Abstract
Summary
Our study showed that serum osteocalcin levels are closely related to glucose metabolism in men of all ages and younger women. This association disappeared in postmenopausal women in which increases bone turnover rates. The association between serum osteocalcin levels and glucose homeostasis should be interpreted according to age and sex.
Introduction
Osteocalcin, a marker of bone formation, appears to be associated with glucose homeostasis. We investigated the age- and sex-specific association of serum osteocalcin level with variables related to glucose metabolism.
Methods
This study was based on cross-sectional analysis from 719 participants aged 20–85 years after excluding patients taking antidiabetic or antiosteoporotic drugs. The subjects were divided into four groups according to age and sex as follows: men <50 years (n = 131), men ≥50 years (n = 191), women <50 years (n = 108), and women ≥50 years (n = 279). Anthropometric and biochemical variables including insulin resistance (HOMA-IR) and β cell function (HOMA-β) from a 75-g oral glucose tolerance test, and serum 25-OH-vitamin D and parathyroid hormone levels were measured.
Results
The serum osteocalcin level was significantly higher in women aged ≥50 years compared with women <50 years (20.4 ± 7.8 vs. 17.9 ± 6.8 ng/ml, p < 0.001), but there was no difference between men aged ≥50 years and men <50 years (16.4 ± 5.9 vs. 16.8 ± 6.0 ng/ml, p = 0.905). The participants diagnosed with diabetes had lower serum osteocalcin levels than normal or prediabetic participants. Multivariable regression analyses including HOMA-IR and HOMA-β indicated that serum osteocalcin levels had a negative and independent association with HbA1c levels in men and women aged <50 years, but not in women ≥50 years.
Conclusions
Low osteocalcin levels are associated with impaired glucose metabolism in men and premenopausal women. The osteocalcin levels may be determined by factors related to bone metabolism in postmenopausal women. Our data suggest that the serum levels of osteocalcin associated with glucose homeostasis should be interpreted according to age and sex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The skeleton is a metabolically active organ and is continuously remodeled throughout life by bone resorption by osteoclasts and bone formation by osteoblasts [1]. During this process, proteins originating from the skeletons are released into the systemic circulation as metabolites of the process, but little is known about their systemic role [2]. Recent accumulating evidence suggests that the proteins released during the bone remodeling process have extraskeletal roles [3, 4].
Osteocalcin is a bone matrix protein preferentially synthesized by osteoblasts [5]. The systemic role of osteocalcin is not yet clear. However, it is increasingly evident that osteocalcin has extraskeletal roles, indicating an association with glucose homeostasis and energy metabolism [6]. In vitro and in vivo studies have shown that osteocalcin exerts physiological effects on pancreatic β cells and adipose tissue [7, 8]. Mice lacking osteocalcin display decreased β cell function, glucose intolerance, and insulin resistance [7]. Conversely, treatment with osteocalcin significantly improves glucose tolerance and insulin sensitivity in mouse models of obesity and glucose intolerance [8]. In humans, osteocalcin is associated with fasting glucose or insulin sensitivity indices [9–12]. By contrast, other studies found no correlation between circulating osteocalcin and insulin resistance [13, 14] or blood glucose levels [14]. Thus, there are still some discrepancies between the study results according to the study design or characteristics of study participants.
Bone turnover rate varies considerably according to individual variables. Among them, age and sex are the most important variables determining bone remodeling [15, 16]. Given that the circulating levels of osteocalcin, a marker of bone formation, differ between sexes and alter with age [17, 18], the relationship between circulating osteocalcin and glucose homeostasis may also differ according to these variables. This variability in circulating osteocalcin levels may be the cause of inconsistent data observed in previous studies of the association between circulating osteocalcin levels and glucose homeostasis [9–14]. Therefore, the aim of the present study was to evaluate the association of circulating osteocalcin levels with variables related to glucose metabolism in men and women with a broad range of ages.
Material and methods
Study population
We included consecutive patients aged ≥20 years who visited the diabetes clinic at Seoul National University Bundang Hospital (SNUBH) between October 2013 and December 2014 for evaluation or treatment of diabetes. Exclusion criteria were as follows: (1) patients who had been treated with oral hypoglycemic agents or insulin; (2) patients taking agents that may affect bone turnover rates, such as bisphosphonates, selective estrogen receptor modulators, or teriparatide; (3) those taking medications that may affect bone or glucose metabolism, such as systemic glucocorticoids; (4) those having a history of metabolic bone diseases including hyperparathyroidism, Paget’s disease, or a recent history of bone fracture; (5) uncontrolled hyper- and hypothyroidism; or (6) major surgery within the previous 6 months.
A total of 719 patient participants who were naïve to drugs affecting glucose metabolism were enrolled in the present study. The participants were divided into four groups by age and sex as follows: men <50 years (n = 131), men ≥50 years (n = 191), women <50 years (n = 108), and women ≥50 years (n = 279). Participants were subsequently classified as those with normal glucose metabolism, those with prediabetes including impaired fasting glucose and impaired glucose tolerance, and those with diabetes mellitus (DM) according to the American Diabetes Association diagnosis definition based on their fasting and postprandial 2-h glucose levels [19]. This observational study was approved by the Institutional Review Board of SNUBH (IRB no. B-1502/286-106) and complied with the Declaration of Helsinki.
Anthropometric and biochemical measurements
Height and body weight were measured by standard methods. Body mass index (BMI) was calculated as body weight divided by height squared (in kilogram per square meter). Waist circumference was measured with a flexible tape at a level midway between the lower rib margin and the iliac crest. Blood pressure measurements were made after subjects had remained seated for 10 min. Measurements were made twice, with a 5-min rest interval between them, and the mean value of the two measurements was used. Participants were classified according to their smoking habits as nonsmokers, ex-smokers, or current smokers. Participants were also classified according to their alcohol consumption habits as abstinent (<20.0 g/week), mild-to-moderate drinkers (20.0–199.9 g/week), or heavy drinkers (≥200 g/week). Physical activity of participants was classified as none, irregular (1–2 bouts/week), or regular (≥3 bouts/week) exercise.
Plasma glucose levels were measured using a Hitachi 747 Clinical Chemistry Analyzer (Hitachi, Tokyo, Japan). HbA1c levels were measured using a Bio-Rad Variant II Turbo HPLC Analyzer (Bio-Rad, Hercules, CA, USA) in the National Glycohemoglobin Standardization Program (NGSP) level II certified laboratory at SNUBH. Plasma insulin concentrations were measured by radioimmunoassay (Linco, St. Louis, MO, USA). Fasting plasma concentrations of lipids, aspartate/alanine aminotransferase (AST/ALT), and creatinine were measured using a Hitachi 747 Clinical Chemistry Analyzer. Serum calcium (corrected for albumin binding) and phosphate were also measured by standard automated laboratory methods (Hitachi 747 Clinical Chemistry Analyzer).
Insulin resistance (HOMA-IR) and β cell function (HOMA-β) were calculated using the homeostasis model assessment (HOMA) described by Matthews et al.[20] as follows: HOMA-IR = fasting insulin (in microunits per milliliter) × fasting plasma glucose (in milligram per deciliter)/405; HOMA-β = 360 × fasting insulin (in microunits per milliliter)/[fasting plasma glucose (in milligram per deciliter) − 63]. Four hundred twenty-five of the 719 participants (71.9 %, 188 men and 237 women) underwent a 75-g oral glucose tolerance test (OGTT). Plasma glucose and insulin levels were determined at 30 and 120 min after the glucose load (PP30, PP120, Insulin30, and Insulin120, respectively). The insulinogenic index (IGI), as an indicator of early-phase insulin release, was calculated according to the following formula: (30-min insulin − fasting insulin)/(30-min glucose − fasting glucose) [21].
Within the definition of prediabetes and diabetes mellitus (DM), impaired fasting glucose (IFG) was defined as a fasting plasma glucose (FPG) level of 100–125 mg/dL, and impaired glucose tolerance (IGT) as PP120 140–199 mg/dL. Participants with IFG or IGT were defined as prediabetic. DM was diagnosed when the FPG was ≥126 mg/dL, or the PP120 was ≥200 mg/dL.
The total serum level of osteocalcin was assayed immunoradiometrically using an Osteo-RIACT kit (Cisbio Bioassays, Saclay, France). The detection limit of the assay was 0.4 ng/mL and intra-assay and interassay coefficients of variation (CVs) were 1.2–2.8 % and 3.6–5.2 %, respectively. Serum c-terminal telopeptide of type I collagen (CTX) was measured by electrochemiluminescence immunoassay using a Modular Analytics E170 platform (Roche, Mannheim, Germany) in those women among the study participants who were aged ≥50 years. Serum 25-OH vitamin D concentrations were measured by Diel–Alder derivatization and ultrahigh-performance liquid chromatography–mass spectrometry (Waters, Milford, MA, USA). Circulating concentrations of intact parathyroid hormone (PTH) were measured using an electrochemiluminescence immunoassay on a Modular Analytics E170 platform (Roche, Mannheim, Germany). Serum levels of C-reactive protein were measured with high-sensitivity (hsCRP) using an automated immunoturbidimetric method (Denka Seiken CRP II Latex X2, Tokyo, Japan). White blood cells (WBC) were counted by flow cytometry (XE-2100, Sysmex, Kobe, Japan). Urinary albumin levels were measured using a nephelometric assay method (A&T 502X, A&T, Tokyo, Japan), and urine creatinine levels were measured using the Jaffe method (Hitachi 7170 analyzer) to calculate the spot urine albumin-to-creatinine ratio (UACR).
Statistical analyses
Differences in anthropometric and biochemical variables between participants of each sex aged <50 and ≥50 years were evaluated using a Student’s t test. Variables such as HOMA-IR, HOMA-β, and hsCRP were log-transformed to approximate a normal distribution. Pearson correlation analysis was used to evaluate the associations between osteocalcin and metabolic variables such as anthropometric indices and glucose metabolism-related parameters. Multiple linear regression analysis was used to determine the association of osteocalcin levels with HbA1c concentrations after adjusting for various factors that may affect glucose homeostasis, bone metabolism, or both. We considered p < 0.05 to be significant. The statistical analyses were performed using IBM SPSS Statistics for Windows (version 20.0, IBM Corp, Armonk, NY, USA).
Results
Association of serum osteocalcin levels with age in men and women
Age-related changes in serum osteocalcin levels are shown in Fig. 1. A scatterplot of the osteocalcin levels and a correlation line for age of men indicated serum levels of osteocalcin decreased with age in the rage of 20 to 49 years (r = –0.311, p < 0.001). However, no significant correlation between age and serum osteocalcin levels was observed in men aged ≥50 years (r = 0.089, p = ns). In women aged between 20 and 49 years, serum osteocalcin levels decreased with increasing age (r = –0.294, p = 0.002). The serum osteocalcin levels increased markedly at age 50 years, the median age of natural menopause, and then decreased with increasing age in women aged ≥50 years (r = –0.197, p = 0.001).
Comparison of baseline characteristics between age groups by sexes
Considering the trend of serum osteocalcin levels according to age and sex, we divided the participants into four groups by age and sex. The anthropometric and biochemical variables related to glucose homeostasis and bone metabolism in the four groups are shown in Table 1. The mean BMI was higher in men than women. Between younger and older age groups, the mean BMI was lower in older men than their younger counterparts, while it was higher in older women than in their younger counterparts.
Between the sexes, serum osteocalcin levels were higher in women than in men (19.7 ± 7.6 ng/mL vs. 16.6 ± 5.9 ng/mL, p < 0.001). Serum osteocalcin levels were not significantly different between men aged <50 years and men aged ≥50 years. By contrast, serum osteocalcin levels were significantly higher in women aged ≥50 years compared with those in women <50 years. The serum osteocalcin levels were not different between sexes in participants <50 years, but were significantly higher in women ≥50 years than in men ≥50 years.
The levels of 25-OH vitamin D reflecting vitamin D levels were lower in people <50 years than in people ≥50 years, regardless of sex. Glucose levels tended to be higher in people in the older age group than in people in the younger groups, regardless of sex. Fasting insulin concentration and HOMA-IR were significantly higher in women aged ≥50 years than in women <50 years old, whereas they were not significantly different by age group in men. Men aged ≥50 years had lower HOMA-β and IGI than men aged <50 years. Serum hsCRP levels were higher in the older people than the younger people, regardless of sex. ALT levels were higher in the younger men than in the older men, while they were lower in the younger women than in the older women.
Association of serum osteocalcin levels with dynamic glucose-related variables
To assess the correlations between serum osteocalcin levels and other variables, simple correlation analyses were conducted, separately for the four groups (Table 2). Serum osteocalcin levels were significantly associated with most parameters related with glucose metabolism including HbA1c, FPG, PP30, and PP120 with varying relationship strengths, whereas correlations between serum osteocalcin levels and fasting insulin levels were not significant, except in older women.
Serum osteocalcin levels were positively correlated with HOMA-β and IGI in the groups of men aged <50 years, men ≥50 years, and women <50 years, but not in women ≥50 years. The levels of osteocalcin were negatively correlated with HOMA-IR in all groups except for in men aged ≥50 years. There were negative correlations between osteocalcin concentrations and hsCRP levels except for men ≥50 years. Similar negative correlations were found between osteocalcin levels and WBC.
CTX levels were measured only in women aged ≥50 years. The CTX levels were positively associated with osteocalcin levels (r = 0.533, p < 0.001), but not with glucose metabolism-related parameters including HbA1c, HOMA-IR, and HOMA-β (Electronic supplementary material (ESM) Supplementary Table 1).
Independent association of serum levels of osteocalcin with HbA1c levels
To identify an independent association of serum osteocalcin levels with HbA1c levels, multivariable linear regression analyses for HbA1c levels were conducted. We adjusted for clinical and biochemical variables including age, BMI, systolic blood pressure (SBP), ALT, serum creatinine, HOMA-IR, HOMA-β, and hsCRP. Other variables related to bone metabolism, such as calcium and 25-OH vitamin D, were included as confounders.
In this study, the HOMA-IR and HOMA-β indices were significantly associated with HbA1c levels in all age groups. The hsCRP levels were also significantly associated with HbA1c levels in both groups of men groups and showed borderline significance in both groups of women. Serum osteocalcin levels were inversely correlated with HbA1c levels in the groups of men aged <50 years, men aged ≥50 years, and women aged <50 years, but not in women ≥50 years (Table 3). When exercise habit, smoking status, and alcohol consumption were included, the association between osteocalcin and HbA1c levels was maintained, but was slightly diminished (data not shown). When CTX was included in the final multivariable regression model for women aged ≥50 years, it was not significantly associated with HbA1c levels (ESM Supplementary Table 2).
Serum osteocalcin levels by glucose metabolism status
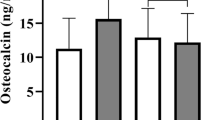
After classifying the participants as normal, prediabetic, or with DM, we compared serum osteocalcin levels according to their glycemic status. Serum osteocalcin levels were significantly lower in those with DM compared with normal or prediabetic men or women, regardless of age group (Fig. 2). There was a significant difference in serum osteocalcin levels between groups of normal and prediabetic men aged ≥50 years.
Discussion
In the present study of a relatively large number of participants with a wide age range, circulating levels of osteocalcin were negatively associated with glucose concentrations obtained from OGTT and HOMA-IR, and positively associated with HOMA-β and IGI in men <50 years, men ≥50 years, and women <50 years, but these associations diminished or disappeared in women ≥50 years whose bone turnover increases and whose serum osteocalcin levels markedly increase. In the multivariable linear regression analyses including 25-OH vitamin D, hsCRP, HOMA-IR, HOMA-β, or IGI, the serum osteocalcin level was significantly associated with HbA1c levels in men aged <50 years, men aged ≥50 years, and women aged <50 years, but not in women ≥50 years.
Bone formation and resorption occur continuously throughout life and the bone turnover rate varies according to age and sex [15]. In women, it is maintained relatively stable at a low level until the early 1950s and the menopause, and then is dramatically increased with increased bone loss. However, in men the pattern is different. Bone turnover rate increases in the early 1920s to build a peak bone mass and declines slightly after then to 50 years of age. Thereafter, it remains at a stable level in older men. Thus, the bone turnover rate in men does not show significant alteration in old age as it does in women at the age of 50 years [16, 17, 22].
Osteocalcin is a marker of bone formation and its circulating concentration changes according to bone turnover rate with aging [18]. In our study, the serum levels of osteocalcin changed with age in men and women in a different fashion: serum osteocalcin levels were inversely correlated with age in men aged 20–49 years, but the association disappeared from age 50 years. Furthermore, the mean levels of serum osteocalcin were not different between younger and older men. By contrast, there was dramatic change in serum osteocalcin levels in women at age 50 years: osteocalcin levels decreased significantly with age in women aged 20–49 years. The levels were then elevated sharply at the age of 50 years, which is consistent with previous reports [17, 18]. In women aged ≥ 50 years, serum osteocalcin levels again decreased significantly with age. Thus, consistent with previous observations, alteration in bone turnover rate leads to age- and sex-specific differences in circulating osteocalcin concentrations.
Osteocalcin might directly affect glucose metabolism; osteocalcin increases β cell proliferation, insulin expression and secretion, and regulates peripheral insulin sensitivity through regulating adiponectin expression [7, 8]. Cross-sectional studies have found that circulating concentrations of osteocalcin are negatively associated with serum levels of HbA1c, fasting glucose, and insulin, and insulin resistance [10–12]. However, other clinical studies have shown that there is no association between circulating osteocalcin and markers of glucose metabolism [13, 14]. Most previous studies that investigated the association between osteocalcin and glucose metabolism were conducted in a specific group such as older men [9, 10, 23] or postmenopausal women [24–26]. Consequently, there was a discrepancy in the associations between osteocalcin and glucose metabolism. This may be caused by the intrinsic bone turnover rates, which are specifically related to age and sex.
In the present study, the serum osteocalcin levels showed a negative correlation with HbA1c levels after adjusting for the other variables related to glucose homeostasis and bone metabolism. Serum osteocalcin was positively associated with insulin sensitivity in men and premenopausal women. However, these associations disappeared in postmenopausal women, when bone loss increase and bone turnover rate increases. In this context, one can speculate that circulating osteocalcin levels in postmenopausal women might be more influenced by bone turnover rates, which should be considered when evaluating the association of serum osteocalcin levels with glucose metabolism. In other words, serum osteocalcin may be able to indicate accurately the glucose metabolism in a state of low bone turnover, but it might reflect bone turnover rates per se in a high bone turnover phase. The associations with glucose metabolism might be attenuated in this phase.
Another study including participants with a broad range of ages showed that an elevated plasma osteocalcin level was associated with improved glucose tolerance [27]. However, the investigators did not examine its association by age or sex specifically. The status of bone turnover rate should be considered when interpreting the serum level of osteocalcin as a marker of glucose metabolism in a specific group.
Considering the influence of bone metabolism on glucose homeostasis, it could be expected that other markers of bone turnover, such as CTX might be associated with glucose metabolism. Some recent studies suggested that CTX levels were associated with glucose metabolism, but the results were inconsistent [23, 28]. In our study, there was no significant relationship between CTX levels and glucose metabolism in women aged ≥50 years (ESM Supplementary Table 3). In previous studies, osteocalcin showed a stronger association with glucose metabolism than did CTX [23, 29]. Moreover, evidence has shown that osteocalcin plays a direct role in pancreas β cell function and insulin secretion [7, 30]. Therefore, osteocalcin might be a more robust marker that reflects both bone turnover and glucose metabolism.
Our study has several limitations. First, we measured total serum osteocalcin levels only. Some studies suggest that carboxylation of osteocalcin is important for its biological properties. More specifically, uncarboxylated osteocalcin was reported to be linked to glucose homeostasis in mice [8]. However, this association in humans is not clear [13, 14, 31]. Furthermore, concentrations of uncarboxylated osteocalcin are highly correlated with total osteocalcin [5]. Second, the present study is cross-sectional. Accordingly, we cannot assume any causal relationship between the serum levels of osteocalcin and glucose metabolism. It remains to be determined whether low osteocalcin concentrations in type 2 diabetes represent a cause and/or a consequence of hyperglycemia. Third, we measured CTX levels only in postmenopausal women. Finally, we did not accurately assess participants’ gonadal status, which has major effects on bone turnover in both men and women. Instead, we arbitrarily used an age of 50 years as a cutoff. This age is commonly used for defining the onset of menopause in many studies [32–34]. In addition, the mean age of menopause in Korean women is 49.8 years [35]. In men, androgen levels decrease gradually with age but tend to decrease more rapidly after the age of 50 years [36]. For these reasons, we chose 50 years as the cutoff for age distribution in both sexes.
This study has several strengths. We investigated the association between serum osteocalcin and glucose metabolism in a large number of both male and female participants. Furthermore, we investigated glucose metabolism comprehensively using dynamic measures with the 75-g OGTT. The negative association between serum osteocalcin levels and poor glycemic control persisted after adjusting for other confounding factors related to glucose and bone metabolism including calcium, 25-OH vitamin D, and PTH.
In conclusion, circulating osteocalcin concentrations are closely related to glucose metabolism in men of all ages and younger women. The association diminished in older women, particularly after menopause. Intrinsic bone turnover rates could affect the intensity of the association between circulating osteocalcin and glucose homeostasis. Our study suggests that the difference in bone turnover rates according to age and sex should be considered when evaluating the association between circulating osteocalcin levels and glucose homeostasis.
References
Hadjidakis DJ, Androulakis II (2006) Bone remodeling. Ann N Y Acad Sci 1092:385–396
Naylor K, Eastell R (2012) Bone turnover markers: use in osteoporosis. Nat Rev Rheumatol 8:379–389
Ducy P (2011) The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia 54:1291–1297
Ng KW (2011) Regulation of glucose metabolism and the skeleton. Clin Endocrinol (Oxf) 75:147–155
Booth SL, Centi A, Smith SR, Gundberg C (2013) The role of osteocalcin in human glucose metabolism: marker or mediator? Nat Rev Endocrinol 9:43–55
Booth SL, Centi AJ, Gundberg C (2014) Bone as an endocrine organ relevant to diabetes. Curr Diab Rep 14:556
Lee NK, Sowa H, Hinoi E et al (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469
Ferron M, Hinoi E, Karsenty G, Ducy P (2008) Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A 105:5266–5270
Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, Mellstrom D (2009) Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res 24:785–791
Iki M, Tamaki J, Fujita Y et al (2012) Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Study. Osteoporos Int 23:761–770
Kanazawa I, Yamaguchi T, Tada Y, Yamauchi M, Yano S, Sugimoto T (2011) Serum osteocalcin level is positively associated with insulin sensitivity and secretion in patients with type 2 diabetes. Bone 48:720–725
Saleem U, Mosley TH Jr, Kullo IJ (2010) Serum osteocalcin is associated with measures of insulin resistance, adipokine levels, and the presence of metabolic syndrome. Arterioscler Thromb Vasc Biol 30:1474–1478
Mori K, Emoto M, Motoyama K, Lee E, Yamada S, Morioka T, Imanishi Y, Shoji T, Inaba M (2012) Undercarboxylated osteocalcin does not correlate with insulin resistance as assessed by euglycemic hyperinsulinemic clamp technique in patients with type 2 diabetes mellitus. Diabetol Metab Syndr 4:53
Lu C, Ivaska KK, Alen M et al (2012) Serum osteocalcin is not associated with glucose but is inversely associated with leptin across generations of nondiabetic women. J Clin Endocrinol Metab 97:4106–4114
Michelsen J, Wallaschofski H, Friedrich N, Spielhagen C, Rettig R, Ittermann T, Nauck M, Hannemann A (2013) Reference intervals for serum concentrations of three bone turnover markers for men and women. Bone 57:399–404
Fatayerji D, Eastell R (1999) Age-related changes in bone turnover in men. J Bone Miner Res 14:1203–1210
Hannemann A, Friedrich N, Spielhagen C, Rettig R, Ittermann T, Nauck M, Wallaschofski H (2013) Reference intervals for serum osteocalcin concentrations in adult men and women from the study of health in Pomerania. BMC Endocr Disord 13:11
Gundberg CM, Looker AC, Nieman SD, Calvo MS (2002) Patterns of osteocalcin and bone specific alkaline phosphatase by age, gender, and race or ethnicity. Bone 31:703–708
(2013) Standards of medical care in diabetes--2013. Diabetes Care 36 Suppl 1:S11-66
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Seltzer HS, Allen EW, Herron AL Jr, Brennan MT (1967) Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 46:323–335
Chubb SA, Byrnes E, Manning L, Beilby JP, Ebeling PR, Vasikaran SD, Golledge J, Flicker L, Yeap BB (2015) Reference intervals for bone turnover markers and their association with incident hip fractures in older men: the Health in Men study. J Clin Endocrinol Metab 100:90–99
Yeap BB, Alfonso H, Chubb SA et al (2015) Higher serum undercarboxylated osteocalcin and other bone turnover markers are associated with reduced diabetes risk and lower estradiol concentrations in older men. J Clin Endocrinol Metab 100:63–71
Im JA, Yu BP, Jeon JY, Kim SH (2008) Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clin Chim Acta 396:66–69
Yang R, Ma X, Pan X, Wang F, Luo Y, Gu C, Bao Y, Jia W (2013) Serum osteocalcin levels in relation to metabolic syndrome in Chinese postmenopausal women. Menopause 20:548–553
Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, Sugimoto T (2009) Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab 94:45–49
Hwang YC, Jeong IK, Ahn KJ, Chung HY (2012) Circulating osteocalcin level is associated with improved glucose tolerance, insulin secretion and sensitivity independent of the plasma adiponectin level. Osteoporos Int 23:1337–1342
Xuan Y, Sun LH, Liu DM, Zhao L, Tao B, Wang WQ, Zhao HY, Liu JM, Ning G (2015) Positive association between serum levels of bone resorption marker CTX and HbA1c in women with normal glucose tolerance. J Clin Endocrinol Metab 100:274–281
Starup-Linde J, Eriksen SA, Lykkeboe S, Handberg A, Vestergaard P (2014) Biochemical markers of bone turnover in diabetes patients—a meta-analysis, and a methodological study on the effects of glucose on bone markers. Osteoporos Int 25:1697–1708
Kover K, Yan Y, Tong PY, Watkins D, Li X, Tasch J, Hager M, Clements M, Moore WV (2015) Osteocalcin protects pancreatic beta cell function and survival under high glucose conditions. Biochem Biophys Res Commun 462:21–26
Shea MK, Gundberg CM, Meigs JB, Dallal GE, Saltzman E, Yoshida M, Jacques PF, Booth SL (2009) Gamma-carboxylation of osteocalcin and insulin resistance in older men and women. Am J Clin Nutr 90:1230–1235
Koitaya N, Sekiguchi M, Tousen Y et al (2014) Low-dose vitamin K2 (MK-4) supplementation for 12 months improves bone metabolism and prevents forearm bone loss in postmenopausal Japanese women. J Bone Miner Metab 32:142–150
Nakamura K, Saito T, Kobayashi R, Oshiki R, Kitamura K, Oyama M, Narisawa S, Nashimoto M, Takahashi S, Takachi R (2012) Effect of low-dose calcium supplements on bone loss in perimenopausal and postmenopausal Asian women: a randomized controlled trial. J Bone Miner Res 27:2264–2270
Crandall CJ, Yildiz VO, Wactawski-Wende J, Johnson KC, Chen Z, Going SB, Wright NC, Cauley JA (2015) Postmenopausal weight change and incidence of fracture: post hoc findings from Women's Health Initiative Observational Study and Clinical Trials. BMJ 350:h25
Choi H LH, Park HM (2003) The Korean menopausal women’s attitudes and awareness on menopause: results of Korean gallup epidemiologic survey on menopause and HRT. J Korean Soc Menopause 9:36-43
Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR (2001) Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86:724–731
Conflicts of interest
Kyong Yeun Jung, Kyoung Min Kim, Eu Jeong Ku, Yoon Ji Kim, Dong-Hwa Lee, Sung Hee Choi, Hak Chul Jang, Chan Soo Shin, Kyong Soo Park, and Soo Lim declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
K. Y. Jung and K. M. Kim contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOCX 18 kb)
Supplementary Table 2
(DOCX 16 kb)
Supplementary Table 3
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Jung, K.Y., Kim, K.M., Ku, E.J. et al. Age- and sex-specific association of circulating osteocalcin with dynamic measures of glucose homeostasis. Osteoporos Int 27, 1021–1029 (2016). https://doi.org/10.1007/s00198-015-3315-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3315-7