leptin is a well-known adipocyte-derived hormone that its systemic administration to animals and humans increases bone mass [195, 196]. These studies suggest that the anabolic effect of leptin is mediated by stimulant osteoblast proliferation and inhibition of osteoclastogenesis [197, 198]. Adiponectin is another adipokine that increases with moderate weight loss [199]. Result from adiponectin knockout mice suggest that this hormone is related to reduced bone mass [200]. Adiponectin by modulating proliferation and mineralization of osteoblastic cells (then, increasing differentiation of osteoblasts to osteoclasts) [201] enhance the expression of osteoclast receptor activator of nuclear factor-κB ligand [202] and by inhibition of osteoprotegerin exacerbate bone turn over [203]. A reduction in the circulating insulin levels through stimulating insulin sensitivity might also contribute to reduce bone density following weight loss. Insulin resistance, with compensating hyperinsulinemia as a result of obesity, has been suggested to have an anabolic effect on bone mass which may be due to a negative effect on sex hormone-binding globulin and the increase of free sex hormone levels [204, 205]. In addition to this mechanism, insulin, by exerting synergistic effects with insulin-like growth factor-I and parathyroid hormone may prevent decline in BMD [206]. It is essential to point out that the progenitor-mesenchymal stem cells of osteoblasts and adipocytes are in common to each other and their differentiation is regulated by peroxisome proliferators activated receptor-γ (PPAR-γ) and leptin [207]. A reduction in estrogen levels and rise in serum cortisol during weight management program may also explain the bone mass induced by weight loss [208, 209]. Finally, weight loss will be associated with less mechanical loading on the bones during locomotion which may lead to bone loss [15, 173]. Thus, a decrease in body weight that induces concomitant reductions in fat and lean mass could have negative effects on bone mass.
Our results showed that loss of hip BMD due to moderate weight loss was reduced similarly in calorie restriction and combination calorie restriction and exercise program, whereas exercise during energy restriction may minimize loss of BMD. Despite weight loss, the exercise group demonstrated an increase in hip BMD. It should be kept in mind that our result has shown exercise-induced weight loss was rather modest compared to diet-induced weight loss. Inclusion of exercise training during weight loss seems to decrease BMD loss [172], probably due to the combination of greater maintenance of lean mass and increased mechanical loading on the bones [210]. Supporting this, a recent meta-analysis showed that FFM has a larger effect on BMD than FM [90]. It is important to point out that BMD loss following weight loss may not always increase risk of fracture. In fact, at least one study showed that BMD relative to body weight actually increased following a weight loss of approximately 12 kg, showing an improved BMD relative to body weight [162]. Of course exercise training such as walking or running will increase mechanical loading, especially on the hip, and have a positive effect on preventing BMD loss [210].
Significant decreases in hip BMD were observed in present study for interventions longer than 4 months. If intervention induced changes in BMD are to be detected, the time interval between evaluations must be long enough to allow bone remodeling to occur. The complete cycle of bone remodeling takes 4 to 6 months, thus indicating the time interval between two measurements in the same patient should be at least that long to be able to detect BMD changes in clinical trials [211].
Our study has several strengths. The main strength of this meta-analysis is that the RCTs are included to the analysis. RCTs is widely regarded as gold standard to evaluate the effects of an intervention. Moreover, the present meta-analysis has investigated the effect of all non-invasive weight loss programs on BMD, allowing us to explain individually the differences between diet/exercise-induced weight loss on bone quality.
Some limitations in the present study are needed to be considered while interpreting our results. First, there is a large heterogeneity in hormone replacement therapy in included studies. Indeed, some studies did not take into consideration hormone therapy as exclusion criteria, which are an effective means of attenuating loss of lean mass and bone. Moreover, the majority of studies did not report menopause status separately within their population. Therefore, it was not possible to assess whether differences in menopause status contributed to differences in primary outcomes. Additionally, the degree of participants’ compliance with the weight loss program (energy restriction and exercise training), in the most included studies, has not been considered. The weight loss following dietary interventions is usually complex, i.e., differences in macronutrient composition of the diets may influence changes in bone mass. The findings from 16 weeks energy restriction program in overweight adults suggests that a high-protein, calcium-replete diet may protect against bone loss during weight reduction [27]. Furthermore, as blinding is impossible for dietary intervention trials and exercise programs, it is possible that observer bias have influenced on finding of studies.
In conclusion, the present meta-analysis reveals that weight reduction results in reduced BMD at the hip, but has less effect on the spine. Both calorie restriction and a combination of calorie restriction and exercise result in a decrease in hip bone density, whereas weight loss response to exercise training without dietary restriction leads to increased hip BMD.
References
Gower BA, Ard JD, Hunter GR, Fernandez J, Ovalle F (2007) Elements of the metabolic syndrome: association with insulin sensitivity and effects of ethnicity. Metab Syndr Relat Disord 5(1):77–86
Williams MJ, Hunter GR, Kekes-Szabo T, Snyder S, Treuth MS (1997) Regional fat distribution in women and risk of cardiovascular disease. Am J Clin Nutr 65(3):855–860
Hunter G, Giger JN, Weaver M, Strickland O, Zuckerman P, Taylor H (2000) Fat distribution and cardiovascular disease risk in African-American women. J Natl Black Nurses Assoc 11(2):7–11
Philipson T (2001) The world-wide growth in obesity: an economic research agenda. Health Econ 10(1):1–7
Edelstein SL, Barrett-Connor E (1993) Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol 138(3):160–169
Felson DT, Zhang Y, Hannan MT, Anderson JJ (1993) Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Metab Res 8(5):567–573
Sukumar D, Schlussel Y, Riedt C, Gordon C, Stahl T, Shapses S (2011) Obesity alters cortical and trabecular bone density and geometry in women. Osteoporos Int 22(2):635–645
Tanaka S, Kuroda T, Saito M, Shiraki M (2013) Overweight/obesity and underweight are both risk factors for osteoporotic fractures at different sites in Japanese postmenopausal women. Osteoporos Int 24(1):69–76
Shapses S, Cifuentes M (2003) Weight reduction and bone health. In: Lanham-New SA, Jean-Phillippe B (eds) Nutritional aspects of bone health. Thomas Graham House, Royal Society of Chemistry, Science Park, pp 589–604
Sukumar D, Ambia‐Sobhan H, Zurfluh R, Schlussel Y, Stahl TJ, Gordon CL, Shapses SA (2011) Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized, controlled trial. J Bone Miner Metab Res 26(6):1339–1348
Jürimäe J, Kums T, Jürimäe T (2009) Adipocytokine and ghrelin levels in relation to bone mineral density in physically active older women: longitudinal associations. Eur J Endocrinol 160(3):381–385
Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE (2004) Adiponectin and its receptors are expressed in bone-forming cells. Bone 35(4):842–849
Silva HG, Mendonça L, Conceição FL, Zahar SE, Farias MLF (2007) Influence of obesity on bone density in postmenopausal women. Arq Bras Endocrinol Metabol 51(6):943–949
Jensen LB, Quaade F, Sørensen OH (1994) Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Metab Res 9(4):459–463
Jensen LB, Kollerup G, Quaade F, SøRensen OH (2001) Bone mineral changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Metab Res 16(1):141–147
Zibellini J, Seimon RV, Lee CM, Gibson AA, Hsu MS, Shapses SA, Nguyen T, Sainsbury A (2015) Does diet‐induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta‐analysis of clinical trials. J Bone Miner Metab Res 30(12):2168–2178
Richardson WS, Wilson MC, Nishikawa J, Hayward RS (1995) The well-built clinical question: a key to evidence-based decisions. ACP J Club 123(3):A12–A13
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8(5):336–341
Dawson-Hughes B, Tosteson A, Melton L III, National Osteoporosis Foundation Guide Committee et al (2008) Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int 9(4494158):6–14
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Egger M, Davey-Smith G, Altman D (2008) Systematic reviews in health care: meta-analysis in context. Wiley, Hoboken, NJ
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Hinton PS, Scott Rector R, Donnelly JE, Smith BK, Bailey B (2010) Total body bone mineral content and density during weight loss and maintenance on a low- or recommended-dairy weight-maintenance diet in obese men and women. Eur J Clin Nutr 64(4):392–399
Movassagbehestani M (2012) Effects of a 12-week aerobic exercise on back spine and thigh bone mineral density in heavy women after menopause. Biosci Biotechnol Res Asia 9(1):149–156
Antonio J, Sanders MS, Ehler LA, Uelmen J, Raether JB, Stout JR (2000) Effects of exercise training and amino-acid supplementation on body composition and physical performance in untrained women. Nutrition 16(11–12):1043–1046
Thorpe MP, Jacobson EH, Layman DK, He X, Kris-Etherton PM, Evans EM (2008) A diet high in protein, dairy, and calcium attenuates bone loss over twelve months of weight loss and maintenance relative to a conventional high-carbohydrate diet in adults. J Nutr 138(6):1096–1100
Bowen J, Noakes M, Clifton PM (2004) A high dairy protein, high-calcium diet minimizes bone turnover in overweight adults during weight loss. J Nutr 134(3):568–573
Soomro RR, Ahmed SI, Khan M, Ali SS (2015) Comparing the effects of osteoporosis prevention exercise protocol (OPEP) versus walking in the prevention of osteoporosis in younger females. Pak J Med Sci 31(2):336–340
Tirosh A, De Souza RJ, Sacks F, Bray GA, Smith SR, LeBoff MS (2015) Sex differences in the effects of weight loss diets on bone mineral density and body composition: POUNDS LOST trial. J Clin Endocrinol Metab 100(6):2463–2471
Von Stengel S, Bebenek M, Engelke K, Kemmler W (2015) Whole-body electromyostimulation to fight osteopenia in elderly females: the randomized controlled training and electrostimulation trial (TEST-III). J Osteoporos
Christensen P, Frederiksen R, Bliddal H, Riecke BF, Bartels EM, Henriksen M, Juul SRT, Gudbergsen H, Winther K, Astrup A, Christensen R (2013) Comparison of three weight maintenance programs on cardiovascular risk, bone and vitamins in sedentary older adults. Obesity (Silver Spring) 21(10):1982–1990
Jesudason D, Nordin BEC, Keogh J, Clifton P (2013) Comparison of 2 weight-loss diets of different protein content on bone health: a randomized trial. Am J Clin Nutr 98(5):1343–1352
Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, Stein RI, Mohammed BS, Miller B, Rader DJ (2010) Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med 153(3):147–157
Noakes M, Keogh JB, Foster PR, Clifton PM (2005) Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am J Clin Nutr 81:1298–1306
Cohen TR, Hazell TJ, Vanstone CA, Plourde H, Rodd CJ, Weiler HA (2013) A family-centered lifestyle intervention to improve body composition and bone mass in overweight and obese children 6 through 8 years: a randomized controlled trial study protocol. BMC Public Health 13(1):383–398
Colleran HL, Wideman L, Lovelady CA (2012) Effects of energy restriction and exercise on bone mineral density during lactation. Med Sci Sports Exerc 44(8):1570–1579
DeBar LL, Ritenbaugh C, Vuckovic N, Stevens VJ, Aickin M, Elliot D, Moe E, Orwoll E, Ernst D, Irving LM (2004) YOUTH: decisions and challenges in designing an osteoporosis prevention intervention for teen girls. Prev Med 39(5):1047–1055
Nakagi Y, Ito T, Hirooka K, Sugioka Y, Endo H, Saijo Y, Imai H, Takeda H, Kayama F, Sasaki S, Yoshida T (2010) Association between lifestyle habits and bone mineral density in Japanese juveniles. Environ Health Prev Med 15(4):222–228
Pieltain C, De Curtis M, Gérard P, Rigo J (2001) Weight gain composition in preterm infants with dual energy x-ray absorptiometry. Pediatr Res 49(1):120–124
Santos LC, Cintra IP, Fisberg M, Martini LA (2009) Effects of weight change on bone mass and metabolic parameters in obese adolescents. E Spen 4(1):e47–e52
Wang Q, Alen M, Nicholson P, Suominen H, Koistinen A, Kroger H, Cheng S (2007) Weight-bearing, muscle loading and bone mineral accrual in pubertal girls—a 2-year longitudinal study. Bone 40(5):1196–1202
Armamento-Villareal R, Sadler C, Napoli N, Shah K, Chode S, Sinacore DR, Qualls C, Villareal DT (2012) Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Metab Res 27(5):1215–1221
Shah K, Armamento-Villareal R, Parimi N, Chode S, Sinacore DR, Hilton TN, Napoli N, Qualls C, Villareal DT (2011) Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res 26(12):2851–2859
Waters DL, Vawter R, Qualls C, Chode S, Armamento-Villareal R, Villareal DT (2013) Long-term maintenance of weight loss after lifestyle intervention in frail, obese older adults. J Nutr Health Aging 17(1):3–7
Armamento-Villareal R, Aguirre L, Napoli N, Shah K, Hilton T, Sinacore DR, Qualls C, Villareal DT (2014) Changes in thigh muscle volume predict bone mineral density response to lifestyle therapy in frail, obese older adults. Osteoporos Int 25(2):551–558
Lipkin EW, Schwartz AV, Anderson AM, Davis C, Johnson KC, Gregg EW, Bray GA, Berkowitz R, Peters AL, Hodges A, Lewis C, Kahn SE (2014) The look AHEAD Trial: bone loss at 4-year follow-up in type 2 diabetes. Diabetes Care 37(10):2822–2829
Silverman NE, Nicklas BJ, Ryan AS (2009) Addition of aerobic exercise to a weight loss program increases BMD, with an associated reduction in inflammation in overweight postmenopausal women. Calcif Tissue Int 84(4):257–265
Dalsky GP, Stocke KS, Ehsani AA, Slatopolsky E, Lee WC, Birge SJ Jr (1988) Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women. Ann Intern Med 108(6):824–828
Shea KL, Gozansky WS, Sherk VD, Swibas TA, Wolfe P, Scherzinger A, Stamm E, Kohrt WM (2014) Loss of bone strength in response to exercise-induced weight loss in obese postmenopausal women: results from a pilot study. J Musculoskelet Neuronal Interact 14(2):229–238
Ryan AS, Nicklas BJ, Dennis KE (1998) Aerobic exercise maintains regional bone mineral density during weight loss in postmenopausal women. J Appl Physiol 84(4):1305–1310
Snow CM, Shaw JM, Winters KM, Witzke KA (2000) Long-term exercise using weighted vests prevents hip bone loss in postmenopausal women. J Gerontol A Biol Sci Med Sci 55(9):M489–M491
Ricci TA, Heymsfield SB, Pierson RN, Stahl T, Chowdhury HA, Shapses SA (2001) Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr 73(2):347–352
Casagrande DS, Repetto G, Mottin CC, Shah J, Pietrobon R, Worni M, Schaan BD (2012) Changes in bone mineral density in women following 1-year gastric bypass surgery. Obes Surg 22(8):1287–1292
Fleischer J, Stein E, Bessler M, Badia MD, Restuccia N, Olivero-Rivera L, McMahon D, Silverberg S (2008) The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab 93(10):3735–3740
Rajamanohara R, Robinson J, Rymer J, Patel R, Fogelman I, Blake GM (2011) The effect of weight and weight change on the long-term precision of spine and hip DXA measurements. Osteoporos Int 22(5):1503–1512
Elaine WY, Bouxsein ML, Putman MS, Monis EL, Roy AE, Pratt JSA, Butsch WS, Finkelstein JS (2015) Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab 100(4):1452–1459
Guney E, Kisakol G, Ozgen G, Yilmaz C, Yilmaz R, Kabalak T (2003) Effect of weight loss on bone metabolism: comparison of vertical banded gastroplasty and medical intervention. Obes Surg 13(3):383–388
Kerr D, Ackland T, Maslen B, Morton A, Prince R (2001) Resistance training over 2 years increases bone mass in calcium-replete postmenopausal women. J Bone Miner Metab Res 16(1):175–181
Shibata Y, Ohsawa I, Watanabe T, Miura T, Sato Y (2003) Effects of physical training on bone mineral density and bone metabolism. J Physiol Anthropol Appl Human Sci 22(4):203–208
Boden H, Adolphson P (2004) No adverse effects of early weight bearing after uncemented total hip arthroplasty: a randomized study of 20 patients. Acta Orthop Scand 75(1):21–29
Bravo G, Gauthier P, Roy PM, Payette H, Gaulin P (1997) A weight-bearing, water-based exercise program for osteopenic women: its impact on bone, functional fitness, and well-being. Arch Phys Med Rehabil 78(12):1375–1380
Bravo G, Gauthier P, Roy PM, Payette H, Gaulin P, Harvey M, Peloquin L, Dubois MF (1996) Impact of a 12-month exercise program on the physical and psychological health of osteopenic women. J Am Geriatr Soc 44(7):756–762
Chan K, Qin L, Lau M, Woo J, Au S, Choy W, Lee K, Lee S (2004) A randomized, prospective study of the effects of Tai Chi Chun exercise on bone mineral density in postmenopausal women. Arch Phys Med Rehabil 85(5):717–722
Goodyear SR, Barr RJ, McCloskey E, Alesci S, Aspden RM, Reid DM, Gregory JS (2013) Can we improve the prediction of hip fracture by assessing bone structure using shape and appearance modelling? Bone 53(1):188–193
Hampson G, Martin FC, Moffat K, Vaja S, Sankaralingam S, Cheung J, Blake GM, Fogelman I (2003) Effects of dietary improvement on bone metabolism in elderly underweight women with osteoporosis: a randomised controlled trial. Osteoporos Int 14(9):750–756
Harris S, Dallal GE, Dawson-Hughes B (1992) Influence of body weight on rates of change in bone density of the spine, hip, and radius in postmenopausal women. Calcif Tissue Int 50(1):19–23
Lord SR, Ward JA, Williams P, Zivanovic E (1996) The effects of a community exercise program on fracture risk factors in older women. Osteoporos Int 6(5):361–367
Manios Y, Moschonis G, Koutsikas K, Papoutsou S, Petraki I, Bellou E, Naoumi A, Kostea S, Tanagra S (2009) Changes in body composition following a dietary and lifestyle intervention trial: the postmenopausal health study. Maturitas 62(1):58–65
McCartney N, Hicks AL, Martin J, Webber CE (1996) A longitudinal trial of weight training in the elderly: continued improvements in year 2. J Gerontol A Biol Sci Med Sci 51(6):B425–B433
McMurdo MET, Mole PA, Paterson CR (1997) Controlled trial of weight bearing exercise in older women in relation to bone density and falls. BMJ 314(7080):569
Nana A, Slater GJ, Hopkins WG, Burke LM (2013) Effects of exercise sessions on DXA measurements of body composition in active people. Med Sci Sports Exerc 45(1):178–185
Rasmussen MH, Jensen LT, Andersen T, Breum L, Hilsted J (1995) Collagen metabolism in obesity: the effect of weight loss. Int J Obes 19(9):659–663
Baker JF, Østergaard M, George M, Shults J, Emery P, Baker DG, Conaghan PG (2014) Greater body mass independently predicts less radiographic progression on X-ray and MRI over 1–2 years. Ann Rheum Dis 73:1923–1928
Cao JJ, Pasiakos SM, Margolis LM, Sauter ER, Whigham LD, McClung JP, Young AJ, Combs GF Jr (2014) Calcium homeostasis and bone metabolic responses to high-protein diets during energy deficit in healthy young adults: a randomized controlled trial. Am J Clin Nutr 99(2):400–407
Elsisi HFEM, Mousa GSM, Eldesoky MTM (2015) Electromagnetic field versus circuit weight training on bone mineral density in elderly women. Clin Interv Aging 10:539–548
Sinaki M, Wahner HW, Bergstralh EJ, Hodgson SF, Offord KP, Squires RW, Swee RG, Kao PC (1996) Three-year controlled, randomized trial of the effect of dose-specified loading and strengthening exercises on bone mineral density of spine and femur in nonathletic, physically active women. Bone 19(3):233–244
Skov AR, Haulrik N, Toubro S, Molgaard C, Astrup A (2002) Effect of protein intake on bone mineralization during weight loss: a 6-month trial. Obes Res 10(6):432–438
Stengel SV, Kemmler W, Pintag R, Beeskow C, Weineck J, Lauber D, Kalender WA, Engelke K (2005) Power training is more effective than strength training for maintaining bone mineral density in postmenopausal women. J Appl Physiol 99(1):181–188
Suh HS, Hwang IC, Lee KS, Kim KK (2013) Relationships between serum osteocalcin, leptin and the effect of weight loss by pharmacological treatment in healthy, nonsmoking Korean obese adults. Clin Chim Acta 418:17–21
Pritchard J, Nowson C, Wark J (1996) Bone loss accompanying diet-induced or exercise-induced weight loss: a randomised controlled study. Int J Obes Relat Metab Disord 20(6):513–520
Heffron M, Davey R, Cochrane T (1997) Weight-training and bone mass in post-menopausal women (Conference Paper). Sports Exerc Inj 3(3):143–149
Avenell A, Richmond P, Lean M, Reid D (1994) Bone loss associated with a high fibre weight reduction diet in postmenopausal women. Eur J Clin Nutr 48(8):561–566
Bartels EM, Christensen R, Christensen P, Henriksen M, Bennett A, Gudbergsen H, Boesen M, Bliddal H (2014) Effect of a 16 weeks weight loss program on osteoarthritis biomarkers in obese patients with knee osteoarthritis: a prospective cohort study. Osteoarthritis Cartilage 22(11):1817–1825
Blain H, Vuillemin A, Teissier A, Hanesse B, Guillemin F, Jeandel C (2001) Influence of muscle strength and body weight and composition on regional bone mineral density in healthy women aged 60 years and over. Gerontology 47(4):207–212
Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR (2003) Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc 51(12):1740–1747
Ensrud KE, Fullman RL, Barrett-Connor E, Cauley JA, Stefanick ML, Fink HA, Lewis CE, Orwoll E (2005) Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab 90(4):1998–2004
Glauber HS, Vollmer WM, Nevitt MC, Ensrud KE, Orwoll ES (1995) Body weight versus body fat distribution, adiposity, and frame size as predictors of bone density. J Clin Endocrinol Metab 80(4):1118–1123
Gomez-Ambrosi J, Rodriguez A, Catalan V, Frühbeck G (2008) The bone-adipose axis in obesity and weight loss. Obes Surg 18(9):1134–1143
Hind K, Burrows M (2007) Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone 40(1):14–27
Ho-Pham LT, Nguyen UDT, Nguyen TV (2014) Association between lean mass, fat mass, and bone mineral density: a meta-analysis. J Clin Endocrinol Metab 99(1):30–38
Knoke JD, Barrett-Connor E (2003) Weight loss: a determinant of hip bone loss in older men and women the Rancho Bernardo Study. Am J Epidemiol 158(12):1132–1138
Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, Van Der Meulen MCH, Lorich DG, Lane JM (2009) Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int 20(8):1353–1362
Macdonald HM, New SA, Campbell MK, Reid DM (2005) Influence of weight and weight change on bone loss in perimenopausal and early postmenopausal Scottish women. Osteoporos Int 16(2):163–171
Pedone C, Napoli N, Pozzilli P, Rossi FF, Lauretani F, Bandinelli S, Ferrucci L, Antonelli-Incalzi R (2011) Dietary pattern and bone density changes in elderly women: a longitudinal study. J Am Coll Nutr 30(2):149–154
Ablove T, Binkley N, Leadley S, Shelton J, Ablove R (2015) Body mass index continues to accurately predict percent body fat as women age despite changes in muscle mass and height. Menopause 22(7):727–730
Clifton PM, Keogh JB, Noakes M (2008) Long-term effects of a high-protein weight-loss diet. Am J Clin Nutr 87(1):23–29
Leskinen T, Kujala UM (2015) Health-related findings among twin pairs discordant for leisure-time physical activity for 32 years: the TWINACTIVE study synopsis. Twin Res Hum Genet 18(3):266–272
Pines A (2012) Weight loss, weight regain and bone health. Climacteric 15(4):317–319
Puntila E, Kröger H, Lakka T, Tuppurainen M, Jurvelin J, Honkanen R (2001) Leisure-time physical activity and rate of bone loss among peri- and postmenopausal women: a longitudinal study. Bone 29(5):442–446
Riggio O, Andreoli A, Diana F, Fiore P, Meddi P, Lionetti R, Montagnese F, Merli M, Capocaccia L, De Lorenzo A (1997) Whole body and regional body composition analysis by dual-energy X-ray absorptiometry in cirrhotic patients. Eur J Clin Nutr 51(12):810–814
Saarelainen J, Kiviniemi V, Kröger H, Tuppurainen M, Niskanen L, Jurvelin J, Honkanen R (2012) Body mass index and bone loss among postmenopausal women: the 10-year follow-up of the OSTPRE cohort. J Bone Miner Metab 30(2):208–216
Sellmeyer DE, Stone KL, Sebastian A, Cummings SR (2001) A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Study of Osteoporotic Fractures Research Group. Am J Clin Nutr 73(1):118–122
Sirola J, Rikkonen T, Tuppurainen M, Honkanen R, Jurvelin JS, Kroger H (2006) Maintenance of muscle strength may counteract weight-loss-related postmenopausal bone loss—a population-based approach. Osteoporos Int 17(5):775–782
Von Thun NL, Sukumar D, Heymsfield SB, Shapses SA (2014) Does bone loss begin after weight loss ends? Results 2 years after weight loss or regain in postmenopausal women. Menopause 21(5):501–508
Waters DL, Ward AL, Villareal DT (2013) Weight loss in obese adults 65 years and older: a review of the controversy. Exp Gerontol 48(10):1054–1061
Williams AR, Weiss NS, Ure CL, Ballard J, Daling JR (1982) Effect of weight, smoking, and estrogen use on the risk of hip and forearm fractures in postmenopausal women. Obstet Gynecol 60(6):695–699
Hendel HW, Gotfredsen A, Andersen T, Højgaard L, Hilsted J (1996) Body composition during weight loss in obese patients estimated by dual energy X-ray absorptiometry and by total body potassium. Int J Obes 20(12):1111–1119
Hyldstrup L, Andersen T, McNair P, Breum L, Transbol I (1993) Bone metabolism in obesity: changes related to severe overweight and dietary weight reduction. Acta Endocrinol 129(5):393–398
Evans EM, Saunders MJ, Spano MA, Arngrimsson SA, Lewis RD, Cureton KJ (1999) Body-composition changes with diet and exercise in obese women: a comparison of estimates from clinical methods and a 4-component model. Am J Clin Nutr 70(1):5–12
Evans EM, Saunders MJ, Spano MA, Arngrimsson SA, Lewis RD, Cureton KJ (1999) Effects of diet and exercise on the density and composition of the fat-free mass in obese women. Med Sci Sports Exerc 31(12):1778–1787
Uusi-Rasi K, Rauhio A, Kannus P, Pasanen M, Kukkonen-Harjula K, Fogelholm M, Sievänen H (2010) Three-month weight reduction does not compromise bone strength in obese premenopausal women. Bone 46(5):1286–1293
Campbell KL, Foster-Schubert KE, Alfano CM, Wang CC, Wang CY, Duggan CR, Mason C, Imayama I, Kong A, Xiao L, Bain CE, Blackburn GL, Stanczyk FZ, McTiernan A (2012) Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J Clin Oncol 30(19):2314–2326
Cifuentes M, Riedt CS, Brolin RE, Field MP, Sherrell RM, Shapses SA (2004) Weight loss and calcium intake influence calcium absorption in overweight postmenopausal women. Am J Clin Nutr 80(1):123–130
Conroy R, Girotra M, Shane E, McMahon DJ, Pavlovich KH, Leibel RL, Rosenbaum M, Korner J (2011) Leptin administration does not prevent the bone mineral metabolism changes induced by weight loss. Metabolism 60(9):1222–1226
Crandall CJ, Yildiz VO, Wactawski-Wende J, Johnson KC, Chen Z, Going SB, Wright NC, Cauley JA (2015) Postmenopausal weight change and incidence of fracture: post hoc findings from Women’s Health Initiative Observational Study and Clinical Trials. BMJ 350:h25
Forsmo S, Aaen J, Schei B, Langhammer A (2006) What is the influence of weight change on forearm bone mineral density in peri- and postmenopausal women? The Health Study of Nord-Trøndelag, Norway. Am J Epidemiol 164(9):890–897
Forsmo S, Langhammer A, Schei B (2009) Past and current weight change and forearm bone loss in middle-aged women: the Nord-Trøndelag Health Study, Norway. Menopause 16(6):1197–1204
Franek E, Wichrowska H, Gozdowski D, Puzianowska-Kuźnicka M (2009) WHO fracture risk calculator (FRAX™) in the assessment of obese patients with osteoporosis. Endokrynol Pol 60(2):82–87
Henriksen M, Christensen R, Hunter DJ, Gudbergsen H, Boesen M, Lohmander LS, Bliddal H (2014) Structural changes in the knee during weight loss maintenance after a significant weight loss in obese patients with osteoarthritis: a report of secondary outcome analyses from a randomized controlled trial. Osteoarthritis Cartilage 22(5):639–646
Hinton PS, LeCheminant JD, Smith BK, Rector RS, Donnelly JE (2009) Weight loss-induced alterations in serum markers of bone turnover persist during weight maintenance in obese men and women. J Am Coll Nutr 28(5):565–573
Hinton PS, Rector RS, Thomas TR (2006) Weight-bearing, aerobic exercise increases markers of bone formation during short-term weight loss in overweight and obese men and women. Metabolism 55(12):1616–1618
Hla MM, Davis JW, Ross PD, Yates AJ, Wasnich RD (2001) The relation between lifestyle factors and biochemical markers of bone turnover among early postmenopausal women. Calcif Tissue Int 68(5):291–296
Hopman WM, Leroux C, Berger C, Joseph L, Barr SI, Prior JC, Harrison M, Poliquin S, Towheed T, Anastassiades T, Goltzman D, Tenenhouse A, Godmaire S, Joyce C, Kovacs C, Sheppard E, Kirkland S, Kaiser S, Stanfield B, Brown JP, Bessette L, Gendreau M, Matthews B, Josse B, Murray T, Gardner-Bray B, Kreiger N, Adachi JD, Papaioannou AA, Pickard L, Olszynski WP, Davison KS, Thingvold J, Hanley DA, Allan J, Vigna Y (2007) Changes in body mass index in Canadians over a five-year period: results of a prospective, population-based study. BMC Public Health 7(1):150–159
Messier SP, Legault C, Loeser RF, Van Arsdale SJ, Davis C, Ettinger WH, DeVita P (2011) Does high weight loss in older adults with knee osteoarthritis affect bone-on-bone joint loads and muscle forces during walking? Osteoarthritis Cartilage 19(3):272–280
Nguyen T, Sambrook P, Eisman J (1998) Bone loss, physical activity, and weight change in elderly women: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Metab Res 13(9):1458–1467
Sakamoto K, Endo N, Harada A, Sakada T, Tsushita K, Kita K, Hagino H, Sakai A, Yamamoto N, Okamoto T, Liu M, Kokaze A, Suzuki H (2013) Why not use your own body weight to prevent falls? A randomized, controlled trial of balance therapy to prevent falls and fractures for elderly people who can stand on one leg for ≤15 s. J Orthop Sci 18(1):110–120
Sharp DS, Andrew ME, Burchfiel CM, Violanti JM, Wactawski-Wende J (2012) Body mass index versus dual energy x-ray absorptiometry-derived indexes: predictors of cardiovascular and diabetic disease risk factors. Am J Hum Biol 24(4):400–405
Uusi-Rasi K, Sievänen H, Heinonen A, Beck TJ, Vuori I (2005) Determinants of changes in bone mass and femoral neck structure, and physical performance after menopause: a 9-year follow-up of initially peri-menopausal women. Osteoporos Int 16(6):616–622
Wand JS, Smith T, Green JR, Hesp R, Bradbeer JN, Reeve J (1992) Whole-body and site-specific bone remodelling in patients with previous femoral fractures: relationships between reduced physical activity, reduced bone mass and increased bone resorption. Clin Sci 83(6):665–675
Weinsier RL, Hunter GR, Zuckerman PA, Redden DT, Darnell BE, Larson DE, Newcomer BR, Goran MI (2000) Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr 71(5):1138–1146
Berenson JR, Yellin O, Boccia RV, Flam M, Wong SF, Batuman O, Moezi MM, Woytowitz D, Duvivier H, Nassir Y, Swift RA (2008) Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin Cancer Res 14(19):6289–6295
Blake GM, Herd RJ, Patel R, Fogelman I (2000) The effect of weight change on total body dual-energy X-ray absorptiometry: results from a clinical trial. Osteoporos Int 11(10):832–839
Cussler EC, Going SB, Houtkooper LB, Stanford VA, Blew RM, Flint-Wagner HG, Metcalfe LL, Choi JE, Lohman TG (2005) Exercise frequency and calcium intake predict 4-year bone changes in postmenopausal women. Osteoporos Int 16(12):2129–2141
Cussler EC, Lohman TG, Going SB, Houtkooper LB, Metcalfe LL, Flint-Wagner HG, Harris RB, Teixeira PJ (2003) Weight lifted in strength training predicts bone change in postmenopausal women. Med Sci Sports Exerc 35(1):10–17
Erlandson KM, Kitch D, Tierney C, Sax PE, Daar ES, Tebas P, Melbourne K, Ha B, Jahed NC, McComsey GA (2013) Weight and lean body mass change with antiretroviral initiation and impact on bone mineral density. AIDS 27(13):2069–2079
Iwamoto J, Takeda T, Sato Y, Uzawa M (2005) Effect of whole-body vibration exercise on lumbar bone mineral density, bone turnover, and chronic back pain in post-menopausal osteoporotic women treated with alendronate. Aging Clin Exp Res 17(2):157–163
Patel R, Blake GM, Herd RJM, Fogelman I (1997) The effect of weight change on DXA scans in a 2-year trial of etidronate therapy. Calcif Tissue Int 61(5):393–399
Prince R, Devine A, Dick I, Criddle A, Kerr D, Kent N, Price R, Randell A (1995) The effects of calcium supplementation (milk powder or tablets) and exercise on bone density in postmenopausal women. J Bone Miner Res 10(7):1068–1075
Ricci TA, Chowdhury HA, Heymsfield SB, Stahl T, Pierson RN, Shapses SA (1998) Calcium supplementation suppresses bone turnover during weight reduction in postmenopausal women. J Bone Miner Metab Res 13(6):1045–1050
Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA (2005) Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Metab Res 20(3):455–463
Ryan CW, Huo D, Demers LM, Beer TM, Lacerna LV (2006) Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J Urol 176(3):972–978
Wagner G, Kindrick S, Hertzler S, DiSilvestro RA (2007) Effects of various forms of calcium on body weight and bone turnover markers in women participating in a weight loss program. J Am Coll Nutr 26(5):456–461
Wood AD, Secombes KR, Thies F, Aucott LS, Black AJ, Reid DM, Mavroeidi A, Simpson WG, Fraser WD, Macdonald HM (2014) A parallel group double-blind RCT of vitamin D3 assessing physical function: is the biochemical response to treatment affected by overweight and obesity? Osteoporos Int 25(1):305–315
Allison SJ, Folland JP, Rennie WJ, Summers GD, Brooke-Wavell K (2013) High impact exercise increased femoral neck bone mineral density in older men: a randomised unilateral intervention. Bone 53(2):321–328
Bosy‐Westphal A, Later W, Schautz B, Lagerpusch M, Goele K, Heller M, Glüer CC, Müller MJ (2011) Impact of intra‐ and extra‐osseous soft tissue composition on changes in bone mineral density with weight loss and regain. Obesity 19(7):1503–1510
Compston J, Croucher P, Laskey M, Coxon A, Kreitzman S (1992) The effect of diet-induced weight loss on total body bone mass. Bone Miner 82(4):429–432
Fogelholm GM, Sievänen HT, Kukkonen-Harjula TK, Pasanen ME (2001) Bone mineral density during reduction, maintenance and regain of body weight in premenopausal, obese women. Osteoporos Int 12(3):199–206
Gossain VV, Rao DS, Carella MJ, Divine G, Rovner D (1998) Bone mineral density (BMD) in obesity effect of weight loss. J Med 30(5–6):367–376
Hinton PS, Rector RS, Linden MA, Warner SO, Dellsperger KC, Chockalingam A, Whaley-Connell AT, Liu Y, Thomas TR (2012) Weight-loss-associated changes in bone mineral density and bone turnover after partial weight regain with or without aerobic exercise in obese women. Eur J Clin Nutr 66(5):606–612
Ramsdale S, Bassey E (1994) Changes in bone mineral density associated with dietary-induced loss of body mass in young women. Clin Sci 87(3):343–348
Ruiz-Montero PJ, Castillo-Rodriguez A, Mikalacki M, Nebojsa C, Korovljev D (2014) 24-Weeks Pilates-aerobic and educative training to improve body fat mass in elderly Serbian women. Clin Interv Aging 9:243–248
Van Loan MD, Johnson HL, Barbieri TF (1998) Effect of weight loss on bone mineral content and bone mineral density in obese women. Am J Clin Nutr 67(4):734–738
Villalon KL, Gozansky WS, Van Pelt RE, Wolfe P, Jankowski CM, Schwartz RS, Kohrt WM (2011) A losing battle: weight regain does not restore weight loss-induced bone loss in postmenopausal women. Obesity (Silver Spring) 19(12):2345–2350
Caillot-Augusseau A, Lafage-Proust MH, Margaillan P, Vergely N, Faure S, Paillet S, Lang F, Alexandre C, Estour B (2000) Weight gain reverses bone turnover and restores circadian variation of bone resorption in anorexic patients. Clin Endocrinol 52(1):113–121
Gerhardt DMJM, Smolders JMH, Rijnders TAJM, Hol A, van Susante JLC (2015) Changes in bone mineral density and femoral neck narrowing in the proximal femur three to five years after hip resurfacing versus conventional total hip arthroplasty. J Arthroplasty 30(2):308–314
KJones KP, Ravnikar VA, Tulchinsky D, Schiff I (1985) Comparison of bone density in amenorrheic women due to athletics, weight loss, and premature menopause. Obstet Gynecol 66(1):5–8
Kujath AS, Quinn L, Elliott ME, Lecaire TJ, Binkley N, Molino AR, Danielson KK (2015) Different health behaviours and clinical factors associated with bone mineral density and bone turnover in premenopausal women with and without type 1 diabetes. Diabetes Metab Res Rev 31(4):421–432
Andersen RE, Wadden TA, Herzog RJ (1997) Changes in bone mineral content in obese dieting women. Metabolism 46(8):857–861
Beavers DP, Beavers KM, Loeser RF, Walton NR, Lyles MF, Nicklas BJ, Shapses SA, Newman JJ, Messier SP (2014) The independent and combined effects of intensive weight loss and exercise training on bone mineral density in overweight and obese older adults with osteoarthritis. Osteoarthritis Cartilage 22(6):726–733
Campbell WW, Tang M (2010) Protein intake, weight loss, and bone mineral density in postmenopausal women. J Gerontol A Biol Sci Med Sci 65 A(10):1115–1122
Chao D, Espeland MA, Farmer D, Register TC, Lenchik L, Applegate WB, Ettinger WH Jr (2000) Effect of voluntary weight loss on bone mineral density in older overweight women. J Am Geriatr Soc 48(7):753–759
Hamilton KC, Fisher G, Roy JL, Gower BA, Hunter GR (2013) The effects of weight loss on relative bone mineral density in premenopausal women. Obesity 21(3):441–448
Jessup JV, Horne C, Vishen RK, Wheeler D (2003) Effects of exercise on bone density, balance, and self efficacy in older women. Biol Res Nurs 4(3):171–180
Kohrt WM, Ehsani AA, Birge SJ Jr (1997) Effects of exercise involving predominantly either joint-reaction or ground-reaction forces on bone mineral density in older women. J Bone Miner Metab Res 12(8):1253–1261
Pop LC, Sukumar D, Tomaino K, Schlussel Y, Schneider SH, Gordon CL, Wang X, Shapses SA (2015) Moderate weight loss in obese and overweight men preserves bone quality. Am J Clin Nutr 101(3):659–667
Redman LM, Rood J, Anton SD, Champagne C, Smith SR, Ravussin E (2008) Calorie restriction and bone health in young, overweight individuals. Arch Intern Med 168(17):1859–1866
Riedt CS, Schlussel Y, von Thun N, Ambia-Sobhan H, Stahl T, Field MP, Sherrell RM, Shapses SA (2007) Premenopausal overweight women do not lose bone during moderate weight loss with adequate or higher calcium intake. Am J Clin Nutr 85(4):972–980
Schwartz AV, Johnson KC, Kahn SE, Shepherd JA, Nevitt MC, Peters AL, Walkup MP, Hodges A, Williams CC, Bray GA (2012) Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res 27(3):619–627
Shapses SA, Von Thun NL, Heymsfield SB, Ricci TA, Ospina M, Pierson RN Jr, Stahl T (2001) Bone turnover and density in obese premenopausal women during moderate weight loss and calcium supplementation. J Bone Miner Metab Res 16(7):1329–1336
Singh JA, Schmitz KH, Petit MA (2009) Effect of resistance exercise on bone mineral density in premenopausal women. Joint Bone Spine 76(3):273–280
Stewart KJ, Bacher AC, Hees PS, Tayback M, Ouyang P, De Beur SJ (2005) Exercise effects on bone mineral density: relationships to changes in fitness and fatness. Am J Prev Med 28(5):453–460
Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K (2011) Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 364(13):1218–1229
Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, Klein S, Holloszy JO (2006) Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med 166(22):2502–2510
Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S (2008) Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab 93(6):2181–2187
Yoo EJ, Jun TW, Hawkins SA (2010) The effects of a walking exercise program on fall-related fitness, bone metabolism, and fall-related psychological factors in elderly women. Res Sports Med 18(4):236–250
Rhodes EC, Martin AD, Taunton JE, Donnelly M, Warren J, Elliot J (2000) Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. Br J Sports Med 34(1):18–22
Salamone LM, Cauley JA, Black DM, Simkin-Silverman L, Lang W, Gregg E, Palermo L, Epstein RS, Kuller LH, Wing R (1999) Effect of a lifestyle intervention on bone mineral density in premenopausal women: a randomized trial. Am J Clin Nutr 70(1):97–103
Warren M, Petit MA, Hannan PJ, Schmitz KH (2008) Strength training effects on bone mineral content and density in premenopausal women. Med Sci Sports Exerc 40(7):1282–1288
Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, Bales C, Rochon J, Pieper C, Huang M (2015) Effect of two‐year caloric restriction on bone metabolism and bone mineral density in non‐obese younger adults: a randomized clinical trial. J Bone Miner Metab Res
Bassey EJ, Ramsdale SJ (1995) Weight-bearing exercise and ground reaction forces: a 12-month randomized controlled trial of effects on bone mineral density in healthy postmenopausal women. Bone 16(4):469–476
De Jong N, Chin A, Paw MJM, De Groot LCPGM, Hiddink GJ, Van Staveren WA (2000) Dietary supplements and physical exercise affecting bone and body composition in frail elderly persons. Am J Public Health 90(6):947–954
Englund U, Littbrand H, Sondell A, Pettersson U, Bucht G (2005) A 1-year combined weight-bearing training program is beneficial for bone mineral density and neuromuscular function in older women. Osteoporos Int 16(9):1117–1123
Svendsen OL, Hassager C, Christiansen C (1993) Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med 95(2):131–140
Kim YS, Nam JS, Yeo DW, Kim KR, Suh SH, Ahn CW (2015) The effects of aerobic exercise training on serum osteocalcin, adipocytokines and insulin resistance on obese young males. Clin Endocrinol 82(5):686–694
Nakata Y, Ohkawara K, Lee DJ, Okura T, Tanaka K (2008) Effects of additional resistance training during diet-induced weight loss on bone mineral density in overweight premenopausal women. J Bone Miner Metab 26(2):172–177
Park HA, Lee JS, Kuller LH, Cauley JA (2007) Effects of weight control during the menopausal transition on bone mineral density. J Clin Endocrinol Metab 92(10):3809–3815
Hui SS-C, Xie YJ, Woo J, Kwok TC-Y (2015) Effects of Tai Chi and walking exercises on weight loss, metabolic syndrome parameters, and bone mineral density: a cluster randomized controlled trial. Evid Based Complement Alternat Med
Hosny IA, Elghawabi HS, Fahmy Younan WB, Sabbour AA, Messih Gobrial MA (2012) Beneficial impact of aerobic exercises on bone mineral density in obese premenopausal women under caloric restriction. Skeletal Radiol 41(4):423–427
Whiteford J, Ackland TR, Dhaliwal SS, James AP, Woodhouse JJ, Price R, Prince RL, Kerr DA (2010) Effects of a 1-year randomized controlled trial of resistance training on lower limb bone and muscle structure and function in older men. Osteoporos Int 21(9):1529–1536
Reid IR (2002) Relationships among body mass, its components, and bone. Bone 31:547–555
Bonnick SL (2010) Skeletal anatomy in densitometry. In: Bone densitometry in clinical practice. Springer, pp 35–78
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377(9773):1276–1287
Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD (2009) Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 94(9):3387–3393
Galic S, Oakhill JS, Steinberg GR (2010) Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316(2):129–139
Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG (2000) Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept 92(1):73–78
Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS (2004) Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351(10):987–997
Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL et al (2002) Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol 175(2):405–415
Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, Gough TJ, Collier GR, Nicholson GC (2002) Leptin inhibits osteoclast generation. J Bone Miner Metab Res 17(2):200–209
Lenchik L, Register T, Hsu F, Lohman K, Nicklas B, Freedman B, Langefeld C, Carr J, Bowden D (2003) Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 33(4):646–651
Williams GA, Wang Y, Callon KE, Watson M, Lin J-m, Lam JB, Costa JL, Orpe A, Broom N, Naot D (2009) In vitro and in vivo effects of adiponectin on bone. Endocrinology 150(8):3603–3610
Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T (2007) Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biol 8(1):51–63
Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM (2002) AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation 106(3):388–391
Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, Liao EY (2006) Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Metab Res 21(10):1648–1656
Dennison E, Syddall H, Sayer AA, Craighead S, Phillips D, Cooper C (2004) Type 2 diabetes mellitus is associated with increased axial bone density in men and women from the Hertfordshire Cohort Study: evidence for an indirect effect of insulin resistance? Diabetologia 47(11):1963–1968
Birkeland KI, Hanssen KF, Torjesen PA, Vaaler S (1993) Level of sex hormone-binding globulin is positively correlated with insulin sensitivity in men with type 2 diabetes. J Clin Endocrinol Metab 76(2):275–278
Thrailkill KM, Lumpkin CK, Bunn RC, Kemp SF, Fowlkes JL (2005) Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metabol 289(5):E735–E745
Pei L, Tontonoz P (2004) Fat’s loss is bone’s gain. J Clin Invest 113(6):805–807
Hawkins J, Cifuentes M, Pleshko NL, Ambia-Sobhan H, Shapses SA (2010) Energy restriction is associated with lower bone mineral density of the tibia and femur in lean but not obese female rats. J Nutr 140(1):31–37
Cifuentes M, Advis JP, Shapses SA (2004) Estrogen prevents the reduction in fractional calcium absorption due to energy restriction in mature rats. J Nutr 134(8):1929–1934
Babatunde O, Forsyth J, Gidlow C (2012) A meta-analysis of brief high-impact exercises for enhancing bone health in premenopausal women. Osteoporos Int 23(1):109–119
Shapses SA, Sukumar D (2012) Bone metabolism in obesity and weight loss. Annu Rev Nutr 32:287–314
Acknowledgments
Each author acknowledges he/she has participated in the work in a substantive way and is prepared to take full responsibility for the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 59 kb)
Rights and permissions
About this article
Cite this article
Soltani, S., Hunter, G.R., Kazemi, A. et al. The effects of weight loss approaches on bone mineral density in adults: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 27, 2655–2671 (2016). https://doi.org/10.1007/s00198-016-3617-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3617-4