Abstract
Summary
A snapshot of current evidence from 6 randomised controlled trials for the effects of short bouts of high-impact exercises in 256 women via meta-analysis reveals that ample osteogenic response could be realised at the femoral neck and trochanter of premenopausal women with rest-inserted bouts of few mechanical bone loading cycles.
Introduction
Exercise is an important means of improving bone health and preventing osteoporosis. Brief bouts of simple exercises may be useful for aiding lifestyle compliance to physical activity. This study aimed to review the evidence on the effect of brief, high-impact exercise on bone health among premenopausal women.
Methods
A structured and comprehensive search of databases was undertaken along with hand searching of key journals and reference lists to locate relevant studies published and unpublished up to January 2011. Six randomised controlled trials met predetermined inclusion criteria. Brief high-impact exercises (<30 min) were examined for their effect on bone mineral density (BMD) among premenopausal women. Trial quality was assessed using the Effective Public Health Practice Project quality assessment tool. Study outcomes for analysis, absolute change (grams per square centimetre) or relative change (in percent) in BMD at femoral neck, trochanter and lumbar spine were compared by calculating standardised mean difference (SMD) using fixed- and random effects models.
Results
Quality of included trials varied from medium to high on a scale of 1 to 3. Brief bouts of exercise led to significant increases in femoral neck BMD (SMD = 0.64, 95% confidence interval (CI) = 0.38, 0.90, overall effect Z value = 4.84, p = 0.001); a modest increase in trochanteric BMD (SMD = 0.36, 95% CI = 0.10, 0.61, Z value = 2.08, p = 0.04) and no increase in spinal BMD (SMD = 0.04, 95% CI = −0.23, 0.31, Z value = 0.26, p = 0.79).
Conclusion
Based on the meta-analysis, brief high-impact exercise improves BMD at the hip but not at the lumbar spine. Effectiveness of this form of exercise as a lifestyle physical activity for prevention of osteoporosis should be explored in larger populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is second only to cardiovascular diseases as a leading health care problem worldwide [1]. Given the absence of somatic symptoms that can easily be attributed to poor bone health, many women who are at risk of osteoporosis are unaware of its existence until later in life, when preventative measures are barely helpful [2]. Based on current understanding of the pathology of osteoporosis and its modification by physical activity, generally accepted strategies to improve bone health and reduce the incidence of osteoporotic fracture in women aim to: maximise peak bone mass during growth (childhood and adolescence), minimise age-related bone loss (middle-aged adults/premenopausal women) and prevent falls and fractures (in older adults/postmenopausal women) [3]. The efficacy of mechanical loading as a potent osteogenetic influence on bone is a well-known issue in bone health research [3], but there is still a wide gap between research and practice in terms of using physical activity as an effective and inexpensive means of preventing osteoporosis [1, 3].
A variety of opportunities (e.g. organized sports, school sports) exist for children and adolescents to increase lifestyle physical activity and thereby optimise peak bone mass during growth [2]. Leisure time physical activities, through community- or group-led initiatives, also exist for the older population [1, 2]. Lack of time has been implicated as one of the most important barriers to physical activity among premenopausal women as women in this age group claim to be busy, due to, for instance, family or paid employment [4]. As a result, regular physical activity has attracted less attention in the next generation of older women who are at risk of developing osteoporosis [1, 3]. Given that 15% to 25% of bone mineral density (BMD) can be lost during the premenopausal years alone [2], if prevention of osteoporosis is not properly addressed in this at-risk population, considerable social and health care costs will continually be directed towards treatment [5]; this undoubtedly will be an economically undesirable outcome. Thus, public health strategies aimed at improving bone health through specification of a practical lifestyle approach to increase physical activity may help to reduce overall prevalence of osteoporosis [6].
Disparate findings have emerged from previous systematic reviews and meta-analyses that sought to evaluate the optimal type and amount of exercise intervention that can significantly alter bone turnover and remodelling in premenopausal women [7–9]. To our knowledge, the effects of short duration and low-frequency high-impact exercises on bone health among premenopausal women are yet to be systematically reviewed. Whilst the role of non-impact exercises in conservation of bone density is yet to be clearly enunciated [9, 10], impact exercises (e.g. aerobic and weight-bearing exercises) have yielded fairly consistent positive results [7, 8]. However, wide variation exists in terms of the frequency and intensity employed in those exercise programmes. Furthermore, most of the exercise intervention programmes that have been demonstrated to be beneficial for enhancing bone health in premenopausal women are extensive, requiring at least tri-weekly sessions that are facility based, reducing the affordability and feasibility on a long-term basis [11–14]. Thus, it appears that precise methods of delivering effective, accessible and sustainable exercise to improve premenopausal bone health is yet to be defined.
Animal studies have found that bone, whilst insensitive to static loading, responds to few dynamic load cycles of high magnitude that produce unusual strain distribution and that brief exposure to strain is sufficient to produce ample osteogenic effects [15–17]. Similar strain patterns in the form of high-impact exercise interventions have been adapted for women and yielded promising results [18–21]. An evidence-based synthesis of these types of intervention, in a bid to specify practical lifestyle exercises for enhancing bone health and preventing osteoporosis, is warranted. The aim of this present study, therefore, was to investigate, through systematic review and meta-analysis, the effects of brief lifestyle exposure to bouts of high-impact exercise on the bone health of premenopausal women.
Methods
A systematic review of published and unpublished literature on the effects of brief bouts of high-impact exercise on bone health among premenopausal women was conducted. In order to specify simple exercise interventions involving little or no technical skills and that could be easily adopted into a lifestyle as well as circumvent potential barriers to exercise such as lack of time, unaffordability of facility-based programs, an exclusion of studies with >30% supervision of exercise intervention, was applied in this review. The inclusion criteria are given in Table 1. Structured computer searches of MEDLINE/PubMed, EMBASE, Web of Science, SportDiscus, Cochrane controlled trials register, ProQuest and CINAHL were undertaken from their inception to January 2011. Search terms, keywords and participant headings in the searches are presented in Appendix 1. The search was supplemented by a search of OpenSigle for Grey literature, hand searching of key journals (Journal of Bone Mineral Research, Bone, Calcified Tissue International, Osteoporosis International and Medicine and Science in Sports and Exercise) and citation tracking and reference lists. Current awareness searches were conducted by contacting expert authors in the field for published and unpublished literature in the area. Full text versions of relevant articles were obtained and assessed by two independent reviewers (OB and CG).
Methodological quality assessment of studies
Quality of studies was assessed independently by two investigators (OB and CG) using the Effective Public Health Practice Project quality assessment tool for quantitative studies [22]. Using this tool, rating opinion was based upon information contained in the study. Differences of opinion regarding scoring of articles were resolved between the two investigators through discussion until consensus was reached.
Data extraction and analysis
Data extraction was completed for all included studies independently by OB and CG. The data extracted included: the participants' age and premenopausal status; number of allocated participants; number of participants followed up; mode of exercise intervention (intensity (number of loading cycles), duration (minutes), frequency (number of times per week), length of intervention (months)); setting (home- or facility based); attrition; compliance; whether exercise was supervised or not; adjuvant pharmacological or nutritional therapy and outcome measures (region of interest (ROI) assessed; BMD values with standard deviation). Where standard deviations were not provided, they were calculated using recommended equations [23].
To provide a measure of the impact of each exercise intervention, as well as a comparison between outcomes reported by individual studies, effect sizes (standardised mean difference (SMD)) after Hedges' g transformation with 95% confidence intervals (CI) were calculated. This transformation was necessary as change in mean BMD scores was reported in different units (absolute change (grams per square centimetre) or relative change (in percent)). Meta-analysis using a fixed effect model was applied to investigate specific ROI (femoral neck, lumbar spine and trochanter). For studies with multi-intervention groups [20, 24], data from the exercise group which were reported to have generated the most osteogenetic response in their respective trials were incorporated into the meta-analysis. Between-study heterogeneity was assessed through visual inspection of funnel plots, Cochran's χ 2 test (p < 0.05 indicating significance) and the I 2 index values (25%, 50% and 75% represents low, moderate and high heterogeneity, respectively). These values were generated using the Cochrane Collaboration Review Manager (RevMan) computer programme, version 5.1.
Results
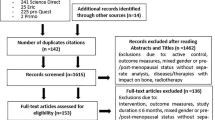
Six randomised controlled trials (RCT) involving 255 participants in total met the predetermined inclusion criteria. A summary of the review process is presented in Fig. 1 and a synopsis of data from the six studies is presented in Table 2.
Description of studies
All of the included trials in this review were duplicated in the electronic databases that were searched. They were also cited in the reference lists of each other and many other prominent articles published in this area of research. One large on-going RCT, which fits into this present review's inclusion criteria, was found through correspondence with experts in the field [25], but no new studies were found through a monthly search alert system that was set up to intimate the author of new publications in the area.
Study quality assessment
Although all of the studies reported randomisation of study participants into exercise intervention groups and control groups, none employed random recruitment or detailed the randomisation process. Only two of the six studies [19, 21] reported concealed allocation of subjects to their respective groups (semi-blinded). Bassey and Ramsdale [18] reported outcome assessor blinding to group allocation of participants. With a ‘2’ rating, on a scale of 1 to 3, most of the studies were classified as having moderate methodological quality [18–20, 24, 26]. Only one study [21] was considered to have high methodological quality with a rating of ‘1’.
All the trials were conducted with apparently healthy premenopausal women between the ages of 18 and 50 years. Premenopausal status was based on self-completed lifestyle and menstrual history questions. The sample size varied from 42 [21] to 91 participants [26]. Only three studies [20, 24, 26] reported a power calculation and estimated achievement of at least 80% power to detect between 2% and 3% differences in BMD of premenopausal women in response to the brief high-impact exercise intervention at the 0.05 alpha level. While there were generally mild variances in the number of participants allocated to each group (intervention versus control) across the studies at baseline, in one trial [19], there were five more subjects in the intervention group due to a higher anticipated dropout rate in the exercise group. Bailey and Brooke-Wavell [20], who had three exercise intervention groups, successfully preserved the required number of participants (n = 16) in each group, according to a priori power calculation.
Intervention
All studies compared the effectiveness of a high-impact exercise intervention with their respective control groups. The control groups in three of the studies received no specific treatment [19–21]. In two of the studies [20, 21], the control group participants were requested to maintain their usual diet and lifestyle, while Bassey et al. [19] gave no information on any form of intervention to control participants. The participants in the control groups of the other three studies [18, 24, 26] performed mild stretching (sham) exercises which followed a similar regime with their respective experimental group in terms of duration, frequency and time commitment. The exercise intervention in three studies [18, 19, 24] comprised of brief bouts of high-impact jumping exercises 6 days in a week. Similar interventions were carried out by two other studies [21, 26] on alternate days (three sessions per week). Bailey and Brooke-Wavell [20] had three intervention groups who completed the same hopping exercise on 2, 4 or 7 days per week. Strong [24] also had 2 intervention groups, 1 of whom performed 10 high-impact jumps and the other who performed 20 jumps. The number of high-impact jumps completed as part of the intervention varied across the studies, but they were commonly completed in five sets of ten high-impact jumps or hops, which were interspersed with rest intervals [18–20, 26]. Kato et al. [21] employed only one set of ten vertical jumps each interspersed with 8–12 s of rest, while Strong [24] employed either one or two sets of ten high-impact jumps. Each jump was interspersed with a 30-s rest interval.
With the exception of one study, which was office based and video guided [26], the exercise interventions of all the studies included in this review were completed without supervision at the participants' homes for 80% of the time. Four of the trials included a few supervised sessions in order to ensure adherence and compliance to precise exercise protocols. The supervision sessions that were introduced in those trials ranged from 0% [20, 21, 24] to 30% supervised sessions [26]. The intervention periods were 4 [24], 6 [18–21] and 12 months [26].
Outcome measures/measurement tools
A number of outcome measures were reported. Measurement of BMD as a function of bone health using dual energy X-ray absorptiometry (DXA) was the primary outcome for all studies. Specific ROIs assessed were the lumbar spine L1–L4 [18, 19, 21, 24, 26], femoral neck and the trochanter [18–21, 24, 26]. Assessors were reported to have been blinded to participants' group allocation in all studies. Secondary outcomes included bone resorption markers, ground reaction forces and accelerometry data. In two of the studies [21, 26], bone resorption markers were measured to complement the BMD assessment; Kato et al. [21] measured deoxypyridinoline, while Niu et al. [26] measured adiponectin concentration and gave evidence of bone metabolism as a result of the exercise intervention. Ground reaction forces were measured in five studies and were found to be four to five times the body mass. Niu et al. [26] and Kato et al. [21] employed accelerometry-based measures of physical activity, which enabled them to classify the physical activity profiles of participants in the exercise intervention groups of their study as ‘average’. The study by Niu et al. [26] also included quality of life (QOL) and cardiovascular risk factor assessments. They found significant decreases in systolic blood pressure and low-density lipoprotein cholesterol and significant increases in high-density lipoprotein cholesterol in both exercise and control groups post-intervention. The authors also reported significant increases in diastolic blood pressure and total cholesterol for the control group after 1 year when compared with baseline values, but the QOL indexes were not perceptibly different between groups. In order to clarify any association with improvement of physical capabilities as a result of the exercise intervention, in three studies [18, 19, 26], leg strength and coordination were measured, but a positive relationship with changes in BMD was not found for either of these two measures.
Dropout rate
Kato et al. [21] had only 1 dropout (2.4% of the total sample) from 36 participants in the control group through lack of interest. In contrast, Bassey and Ramsdale [18] reported 48% dropout rate but did not specify the reasons and the group from which they had dropped out. The office-based intervention of Niu et al. [26] accounted for a 26.4% dropout rate which was mostly due to job relocation. Bailey and Brooke-Wavell [20] lost 24.7% of the participants for reasons mostly related to personal or changed circumstances (17.9%) and adverse events (6.8%) including ankle sprains and back discomforts.
Meta-analyses
The included trials represented evidence from 255 premenopausal women who participated in between 4 and 12 months of non-facility based, brief bouts of high-impact jumping or hopping exercises. A significant improvement in femoral neck BMD (0.64 SMD; 95% CI 0.38, 0.90) (Fig. 2a) and a 0.36 SMD (95% CI 0.10, 0.61) (Fig. 2b) significant improvement in trochanteric BMD were apparent. Significant BMD improvements were not evident for the lumbar spine (Fig. 2c). The I 2 parameter indicated a substantial amount of unexplained heterogeneity at the femoral and trochanteric sites owing to a significantly large effect observed in the study by Bassey & Ramsdale [18]. On the exclusion of this study from the meta-analysis, the I 2 became 0% and the summary effect size remained significant at these ROIs (Table 3). Both fixed and random effects' models were unchanged in the subgroup analysis for all outcomes (Table 3). Funnel plots, which may not be easily depicted due to the small number of studies, were produced for all the ROIs. Visual inspection of these plots shows some asymmetry indicating the possibility of publication bias. The funnel plot for the femoral neck is presented in Fig. 3.
Discussion
The aim of this meta-analysis was to evaluate the effect of brief bouts of high-impact exercise on the bone health of premenopausal women. The six RCTs synthesised in this review were of medium to high methodological quality. There was evidence to support the efficacy of high-impact exercises in bringing about 0.36 SD and 0.64 SD respective increases in trochanteric and femoral BMD among premenopausal women. Regarding clinical significance, each 1 SD decrease in BMD is thought to be associated with a 10% increase in fracture risk [27]; findings from this present study implies that an increase in BMD of these extent (0.36 and 0.64 SD) if sustained could affect an almost 3.6% and 6.4% decrease in hip fracture risk. The overall effect could be greater considering the added benefits of augmenting muscle mass, strength gain and dynamic balance, all of which are independent risk factors for fracture [27].
Significant heterogeneity was brought into the meta-analysis by the study of Bassey et al. [19]; this suggests that the result of this particular study must be interpreted with caution. Possible sources of bias could be due to method of recruitment of participants into this study [19] which involves women registered with a particular GP practice. These women may not be representative of a larger population. However, exploration of heterogeneity due to the large variance effect from the study of Bassey et al. [19] was explored via subgroup analysis (Table 3) and the results remained statistically significant for the trochanteric SMD, 0.22 (−0.04, 0.49) and femoral neck SMD, 0.53 (0.26, 0.79) BMD.
Based on this meta-analysis, no significant beneficial effect of high-impact exercise was found at the lumbar spine. The individual studies in this review also showed no consistent benefit of this type of exercise on spinal BMD. It has been suggested that the loads engendered during high-impact exercise are hinged upon the interaction between the targeted bone's morphology and the amount and orientation of mechanical load applied to it [28]. It appears that the mechanical load generated during the jumping exercises could have been attenuated before being translated to the spine, and as such, does not generate sufficient osteogenic stimulus for bone formation [29]. Moreover, different bone regions may respond differently to mechanical loads due to similar movements [28]. It could also be that the lumbar spine responded to this type of exercise intervention in a manner that had not been captured in the given time frame or by BMD which is the efficacy criterion that was employed by all the trials in this review. Further research is required to bridge the gap of knowledge in this regard.
Intention-to-treat analysis was not used in any of the studies; thus, potential bias as a result of attrition failed to be accounted for. The participants were followed up post-intervention in only one of the trials [18] and it was reported that the group maintained their improvement relative to baseline in the 6 months following exercise intervention. This finding is consistent with that of Kontulainen et al. [30], who demonstrated a significant increase in BMD in response to an 18-month high-impact exercise intervention as well as a relative maintenance of this gain 3.5 years after the intervention. The accompanying neuromuscular performance improvements, in contrast, disappeared during the post-intervention follow-up [30]. Due to the limited post-intervention data in the present analysis, recommendation on the long-term effect of brief, high-impact exercises on bone health cannot be presented.
Measurements of BMD in all the studies in this review were made with the aid of DXA; however, possible variations in BMD measurements cannot be excluded due to inherent differences in instrument models, manufacturers and operators [31]. Though not synonymous with bone strength, BMD, as an expression of bone mineral, has a widespread use in bone research as an outcome measure. There remains equivocal criticism of BMD measurement on account of its non-consideration of vital components of bone strength (bone architecture and material properties), which are similarly influenced by mechanical loading [31]. Aggregated data from a recent meta-analysis by Nikander et al. [32] indicate that BMD and geometrical adaptations of bones to mechanical loading vary by age, skeletal site and sex. There is, therefore, a possibility that measurement of alternative endpoints by the trials in this study could have given a more vivid picture of bone adaptation in response to brief bouts of high-impact exercises among premenopausal women.
Only one of the trials included in this review reported associated injuries as a result of participation in brief high-impact exercises [19] which may be due to the unilateral nature of the exercise intervention. All the other studies in the review involved two-legged jumps with countermovement style. It may be that the predominant style offers more support to the back and lower limbs and as such carries less risk to the participants. Moreover, the exercises, though high impact in nature were submaximal and probably too brief for likelihood of significant injury. It should be noted that the participants were young to middle-aged adults who as at the time of baseline measurements were not shown to be at risk of developing osteoporosis or sustaining an osteoporotic fracture. Bassey et al. [19] had an exercise arm comprising of postmenopausal women: some of whom were on hormone replacement therapy, none of whom reported exercise related injury. However, caution would need to be exercised in recommending this type of exercise to postmenopausal women or women at high risk of fracture.
For many chronic diseases, low adherence to therapy is a public health issue, especially for initially asymptomatic conditions like osteoporosis [33, 34]. The heavy reliance of the efficacy of exercise intervention on life-long compliance has long been described [35, 36]. Poor rates of adherence to exercise prescription and non-sustainability of exercise regimes beyond study lifetime present a potential barrier to the enhancement of bone health in susceptible populations. A lifestyle approach to physical activity that promotes integration of simple exercises into everyday life would be valuable [37–39]. The RCTs included in this review were either home- or office based, without associated travel and facility cost, required minimal or no equipment and involved relatively small time commitments. The regimes of the RCTs [18–21, 24, 26] simulated practical lifestyle interventions that contrast favourably with other similar regimes [11–14, 28, 30] that were also excluded from this study when considering feasibility in terms of the minimal obligations of the participants and the potential for sustainability beyond the study lifespan.
The nature of exercise intervention (brief, rest inserted, mechanical loading through high-impact exercise) employed in each of the trials warrants examination in light of current understanding of bone physiology. Bone's response to mechanical loading, according to this review and that of Nikander et al. [32] does not seem to be entirely load dependent. In the animal studies by Rubin and Lanyon [15] and by Umemura et al. [17], a 50-fold increase in the number of loading cycles (from 36 to 1,800) yielded no increase in osteogenesis, and the adaptive response from 100 jumps per day was not significantly different from that realised with 40 jumps per day. These findings suggest that tissue sensitivity to mechanical loading after a minimum threshold [40] is inversely proportional to the number of consecutive loading cycles, giving a strong indication that undue levels of exercise frequency and duration may not confer additional benefits on bone health. In more recent studies on bone health [41, 42], the enhanced osteogenic response to loading with discrete bouts of high-impact exercises has been established. Based on increased intracellular calcium signalling occurring 15 s post-recurrent fluid stimuli [43, 44] in bone cell mechanotransduction [45], it was presumed that the insertion of rest periods between loading cycles would reduce inertial fluid flow effects and enhance fluid flow near the osteocytes in succeeding cycles. Srinivasan et al. [46] blamed recurrent mechanical loading as a primary contributor to the poor efficacy of exercise interventions and highlighted rest insertion as a vital tool for making reduced amounts of mechanical loading a potent osteogenic stimulus. The rest-inserted interval between bouts of high-impact exercise in this review mostly ranged from 8 to 15 s. This rest interval is consistent with the 15 s elevation in intracellular calcium signalling prescribed for resensitising bone cells to mechanical stimuli in animal studies [42, 43]. It appears that the insertion of a short rest period between each loading cycle, as employed by the studies in this review, enhanced fluid flow near the bone forming cells and optimised their response to subsequent cycles of mechanical loading. It may be that the rest insertions played an important role in amplifying the effectiveness of this type of low-frequency, high-impact exercise. Examination of cellular processes by the RCTs included in this study could have given more credence to the elucidation of the underlying physiological mechanism of these exercise interventions.
Limitations
The strength of evidence from this review is limited to data from only six RCTs. Another inherent limitation of this review is the lack of cost/economic analysis. Future researchers may want to address this gap in body of knowledge through cost benefit analysis of traditional structured exercises versus brief, home- and or office-based exercises for enhancing bone health among premenopausal women.
Conclusion
The meta-analysis showed that brief high-impact exercise improved BMD at the hip but not at the lumbar spine. Based on the studies included in this review, the insertion of rest periods between consecutive loading bouts of high-impact exercise may optimise the response of bone to mechanical loading and thus serve to enhance bone health among premenopausal women. Practitioners may now consider brief, rest-inserted, high-impact exercises as an opportunity to enhance bone health of their clients through appropriate prescription and adoption of simple, home- or office-based, lifestyle exercises. Further evidence on the cost effectiveness of this form of exercise as a lifestyle physical activity for enhancing bone health of premenopausal women should be explored in larger populations.
References
Hardman AE, Stensel DJ (2009) Physical activity and health: the evidence explained. Taylor Francis Ltd, London
Dolan SH, Williams DP, Ainsworth BE, Shaw JM (2006) Development and reproducibility of the Bone Loading History Questionnaire. Med Sci Sports Exerc 38(6):1121–1131. doi:10.1249/01.mss.0000222841.96885.a8
Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR (2004) American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc 36(11):1985–1996. doi:10.1249/01.MSS.0000142662.21767.58
Clark J (2003) Women too busy to exercise. BMJ 326:467. doi:10.1136/bmj.326.7387.467
National Osteoporosis Society (2010) Key facts and figures. Available at http://www.nos.org.uk/NetCommunity/Page.aspx?pid=328. Accessed 13 May 2010
World Health Organization (2009) Global health risks report: mortality and burden of disease attributable to selected major risks. WHO, Geneva
Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW (1999) The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int 9:1–12. doi:10.1007/s001980050109
Wallace BA, Cumming RG (2000) Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int 67:10–18. doi:10.1007/s00223001089
Kelley GA, Kelley KS (2004) Efficacy of resistance exercise on lumbar spine and femoral neck bone mineral density of premenopausal women: a meta-analysis of individual patient data. J Womens Health (Larchmt) 13:293–300. doi:10.1089/154099904323016455
Martyn-St James M, Carroll S (2006) Progressive high-intensity resistance training and bone mineral density changes among premenopausal women: evidence of discordant site-specific skeletal effects. Sports Med 36(8):683–704. doi:0112-1642/06/0008-0683
Snow-Harter C, Bouxsein ML, Lewis BT, Carter DR, Marcus R (1992) Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res 7:761–769. doi:10.1002/jbmr.5650070706
Lohman T, Going S, Pamenter R, Hall M, Boyden T, Houtkooper L, Ritenbaugh C, Bare L, Hill A, Aickin M (1995) Effects of resistance training on regional and total bone mineral density in premenopausal women. J Bone Miner Res 10:1015–1024. doi:10.1002/jbmr.5650100705
Friedlander AL, Genant HK, Sadowsky S, Byl NN, Gluer CC (1995) A two-year program of aerobics and weight training enhances BMD of young women. J Bone Miner Res 10:574–585. doi:10.1002/jbmr.5650100410
Heinonen A, Kannus P, Sievänen H, Oja P, Pasanen M, Rinne M, Uusi-Rasi K, Vuori I (1996) Randomised controlled trial of effect of high impact exercise on selected risk factors for osteoporotic fractures. Lancet 348:1343–1347. doi:10.1016/S0140-6736(96)04214-6
Rubin CT, Lanyon LE (1984) Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am 66:397–402
Rubin CT, Lanyon LE (1985) Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int 37:411–417
Umemura Y, Ishiko T, Yamauchi T, Kurono M, Mashiko S (1997) Five jumps per day increase bone mass and breaking force in rats. J Bone Miner Res 12:1480–1485. doi:10.1359/jbmr.1997.12.9.1480
Bassey EJ, Ramsdale SJ (1994) Increase in femoral bone density in young women following high-impact exercise. Osteoporos Int 4(2):72–75
Bassey EJ, Rothwell MC, Littlewood JJ, Pye DW (1998) Pre-and postmenopausal women have different bone mineral density responses to the same high-impact exercise. J Bone Miner Res 13(112):1805–1813. doi:10.1359/jbmr.1998.13.12.1805
Bailey CA, Brooke-Wavell K (2010) Optimum frequency of exercise for bone health: randomised controlled trial of a high-impact unilateral intervention. Bone 46:1043–1049. doi:10.1016/j.bone.2009.12.001
Kato T, Terashima T, Yamashita T, Hatanaka Y, Honda A, Umemura Y (2006) Effect of low-repetition jump training on bone mineral density in young women. J Appl Physiol 100(3):839–843. doi:10.1152/japplphysiol.00666.2005
Effective Public Health Practice Project (2010) Quality assessment tool for quantitative studies. Available at http://www.ephpp.ca/PDF/Quality%20Assessment%20Tool_2010_2.pdf. Accessed 20 November 2010
Review Manager (RevMan) Version 5.1. Computer program (2011) The Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen
Strong JE (2004) Effects of different jumping programs on hip and spine bone mineral density in pre-menopausal women. Dissertation, Brigham Young University, Utah
Witzke KA (2009) Dose-dependent effects and feasibility of a home-based jumping program for bone health in women. Med Sci Sports Exerc 41(5):289. doi:10.1249/01.MSS.0000355432.86381.ac
Niu K, Ahola R, Guo H, Korpelainen R, Uchimaru J, Vainionpa A, Sato K, Sakai A, Salo S, Kishimoto K, Itoi E, Komatsu S, Jamsa T, Nagatomi R (2010) Effect of office-based brief high-impact exercise on bone mineral density in healthy premenopausal women: the Sendai Bone Health Concept Study. J Bone Miner Metab 28:568–577. doi:10.1007/s00774-010-0163-6
Cummings SR, Black DM, Nevitt MC (1993) Bone density at various sites for prediction of hip fractures. Lancet 341:72–75. doi:10.1016/0140-6736(93)92555-8
Vainionpää A, Korpelainen R, Sievänen H, Vihriälä E, Leppäluoto J, Jämsä T (2007) Effect of impact exercise and its intensity on bone geometry at weight-bearing tibia and femur. Bone 40(3):604–611. doi:10.1016/j.bone.2006.10.005
Coventry E, O'Connor KM, Hart BA, Earl JE, Ebersole KT (2006) The effect of lower extremity fatigue on shock attenuation during single-leg landing. Clin Biomech 21(10):1090–1097. doi:10.1016/j.clinbiomech.2006.07.004
Kontulainen S, Heinonen A, Kannus P, Pasanen M, Sievanen H, Vuori I (2004) Former exercisers of an 18-month intervention display residual aBMD benefits compared with control women 3.5 years post-intervention: a follow-up of a randomized controlled high-impact trial. Osteoporos Int 15:248–251. doi:10.1007/s00198-003-1559-0
Kahn K, McKay H, Kannus P, Bailey D, Wark J, Bennell K (2001) Physical activity and bone health. Human Kinetics, Illinois
Nikander R, Sievänen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P (2010) Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med 21(8):47. doi:10.1186/1741-7015-8-47
Imaz I, Zegarra P, González-Enríquez J, Rubio B, Alcazar R, Amate J (2010) Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int 21:1943–1951. doi:10.1007/s00198-009-1134-4
Cramer JA, Gold D, Silverman S, Lewiecki EM (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 18:1023–1031. doi:10.1007/s00198-006-0322-8
Dalsky GP, Stocke KS, Ehsani AA, Slatopolsky F, Lee WC, Birge SJ (1988) Weight-bearing exercise training and lumbar bone mineral content (BMC) in postmenopausal women. Ann Intern Med 108:824–828
Pisters MF, Veenhof C, van Meeteren NLU, Ostelo RW, de Bakker DH, Schellevis FG, Dekker J (2007) Long-term effectiveness of exercise therapy in patients with osteoarthritis of hip or knee: a systematic review. Arthritis Rheum 57:1245–1253. doi:10.1002/art.23009
Winters KM, Snow CM (2000) Detraining reverses positive effects of exercise on the musculoskeletal system in premenopausal women. J Bone Miner Res 15:2495–2503. doi:10.1359/jbmr.2000.15.12.2495
Blair SN, Kohl HW III, Gordon NF (1992) Physical activity and health: a lifestyle approach. Med Exerc Nutr Health 1:54–57
Blair SN, Brownell KD, Hager DL, Marlatt GA, O'Neil PM, Rhodes SK, St. Jeor S, Wolfe BL (1996) Exercise and physical activity. In: Blair SN (ed) The lifestyle counselor's guide for weight control. American Health Pub. Co., Dallas, pp 262–319
Turner CH, Robling AG (2003) Designing exercise regimens to increase bone strength. Exerc Sport Sci Rev 31:45–50
Robling AG, Burr DB, Turner CH (2000) Partitioning a daily mechanical stimulus into discrete loading bouts improves the osteogenic response to loading. J Bone Miner Res 15:1596–1602. doi:10.1359/jbmr.2000.15.8.1596
Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS (2002) Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res 17:1613–1620. doi:10.1359/jbmr.2002.17.9.1613
Donahue SW, Donahue HJ, Jacobs CR (2003) Osteoblastic cells have refractory periods for fluid-flow-induced intracellular calcium oscillations for short bouts of flow and display multiple low-magnitude oscillations during long-term flow. J Biomech 36:35–43. doi:10.1016/S0021-9290(02)00318-4
Donahue SW, Jacobs CR, Donahue HJ (2001) Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent. Am J Physiol Cell Physiol 281:C1635–C1641
Lanyon LE (1987) Functional strain in bone tissue as an objective and controlling stimulus for adaptive bone remodelling. J Biomech 20:1083–1093. doi:10.1016/0021-9290(87)90026-1
Srinivasan S, Weimer DA, Liu CC, Bain S, Gross TS (2004) The osteogenic potential of rest-inserted loading In: LaMothe JM, Zernicke RF Rest insertion combined with high-frequency loading enhances osteogenesis. J Appl Physiol 96:1788–1793. doi:10.1152/japplphysiol.01145.2003
Acknowledgements
The guidance of Professor Nachiappan Chockalingam in the conduct of this study is graciously appreciated.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Appendix 1. Search strategy
Appendix 1. Search strategy
Databases searched: MEDLINE/PubMed, EMBASE, Web of Science, SportDiscus, Cochrane controlled trials register, ProQuest and CINAHL.
-
1.
exercis*.ti,ab
-
2.
exp EXERCISE/
-
3.
(high AND impact AND exercise).af
-
4.
(brief AND exercise).ti,ab
-
5.
1 OR 2 OR 3 OR 4
-
6.
(bone AND m*).ti,ab
-
7.
BONE DENSITY/
-
8.
6 OR 7
-
9.
5 AND 8
-
10.
9 [Limit to: (Gender Female)]
-
11.
9 [Limit to: (Gender Female) and (Age Groups Adults)]
Search format was repeated for all the databases.
Rights and permissions
About this article
Cite this article
Babatunde, O.O., Forsyth, J.J. & Gidlow, C.J. A meta-analysis of brief high-impact exercises for enhancing bone health in premenopausal women. Osteoporos Int 23, 109–119 (2012). https://doi.org/10.1007/s00198-011-1801-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1801-0