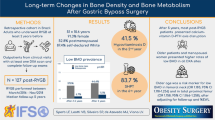
Abstract
Background
Roux-en-Y gastric bypass (RYGB) surgery is the gold standard surgical treatment for obesity. However, unintended nutritional deficiencies following this surgery are common, including changes in bone metabolism. We assessed changes in bone mineral density (BMD), nutritional compounds, and bone resorption markers before and 1 year following RYGB surgery.
Methods
Our study included 22 female patients with class II/III obesity. A clinical questionnaire, a 24-h recall, blood and urine samples, and dual-energy X-ray absorptiometry were provided.
Results
Mean age was 37.2 ± 9.6 years; 86 % were Caucasian and 77.2 % were premenopausal. Mean preoperative body mass index was 44.4 ± 5.0 and 27.5 ± 4.5 kg/m2 at 1-year follow-up (p < 0.001). 25-OH-vitamin D-levels were similar in both periods [11.7 (9.7–18.0) vs. 15.7 (10.2–2.7) pg/dL, p = 0.327]. Serum N-telopeptide (16.3 ± 3.4 vs. 38.2 ± 7.0 nM BCE, p < 0.001) and parathyroid hormone (45.4 ± 16.7 vs. 62.7 ± 28.9 pg/mL, p = 0.026) increased after RYGB surgery, reflecting bone resorption. BMD decreased after RYGB surgery in the lumbar spine (1.13 ± 0.11 vs. 1.04 ± 0.09 g/cm2, p = 0.001), femoral neck (1.03 ± 0.15 vs. 0.94 ± 0.16 g/cm2, p = 0.001), and total femur (1.07 ± 0.11 vs. 0.97 ± 0.15 g/cm2, p = 0.003).
Conclusions
Decreased BMD in the lumbar spine, femoral neck, and total femur is detectable in women 1 year after RYGB surgery. Calcium malabsorption, caused by vitamin D deficiency and increased bone resorption, is partially responsible for these outcomes and should be targeted in future clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has dramatically increased over the last several years throughout the world [1] and reached a prevalence of about 30 % in the USA in 2008 [2] and 13.5 % in Brazil in 2009 [3]. Currently, bariatric surgery is the most effective treatment to lose weight, improve quality of life, and reduce morbidity and mortality among obese individuals who could not achieve weight loss with lifestyle interventions and/or anti-obesity medication [4]. Roux-en-Y gastric bypass (RYGB) surgery is considered as the gold standard of surgical treatment for these patients because it leads to less severe malabsorption and malnutrition than traditional malabsorptive procedures (i.e., jejuno–ileal bypass) [5, 6] and to a significantly higher excess weight loss than pure restrictive procedures [7]. However, unintended nutritional deficiencies following gastric bypass surgery are a major adverse outcome, including changes in bone metabolism [8–11]. Weight loss induced by malabsorptive procedures can be accompanied by increased levels of parathyroid hormone (PTH), resulting from an inadequate intake and absorption of calcium and vitamin D [12]. Increased PTH and decreased vitamin D are known to cause osteoporosis [13] and are highly prevalent after any kind of caloric restriction [14]. This is most prominent if routine exercise is not associated with dietary interventions [15].
Although some previous studies show a decrease in bone mineral density in the first year following bariatric surgery [8, 16, 17], others show that RYGB is associated with high bone resorption and hyperparathyroidism, without a reduction in bone mineral density measured in the lumbar spine and femoral neck [18].
The aim of this 1-year follow-up study in obese women undergoing RYGB is twofold: first, to quantify the change in bone mineral density using dual-emission X-ray absorptiometry (DXA), the standard technique for bone mineral density measurement in non-obese women, and second, to assess changes in bone serum markers and nutritional components between preoperative and 1-year follow-up measurements.
Methods
We performed a prospective cohort study of patients undergoing RYGB surgery. All patients were recruited from the outpatient clinic of the Obesity and Metabolic Syndrome Center of Hospital São Lucas of Pontificia Universidade Católica do Rio Grande do Sul (PUCRS) in Porto Alegre, Brazil. The Internal Review Board of Hospital São Lucas of PUCRS approved the study and all subjects gave written informed consent before participating in it (Protocol 06/02985). The RYGB patients were recruited between January 2007 and July 2008 and met the inclusion criteria to undergo bariatric surgery (patients who maintain a body mass index (BMI) >40 kg/m2 or a BMI >35 kg/m2 with at least one comorbidity) [19]. Patients were excluded if they had preoperative gut malabsorption syndrome, if they had any gastric, kidney, or liver disease, or if they used any medication affecting bone metabolism (e.g., glucocorticoids, calcium supplements, vitamin D derivate, diuretics, and anti-epileptic drugs). All RYGB procedures were performed at the Obesity and Metabolic Syndrome Center of Hospital São Lucas of Pontificia Universidade Católica do Rio Grande do Sul. All procedures entailed the creation of a 30-ml gastric pouch, a 150-cm “Roux limb” (alimentary limb), and a 50-cm biliopancreatic limb.
Patient clinical history, blood samples, anthropometric measurements (body weight, height, abdominal and hip circumference), dietary intake (obtained through 24-h recall), and DXA were collected before and at 1 year following the RYGB procedure. Nutrient analysis of the 24-h recall was performed using the food analysis software Dietwin® Professional 2.0 (Brubins and Dataweb Tecnologia, Porto Alegre, Brazil). The percent of excess weight loss was calculated using the following formula [20]: percent excess weight loss = [(operative weight − follow-up weight)/operative excess weight] × 100], where excess weight = actual weight − ideal weight.
Bone mass density was measured using DXA (Hologic QDR-4500 Acclaim, Boston, MA, USA) at the lumbar spine (L1–L4), the total femur, and the femoral neck in all subjects. Low bone density was defined as a Z-score lower than −2.0 for premenopausal women and a T-score lower than −1.0 for post-menopausal women at any of the sites of bone mineral density studied [21].
Insulin and PTH were measured by the chemiluminescence method (Advia Centaur, Bayer Corporation, Tarrytown, NY, USA). Serum and urinary calcium, alkaline phosphatase, liver enzymes, fasting plasma glucose, creatinine, and albumin were measured by the dry chemical system (Fusion FS 5.1, Johnson & Johnson, Buckinghamshire, UK). Serum thyroxin (T4), thyrotropin (TSH), and 17β-estradiol were measured using a chemiluminescence immunoassay (Vitros ECi Immunodiagnostic System, Ortho-Clinical Diagnostics, Rochester, NY, USA). Insulin resistance was assessed by homeostasis model assessment of insulin resistance (HOMA-IR) in individuals who were not on hypoglycemic agents or insulin, as described using the formula: HOMA1–IR = fasting plasma insulin (μU/ml) × fasting plasma glucose (mM/22.5) [22]. Serum 25-hydroxyvitamin D levels were determined by radioimmunoassay based on an antibody with specificity for 25-OH-D (DiaSorin, Stillwater, MN, USA). Serum collagen-type I N-telopeptide (NTX-s) was measured by means of enzyme-linked immunosorbent assay using Osteomark NTx® serum test (Whampole Laboratories Inc., Princeton, NJ, USA). The manufacturer's recommendation of reference values for women range from 6.2 to 19.0 nM bone collagen equivalents (nM BCE). Some tests were used only as criterion for exclusion and were not included in the results.
Statistical Analysis
Data are expressed as counts and relative frequencies, means and standard deviations, or medians and interquartile ranges (P25–75) where appropriate. Fisher’s exact test was used to compare categorical variables; the paired t-test was used to compare pre- and postoperative outcomes of continuous variables. P values below 0.05 were considered as statistically significant; all tests performed were two-sided. All analyses were performed using SAS Enterprise Guide 4.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Initially, 33 patients were included in this prospective study, but only 22 patients (67 %) completed the 1-year follow-up examinations. Patients dropped out of the study for the following reasons: six patients missed the follow-up appointments, four patients refused the DXA and bone marker measurements at 1-year follow-up, and one woman died from pulmonary embolism. To evaluate the significance of the attrition rate (33 % of our patients), we compared the initial clinical data of the 11 patients who dropped out of our study to the initial clinical data of the 22 patients who remained in our study. The clinical characteristics (age, BMI, blood pressure levels) and laboratory test results (fasting plasma glucose, HOMA-IR, lipid levels) were similar (p < 0.05) between the groups.
The mean preoperative age of the 22 women was 37.2 ± 9.9 years; most were Caucasian (n = 19, 86 %) and premenopausal (n = 17, 77.2 %). The mean BMI was 44.4 ± 5.0 kg/m2 prior to RYGB surgery and 27.5 ± 4.5 kg/m2 at 1-year follow-up (p < 0.001). The mean percent excess weight loss was 89.2 ± 18.1 %. The general clinical characteristics of the subjects studied before and after the procedure are summarized in Table 1.
The mean serum albumin level remained unchanged over the first year. Fasting plasma glucose and HOMA-IR decreased significantly in the same period of observation.
Differences in the nutritional composition of the pre- and postoperative period are presented in Table 2. Overall, we observe a significant decrease in total energy intake from 3,197 to 1,257 kilocalories (p < 0.001). Reported carbohydrates, lipids, phosphorus, and calcium intake decreased at the 1-year follow-up period, while cholecalciferol intake remained unchanged over time. Although the total amount of protein intake in grams decreased from 141.1 to 68.1 (p < 0.001), protein as a percentage of total diet increased from 18.7 to 22.4 % (p < 0.050).
Preoperative and 1-year follow-up summaries of bone serum and urine markers are provided in Table 3. No differences were detected in the group’s 25-OH-vitamin D, alkaline phosphatase, serum calcium, or 24-h urinary calcium levels. Increases in NTX-s levels reflect evidence of bone resorption (p < 0.001).
BMD, measured with the DXA method, significantly decreased after RYGB surgery (lumbar spine 1.13 ± 0.11 vs. 1.04 ± 0.09 g/cm2, p < 0.001; femoral neck 1.03 ± 0.15 vs. 0.94 ± 0.16 g/cm2, p = 0.001; total femur 1.07 ± 0.11 vs. 0.97 ± 0.15 g/cm2, p = 0.003) as reflected in Fig. 1. One year following RYGB surgery, the mean percentage of bone mineral loss was 7.26 % (p < 0.001) in the lumbar spine, 8.59 % (p = 0.001) in the total femur, and 8.78 % (p = 0.003) in the femoral neck.
Comparison of the bone mineral density in the lumbar spine, femoral neck, and total femur preoperative versus the 1-year follow-up. The measurement of BMD was performed by dual energy X-ray absorptiometry. The value of P is the minimum level of significance of the paired t-test for the comparison between pre- vs. postoperative Roux-en-Y gastric bypass
Discussion
In this study, we simultaneously compare bone mineral density, bone resorption markers, nutritional components, and metabolic markers prior to and following RYGB surgery. We provide compelling evidence that bone mineral density compared to the preoperative period decreased after the 1-year follow-up in all evaluated bone segments. The decrease in BMD was accompanied by a decrease in bone serum resorption markers and low vitamin D levels, indicating increased turnover, and overall resorption, of bone substance. However, the lower energy intake, weight loss, and metabolic improvements attained were beneficial for the patients.
Bone mass density in the lumbar spine, femoral neck, and total femur decreased by an average of 8.2 % a year following bariatric surgery. Our findings are in accordance with a recent study performed by Vilarrasa et al. that monitored 59 patients before and after RYGB surgery. They report a decrease of BMD in the femoral neck of about 10 %, but only a 3-% decrease of BMD in the column 1 year after RYGB surgery [23]. In addition, Fleisher et al. support the decrease in BMD in the femoral neck (9.2 %) and the total hip (8.0 %) but similarly do not confirm the decrease of BMD in the lumbar spine 1 year after RYGB surgery [8]. A potential explanation for why they were unable to detect a decrease of BMD in the lumbar spine might be associated with the DXA instrument. DXA may be unable to accurately measure changes in adipose tissue density and distribution following bariatric surgery, as previously described by Tothill et al. [24].
To adequately evaluate bone metabolism after surgery, it is crucial to measure BMD, the specific bone serum markers, vitamin D levels, and also calcium and phosphorus intake. We found that NTX-s and PTH levels were increased 1 year following RYGB surgery compared to preoperative measurements. The high level of NTX-s, a very specific marker for bone resorption, indicates bone mineral loss [25, 26]. This loss may be caused by either low calcium and phosphorus intake or absorption [27] or inadequate bone metabolism [28, 29]. We believe that the combination of both factors is responsible for bone mineral loss in RYGB patients. Other studies used NTX-u (NTX measured in the urine), instead of NTX-s, to show increased bone resorption [8, 9, 30], but serum-based markers of bone turnover (NTX-s) tend to show less variability compared to the urine-based markers (NTX-u) [26]. Further, Eastell et al. showed that NTX-s can be a good marker for monitoring of bone resorption [26].
As we previously reported, most of our patients had a deficiency of serum vitamin D levels in the preoperative period [31], which were lower than the initial vitamin D levels recorded in other research [8, 23, 32]. In the postoperative period, the vitamin D levels of our patients increased by 50 % through the use of a multivitamin supplement and regular doses of cholecalciferol. However, these levels were still lower than those observed in the postoperative period of the previously mentioned studies [8, 23]. We suspect that a few reasons account for the lower vitamin D levels found in our patients. First, the characteristics of patients vary from study to study, and different serum vitamin D levels vary across differing age groups, genders, body fat distributions, and smoking habits [33]. Second, it is known that vitamin D levels are associated with sun exposure, which is often lower in obese patients due to lack of outdoor activity [34, 35]. Third, obese patients often suffer from systemic inflammation, which may interfere with vitamin D metabolism [33, 36]. This research reinforces the importance of strict supplementation of vitamin D in the postoperative period following RYGB surgery in doses well above the recommended daily allowance [37] to achieve beneficial effects on BMD [38].
Additionally, the intake of calcium and phosphorus among our patients following surgery did significantly decrease below the recommended daily intake [37], and this is another important risk factor in BMD loss [39]. Other studies confirm decreased intake of calcium following RYGB surgery, which can persist up to 8 years after surgery [40–42]. In contrast, Fleischer et al. showed that, with additional micronutrient supplementation, an increase of calcium and phosphorus intake can be achieved [8].
We would like to acknowledge the limitations of our study. As with other prospective studies in bariatric surgery evaluating BMD, the sample size of our study is limited. The primary limitation to patient participation relates to the fact that DXA equipment is often unable to measure patients exceeding a weight of 150 kg. Furthermore, we experienced a high postsurgery attrition rate, which is similar to other obesity studies [43, 44]. Moreover, a high prevalence of vitamin D deficiency was observed in the preoperative evaluation, which could account, at least in part, for the bone mineral density reduction observed in our bariatric surgery patients. However, the clinical characteristics and lab results of both groups were similar, which reinforces our belief that our findings were not skewed by patient attrition.
Conclusion
Our study provides preliminary evidence that women with class II and III obesity may have early increases in bone resorption and bone mineral density loss 1 year following RYGB surgery. Calcium malabsorption caused by vitamin D deficiency and increased bone resorption are partially responsible for these outcomes and thus should be measured in future clinical trials involving a larger number of patients being submitted to bariatric surgery. We suggest that routine follow-up measurements should be performed to monitor changes in patient bone metabolism and bone mineral density over time.
References
Rokholm B, Baker JL, Sorensen TI. The levelling off of the obesity epidemic since the year 1999—a review of evidence and perspectives. Obes Rev. 2010;11(12):835–46.
Flegal KM, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–41.
Moura EC , Claro RM. Estimates of obesity trends in Brazil, 2006–2009. Int J Public Health. 2011.
Colquitt JL, et al. Surgery for obesity. Cochrane Database Syst Rev. 2009(2): p. CD003641.
Barrow CJ. Roux-en-Y gastric bypass for morbid obesity. AORN J. 2002;76(4):590. 593–604; quiz 606–8.
Garcia OP, Long KZ, Rosado JL. Impact of micronutrient deficiencies on obesity. Nutr Rev. 2009;67(10):559–72.
Campos GM, et al. Better weight loss, resolution of diabetes, and quality of life for laparoscopic gastric bypass vs banding: results of a 2-cohort pair-matched study. Arch Surg. 2011;146(2):149–55.
Fleischer J, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735–40.
Sinha N, et al. Increased PTH and 1.25(OH)(2)D levels associated with increased markers of bone turnover following bariatric surgery. Obesity (Silver Spring). 2011;19:2388–93.
Higa K, et al. Laparoscopic Roux-en-Y gastric bypass: 10-year follow-up. Surg Obes Relat Dis. 2010;7:516–25.
Gehrer S, et al. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)—a prospective study. Obes Surg. 2010;20(4):447–53.
Riedt CS, et al. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res. 2005;20(3):455–63.
Mezquita-Raya P, et al. Relation between vitamin D insufficiency, bone density, and bone metabolism in healthy postmenopausal women. J Bone Miner Res. 2001;16(8):1408–15.
Ricci TA, et al. Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr. 2001;73(2):347–52.
Villareal DT, et al. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93(6):2181–7.
Mahdy T, et al. Effect of Roux-en Y gastric bypass on bone metabolism in patients with morbid obesity: Mansoura experiences. Obes Surg. 2008;18(12):1526–31.
Vilarrasa N, et al. Evaluation of bone mineral density loss in morbidly obese women after gastric bypass: 3-year follow-up. Obes Surg. 2011;21(4):465–72.
Valderas JP, et al. Increase of bone resorption and the parathyroid hormone in postmenopausal women in the long-term after Roux-en-Y gastric bypass. Obes Surg. 2009;19(8):1132–8.
Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992. 55(2 Suppl): p. 615S–619S.
Deitel M, Greenstein RJ. Recommendations for reporting weight loss. Obes Surg. 2003;13(2):159–60.
Baim S, et al. Official positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J Clin Densitom. 2008;11(1):75–91.
Matthews DR, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Vilarrasa N, et al. Evaluation of bone disease in morbidly obese women after gastric bypass and risk factors implicated in bone loss. Obes Surg. 2009;19(7):860–6.
Tothill P. Dual-energy X-ray absorptiometry measurements of total-body bone mineral during weight change. J Clin Densitom. 2005;8(1):31–8.
Yoshimura, N, et al. Biochemical markers of bone turnover as predictors of osteoporosis and osteoporotic fractures in men and women: 10-year follow-up of the Taiji cohort. Mod Rheumatol. 2011.
Eastell R, et al. Biological variability of serum and urinary N-telopeptides of type I collagen in postmenopausal women. J Bone Miner Res. 2000;15(3):594–8.
Singhellakis PN, et al. Vitamin D deficiency in White, apparently healthy, free-living adults in a temperate region. Hormones (Athens). 2011;10(2):131–43.
Flores L, et al. Calcium and vitamin D supplementation after gastric bypass should be individualized to improve or avoid hyperparathyroidism. Obes Surg. 2010;20(6):738–43.
Williams SE. Metabolic bone disease in the bariatric surgery patient. J Obes. 2011;2011:634614.
Nogues X, et al. Bone mass loss after sleeve gastrectomy: a prospective comparative study with gastric bypass. Cir Esp. 2010;88(2):103–9.
Casagrande DS, et al. Bone mineral density and nutritional profile in morbidly obese women. Obes Surg. 2010;20(10):1372–9.
Gemmel K, et al. Vitamin D deficiency in preoperative bariatric surgery patients. Surg Obes Relat Dis. 2009;5(1):54–9.
Lee JH, et al. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52(24):1949–56.
Saraiva GL, et al. Influence of ultraviolet radiation on the production of 25 hydroxyvitamin D in the elderly population in the city of Sao Paulo (23 degrees 34′S), Brazil. Osteoporos Int. 2005;16(12):1649–54.
Silva BC, et al. Prevalence of vitamin D deficiency and its correlation with PTH, biochemical bone turnover markers and bone mineral density, among patients from ambulatories. Arq Bras Endocrinol Metabol. 2008;52(3):482–8.
Wortsman J, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3.
Aills L, et al. ASMBS Allied Health nutritional guidelines for the surgical weight loss patient. Surg Obes Relat Dis. 2008;4(5 Suppl):S73–S108.
Jackson RD, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–83.
Whitehead CC, Fleming RH. Osteoporosis in cage layers. Poult Sci. 2000;79(7):1033–41.
Duran de Campos C, et al. Calcium intake and metabolic bone disease after eight years of Roux-en-Y gastric bypass. Obes Surg. 2008;18(4):386–90.
Ott MT, et al. biochemical evidence of metabolic bone disease in women following Roux-Y gastric bypass for morbid obesity. Obes Surg. 1992;2(4):341–8.
Colossi FG, et al. Need for multivitamin use in the postoperative period of gastric bypass. Obes Surg. 2008;18(2):187–91.
Karlsson J, et al. Psychosocial functioning in the obese before and after weight reduction: construct validity and responsiveness of the Obesity-Related Problems Scale. Int J Obes Relat Metab Disord. 2003;27(5):617–30.
Garb J, et al. Bariatric surgery for the treatment of morbid obesity: a meta-analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass. Obes Surg. 2009;19(10):1447–55.
Acknowledgments
We thank Dr. Melissa Markoski from Instituto de Cardiologia do Rio Grande do Sul/Fundação Universitária de Cardiologia, Porto Alegre, Brazil, for the bone resorption markers' measurements. This work was supported by a grant from the Brazilian Federal Agency for Support and Evaluation of Graduate Education—Capes and PBBEP3-131567 from the Swiss National Science Foundation (MW). The authors have no other potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s11695-015-1793-5.
Rights and permissions
About this article
Cite this article
Casagrande, D.S., Repetto, G., Mottin, C.C. et al. Changes in Bone Mineral Density in Women Following 1-Year Gastric Bypass Surgery. OBES SURG 22, 1287–1292 (2012). https://doi.org/10.1007/s11695-012-0687-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-012-0687-z