Abstract
Menaquinone-4 (MK-4) administered at a pharmacological dosage of 45 mg/day has been used for the treatment of osteoporosis in Japan. However, it is not known whether a lower dose of MK-4 supplementation is beneficial for bone health in healthy postmenopausal women. The aim of this study was to examine the long-term effects of 1.5-mg daily supplementation of MK-4 on the various markers of bone turnover and bone mineral density (BMD). The study was performed as a randomized, double-blind, placebo-controlled trial. The participants (aged 50–65 years) were randomly assigned to one of two groups according to the MK-4 dose received: the placebo-control group (n = 24) and the 1.5-mg MK-4 group (n = 24). The baseline concentrations of undercarboxylated osteocalcin (ucOC) were high in both groups (>5.1 ng/ml). After 6 and 12 months, the serum ucOC concentrations were significantly lower in the MK-4 group than in the control group. In the control group, there was no significant change in serum pentosidine concentrations. However, in the MK-4 group, the concentration of pentosidine at 6 and 12 months was significantly lower than that at baseline. The forearm BMD was significantly lower after 12 months than at 6 months in the control group. However, there was no significant decrease in BMD in the MK-4 group during the study period. These results suggest that low-dose MK-4 supplementation for 6–12 months improved bone quality in the postmenopausal Japanese women by decreasing the serum ucOC and pentosidine concentrations, without any substantial adverse effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that vitamin K is responsible for the γ-carboxylation of peptide-bound glutaminate (Glu) residues in target proteins that are synthesized in a limited number of tissues such as the liver, bone, and vascular tissues, producing γ-carboxyglutaminate (Gla) [1, 2].
There are two major forms of dietary vitamin K: phylloquinone (PK) and menaquinone (MK). PK is present in green and leafy vegetables, whereas MK is either produced by bacteria or is present in animal tissues. However, there is evidence that MK-4 is synthesized from dietary PK and menadione in certain animal tissues [3, 4].
Previous studies have reported that a 45-mg daily intake of MK-4 prevents osteoporotic fractures and postmenopausal bone loss [5–9]. Therefore, this dose is prescribed for the treatment of osteoporosis in Japan. On the other hand, Binkley et al. [10] reported that a daily intake of approximately 1 mg was required to maximally γ-carboxylate the circulating osteocalcin (OC) in postmenopausal women. Furthermore, recent meta-analysis of randomized controlled trials of vitamin K on bone mineral density (BMD) demonstrated that no study using a low dose of MK-4 in healthy women has been performed, although many studies using 45 mg/day MK-4 in Japanese postmenopausal women or 0.18–10 mg PK in postmenopausal women in Western countries had been conducted [11]. Furthermore, detailed information on the additional health-related effects of MK-4 supplementation in postmenopausal women is lacking. Hence, previously, we conducted a study that examined the effects of low-dose MK-4 supplementation for 4 weeks on postmenopausal Japanese women. We demonstrated that MK-4 supplementation at 1.5 mg/day accelerated the rate of osteocalcin (OC) γ-carboxylation [12]. However, it is not clear whether this is the effective dose for improving the bone health of postmenopausal women. Moreover, we wanted to determine the currently unknown effects of MK-4 on other bio-indices and on bone metabolism.
According to the Dietary Reference Intakes (DRIs) for Japanese (2010), the adequate intake (AI) of vitamin K for Japanese women aged at least 30 years was 65 μg/day [13]. However, a previous study suggested that the amount of vitamin K required for maintaining bone health is higher than the amount required for blood coagulation [14]. Indeed, in our previous study, the serum concentrations of undercarboxylated OC (ucOC) in healthy postmenopausal Japanese women were nearly at fracture-inducing levels [12]. Furthermore, it has been reported that higher concentrations of circulating vitamin K are required to prevent osteoporosis in elderly people [15, 16].
Thus, the aims of the present study was to examine the effects of daily intake an additional 1.5 mg MK-4 for 12 months on bone mineral density (BMD), biomarkers of bone turnover, and other bio-indices in postmenopausal Japanese women.
Materials and methods
Subjects
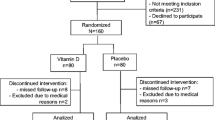
In total, 50 postmenopausal women aged 50–65 years (mean age, 58.4 ± 3.8 years) were recruited from the Kanto area of Japan. Subjects were recruited from the volunteers registered on a contract research organization. Of these women, 48 were selected as participants using the exclusion criteria, and all the subjects were monitored until the end of the study (12 months). Sample size calculation, performed before the start of the study, showed that with 18 subjects in each group, a difference of 1.5 ng/ml between the means of ucOC can be shown with a power of 0.80 and a two-sided type 1 error of 0.05. Thus, it seemed that 25 subjects in each group were sufficient for the study. None of the subjects participated in another study or took any dietary supplements other than those prescribed by the study.
The exclusion criteria were a history of metabolic bone disease, cancer, ovariectomy or hysterectomy, or a daily intake of vitamin K-containing concentrates.
The institutional review board of the National Institute of Health and Nutrition, Japan, approved the protocol, and the study was carried out according to the guidelines of the Declaration of Helsinki. Informed consent was obtained from all the subjects.
Experimental design
The study was a randomized, double-blind, placebo-controlled parallel-group trial performed as an intervention study in free-living subjects. The total duration of the study was 12 months, starting in October 2009 and terminating in October 2010. A single randomization number, which was generated using random permutation by the SPSS software version 18.0J, was used to identify the participants, who were randomly assigned to one of two groups: the 1.5 mg/day MK-4 group and the placebo-control group (n = 24 for both groups). The subjects were instructed to take six tablets of MK-4 or placebo (prepared by Kyowa Hakko Bio, Tokyo, Japan) after breakfast and were requested to return any tablets left at the end of the study; therefore, compliance could be determined from the number of tablets remaining. Natto (MK-7-containing fermented soybean) intake was restricted to once or twice a week during the study period, and the subjects refrained from consuming the product 1 week before the start of the study. The randomization codes were allocated sequentially to the participants in the order in which they were enrolled. After all the analyses had been conducted, the randomization code was disclosed to the investigators.
Blood and urine samples
Blood and urine samples were collected at the start of the study and again after 6 and 12 months. Fasting (>12 h) blood samples were collected with or without ethylenediaminetetraacetic acid (EDTA)-containing tubes by venipuncture. They were refrigerated immediately and centrifuged within 2 h at 1,500 rpm for 30 min at 4 °C. The serum samples from each participant were stored at −80 °C.
The serum concentrations of total cholesterol (TC) and triacylglycerol (TG) were determined using commercial kits (cholesterol C-test and triglyceride G-test, respectively; Wako Pure Chemical, Osaka, Japan), and serum high density lipoprotein (HDL) cholesterol levels were measured using an enzymatic method (HDL-Cholesterol Test; Wako Pure Chemical). The serum low density lipoprotein (LDL) cholesterol levels were determined using the following calculation: TC (mg/dl) − HDL cholesterol (mg/dl) − TG (mg/dl) × 0.2. The 17β-estradiol (E2) levels were assessed using a radioimmunoassay (Amersham Biosciences, Piscataway, NJ, USA).
The serum concentrations of PK, MK-4, and MK-7 were measured using high performance liquid chromatography [17], and the detection limits of serum PK, MK-4, and MK-7 were less than 0.05, 0.1, and 0.1 ng/ml, respectively. The levels of serum bone-specific alkaline phosphatase (BS-ALP), a bone formation marker, were analyzed using an enzyme immunoassay (EIA) (Alkphase-B; Metra Biosystems, Mountain View, CA, USA).
Serum ucOC concentrations and γ-carboxylated OC (GlaOC) levels were determined using an electrochemiluminescence immunoassay with the ECLIA kit (Sanko Junyaku, Ibaraki, Japan) and an immunoassay with the ELISA kit (Takara Bio, Otsu, Shiga, Japan), respectively.
The urine samples were collected and stored at −80 °C on the same day that the blood samples were taken. The levels of urine free deoxypyridinoline (DPD), a bone resorption marker, were determined by sandwich EIA (Pyrilinks-D; Metra Biosystems) and corrected for urinary creatinine concentration.
Serum 25-hydroxyvitamin D [25(OH)D] was analyzed using a radioimmunoassay [25-hydroxyvitamin D 125 IRIA KIT (100); DiaSorin, Stillwater, MN, USA], and finally, pentosidine, leptin, and adiponectin were analyzed using a FSK-pentosidine ELISA kit (Fushimi Seiyakusyo, Kagawa, Japan), an RIA kit (Cosmic, Tokyo, Japan), and a latex particle-enhanced turbidimetric immunoassay kit (Mitsubishi Chemical Medience, Tokyo, Japan), respectively.
Blood pressure measurement
The chronic levels of arterial blood pressure at rest were measured in the brachial and dorsalis arteries, using a semiautomated device (Form PWV/ABI; Colin Medical Technology, San Antonio, TX, USA). The measurement was recorded three times for each patient while lying in the supine position.
Measurement of BMD
Bone mineral density (BMD) of the total body and total hip region were assessed by dual-energy X-ray absorptiometry (DXA) at baseline and after 6 and 12 months using a Hologic QDR-4500A scanner (Hologic, Waltham, MA, USA). BMD of the forearm, one-third distal point of the radius between the styloid process and the tip of the olecranon of the elbow, was assessed by DXA at baseline and after 6 and 12 months using an ALOKA DCS-600EX (ALOKA, Tokyo, Japan). To minimize interobserver variation, all the scans and analysis were carried out by the same investigator. The day-to-day coefficients of variation (CVs) of these observations were <0.3, 0.8, and 1.0 % for BMD in the total hip, whole body, and forearm, respectively. Long-term precision was 0.35 % by testing the spine phantom daily over the previous 1 year for the QDR-4500A scanner.
Questionnaire interview
The trained interviewers collected information from each individual during the face-to-face interviews. The questions were based on the structure of a previously validated questionnaire that included information on sociodemographic data, years since menopause, level of physical activity, medication, and smoking and drinking alcohol habits, as well as several other factors that may have possible confounding effects on the relationship between dietary vitamin K consumption and lipid metabolism.
Dietary analysis
At baseline and at 6 and 12 months after the start of the study, each participant’s dietary intake of energy, protein, vitamin K, and calcium, as well as other nutritional components, was estimated from a 3-day food record and the Fifth Revision of the Standard Tables of Food Composition in Japan [18]. The trained dieticians confirmed each food record during the face-to-face interviews.
Statistical analysis
Descriptive statistics were used to analyze all the variables at baseline and 6 and 12 months after the onset of the study. The data were transformed to normality, and parametric tests were used, with a p < 0.05 being statistically significant. The variables of the two groups at baseline and after 6 and 12 months and the 12-month changes (%) were compared using repeated-measures analysis of variance (ANOVA). We studied the changes in serum E2, 25(OH)D, PK, MK-4, OC, bone metabolic markers, and lipids during the study period, using ANOVA and the Scheffe post hoc test. The significant differences and percent changes in BMD between the groups were determined by repeated-measures ANOVA with covariate adjustment. To adjust for the possible confounding factors for bone metabolism, body weight, height, and daily intake levels of calcium, vitamin D, protein, and total energy were used as covariates for the analyses of BMD. The components measured at baseline and at 6 and 12 months were compared using the paired t test.
All analyses and calculations were performed using the SPSS 18.0J software for Windows.
Results
Characteristics of the subjects
The baseline characteristics of the subjects are displayed in Table 1, which shows that the age and physique of the subjects were not significantly different between the two groups (Table 1).
The daily nutrient intakes are shown in Table 2.
There were some significant differences between the daily intakes of nutrients at baseline and those at 12 months. In the control group, the mean energy and vitamin D intakes were higher at 12 months than at baseline. Furthermore, in the MK-4 group, the mean protein intake was significantly higher at 12 months than at baseline (Table 2).
The level of physical activity of the subjects was classified into three groups according to the DRIs for Japanese (2010), as low, middle, and high [13]. The percent of each level was 4, 79, and 17 % in the control group and 13, 67, and 17 % in the MK-4 group, respectively, at baseline. The percent of each level was 13, 79, and 8 % in the control group and 13, 75, and 12 % in the MK-4 group, respectively, at 12 months.
Effect of MK-4 supplementation on serum hormone, vitamin K, and vitamin D concentrations
Serum E2, PK, MK-4, MK-7, and 25(OH)D concentrations in both groups at baseline and at 6 and 12 months are shown in Table 3. The MK-4 concentration was significantly higher in the MK-4 group than that in the control group at 12 months. The within-group concentrations of 25(OH)D, MK-4, and MK-7 did not change after MK-4 supplementation. However, in the MK-4 group, the mean serum concentration of E2 was significantly higher at 12 months than at baseline. Furthermore, the PK concentrations decreased in the control group and were significantly lower at 6 months than at baseline. However, the PK concentration was not significantly different at 12 months than at baseline.
Effect of MK-4 supplementation on serum OC
The serum concentrations of OC in the two groups are shown in Table 4. The concentrations of ucOC at baseline were high in both groups compared with the values that are usually observed in young women [19]. The concentrations of ucOC and GlaOC in the control group compared with the MK-4 group were not significantly different at baseline. The ucOC concentration in the MK-4 group was significantly lower than that in the control group at 6 and 12 months. In addition, ucOC concentration between the 6- and 12-month evaluations decreased more substantially in the MK-4 group compared with the control group. The GlaOC was still significantly higher in the MK-4 group at 6 and 12 months compared with the control group. In the control group, the percent changes in GlaOC concentration at 6 and 12 months were negative; however, these changes were positive in the MK-4 group, and the difference in percent changes between the two groups was significant.
In the control group, the serum concentrations of ucOC and GlaOC at 12 months were significantly lower than those at baseline; in the MK-4 group, the only ucOC concentrations were significantly lower at 6 and 12 months than at baseline. The levels of GlaOC in the MK-4 group did not change throughout the study period.
Effect of MK-4 supplementation on serum bone metabolic markers
The serum concentrations of the bone markers in the control and MK-4 groups are shown in Table 5. In both groups, the concentration of BS-ALP was significantly higher at 12 months than that at baseline. The serum concentrations of DPD, a bone resorption marker, in the two groups at 6 months were significantly different; however, the difference was not significant at 12 months. The percent change in the serum concentration of pentosidine at 6 and 12 months was significantly different between the control and MK-4 group. Within the group, although there was no significant change in the pentosidine concentrations through the study period in the control group, the concentrations in the MK-4 group were significantly lower at 6 and 12 months than at baseline.
Regarding the leptin concentration, there were no significant differences between the control group and MK-4 group at baseline, 6, or 12 months. However, the leptin concentration at 12 months in the control group was significantly higher than that at baseline; in the MK-4 group, the concentration was significantly higher at the 6- and 12-month evaluations. There was no significant change in the serum adiponectin concentration (Table 5).
Effect of MK-4 supplementation on BMD
The total body, hip, and forearm BMDs of the control and MK-4 groups at baseline and at 6 and 12 months are shown in Table 6
There was no significant difference in hip BMD between the two groups at baseline and at 6 and 12 months (Table 6). The percent change in total body BMD at 6 months was significantly different between the two groups. However, at the 12-month evaluation, this difference was smaller and was no longer significant. On one hand, the forearm BMD in the control group was significantly lower at 12 months than at 6 months; on the other hand, there was no significant decrease in the MK-4 group.
MK-4 supplementation effect on other bio-indices
There was no significant difference in blood pressure between the groups at baseline or 6 or 12 months. In addition, there was no significant change in blood pressure during the 12-month study period in both groups (data not shown). The serum concentrations of lipids such as TC, HDL-cholesterol, LDL-cholesterol, and TG were not significantly different between the groups. In addition, no adverse events were observed in any participant throughout the trial period (data not shown).
Discussion
Previously, we reported that daily 1.5-mg MK-4 supplementation may lead to an increase in the rate of OC γ-carboxylation [12]. In this study, we examined the long-term effects of MK-4 administration on BMD, bone turnover markers, and other bio-indices in healthy postmenopausal Japanese women between 50 and 65 years of age.
When the recorded mean energy and nutrient dietary intakes of the subjects were compared with the AI levels of the DRIs for the Japanese (2010) [13], we determined that their daily intakes of energy, calcium, iron, and magnesium were insufficient. However, their vitamin K intake was almost twice the recommended level, which is stated to be 65 μg/day (Table 2), reflecting the tendency of Japanese to have a high vitamin K intake. In 2008, the National Health and Nutrition Survey reported that older Japanese people are likely to consume greater quantities of vitamin K, and the mean intake of women aged 50 years was estimated to be 260 μg/day. The vitamin K intake of our subjects was relatively low in comparison with the national average. In other words, in general, Japanese tend to consume more vitamin K than the AI level.
In this study, the serum concentration of MK-4 in the MK-4 group had increased at 6 and 12 months; the concentrations were significantly different between the MK-4 and control groups. In our previous study, daily 1.5-mg MK-4 supplementation for 2 or 4 weeks resulted in a significant increase in the serum level of MK-4 [12]. Thus, supplementation at this dose for 2 weeks to 12 months may increase the serum level of MK-4 in postmenopausal women. There was no significant difference in the serum concentration of MK-7 at baseline or after 12 months in either of the two groups.
Vitamin K is thought to maintain bone strength via the γ-carboxylation of OC [2]. It has been reported that high levels of incompletely carboxylated serum OC are an indicator of vitamin K deficiency in the bone in a healthy population [20]. In individuals with vitamin K insufficiency/deficiency, small volumes of OC are released from the osteoblasts into the circulation. Therefore, the serum concentration of ucOC is considered to be a sensitive marker of vitamin K deficiency in the bone [21, 22]. In previous studies, high serum concentrations of ucOC were associated with high skeletal turnover [23], low BMD [24], and an increased risk of osteoporotic fractures [22, 24, 25]. Several reports have been published on the pharmacological MK-4 dose that enhances carboxylation in healthy elderly people [5, 6] and osteoporotic postmenopausal women [26]. In a previous report, it was indicated that MK-7 has greater efficacy than PK for the carboxylation of bone Gla proteins [9]. However, the efficacy of MK-4 for the carboxylation of these proteins remains unclear. In this study, the serum concentration of ucOC decreased after 6 months of daily 1.5-mg MK-4 supplementation.
Tsugawa et al. [19] suggested that the concentration of circulating vitamin K should be maintained at a higher level in elderly people than in young people. They also reported that the mean serum level of ucOC in their cohort of Japanese elderly women (aged 63 years) was 4.68 ± 3.15 ng/ml [16]. In our study, the serum ucOC level at baseline (group mean, 5.7–6.4 ng/ml) was found to be higher than the levels that have been published in other reports [16, 19, 22]. Shiraki et al. [27] reported that the bone is deficient in vitamin K if the concentration of ucOC is 4.5 ng/ml, and the cutoff value for the risk of fracture is 5.5 ng/ml in postmenopausal Japanese women. The intake of vitamin K by our subjects was 166–182 μg/day (group mean), which is considerably higher than the AI level (65 μg/day) that is recommended by the DRIs for Japanese (2010). However, the serum levels of ucOC recorded in our subjects were similar to the cutoff value for the risk-of-fracture. Given that there is evidence indicating that a high concentration of serum vitamin K might be beneficial for maintaining the bone mass of postmenopausal women, the DRI for this Japanese subpopulation should be higher than the current AI. Similarly, a report by Bügel [28] indicated that vitamin K requirements might be higher than the present AI value because dietary references are based on the saturation of the coagulation system, and in most countries, the dietary intake of vitamin K is sufficient to obtain the amount recommended.
We assessed the serum level of BS-ALP as an indicator of bone formation and the level of urinary DPD as an indicator of bone resorption. These concentrations were not affected by MK-4 supplementation during the 12-month study period. Previous studies have reported similar results concerning the bone resorption marker [26]. However, the serum levels of pentosidine [a marker for advanced glycation end products (AGEs)] [29] were high in our subjects, and at 6 months, the MK-4 supplementation had decreased the levels of this biomarker. The subgroup analysis demonstrated that only the higher-level (>50th percentile) subgroup of the MK-4 group demonstrated a decrease in the levels of pentosidine (data not shown). Recently, it was reported that the nonenzymatic cross-linking of collagen by AGEs, such as pentosidine, is associated with an increased fracture risk [29, 30]. Although the efficacy of vitamin K on the maintenance of bone quality has been proposed to be dependent on the stimulation of collagen synthesis [31] and OC carboxylation, the inhibitory effect of MK-4 against pentosidine might contribute to the sustaining of bone quality. The mechanisms causing serum levels of pentosidine to decrease in individuals receiving MK-4 supplementation are currently unclear. However, it is possible that this decrease may be caused by the antioxidant activity of vitamin K [32]. This hypothesis should be examined in future studies.
It has been reported that serum leptin level correlated with bone resorption in healthy postmenopausal women [33]. In contrast, circulating adiponectin levels showed significant association with indices on bone mass in middle-aged postmenopausal women [34, 35]. Thus, we examined these lipid-related markers correlated with bone metabolism. Regarding blood pressure, there is a report that vitamin K-dependent γ-carboxylation of matrix Gla protein in blood vessels is essential for its inhibitory effects on calcification [2]. In the results, there were no differences in these indices between control and MK-4 groups. Although the systolic blood pressure (SBP) and diastolic blood pressure (DBP) were not different between the groups at any experimental periods in this study, the effects of vitamin K on blood pressure might be confirmed in the near future.
There was a significant difference in the percent change at 6 months in the total body BMD between control group and the MK-4 group. Although the exact rationale for this is not clear, it is possible that there is a site specificity of the effect of MK-4 on BMD. Fang et al. [11] have shown that vitamin K supplementation was effective in increasing lumbar spine BMD but not in femoral neck BMD by meta-analysis of postmenopausal women. This site specificity may induce a significant difference at 6 months in the percent change in the total body BMD between the two groups, although the total body and total hip BMDs did not change significantly in either the control or MK-4 group at 12 months.
The forearm BMD in the control group had significantly decreased after 12 months. However, there was no significant decrease in BMD in the MK-4 group during the study period. These results suggest that MK-4 sustained bone strength in postmenopausal healthy Japanese women by maintaining bone quality and by inhibiting bone degradation.
On the efficacy of therapy, the concept of minimal significant change (MSC) was applied. The Japanese 2011 guidelines for prevention and treatment of osteoporosis indicates the MSC for percentage change for BMD at lumbar spine [36], femur, forearm, and bone metabolism markers [36]. Although the aim of this study was not therapy for the patients with osteoporosis, percent change of ucOC at 6 and 12 months in MK-4 group satisfied the MCS (%) of ucOC, 32.2 %, in the guideline.
The limitation of this study is that the intake of MK-4 and MK-7 from daily meals was not restricted.
In conclusion, our results suggest that the 6- to 12-month low-dose MK-4 supplementation in the postmenopausal Japanese women improved bone quality without substantial adverse effects by decreasing the serum concentrations of ucOC and pentosidine.
Abbreviations
- Gla:
-
γ-Carboxyglutaminate
- PK:
-
Phylloquionone
- MK:
-
Menaquinone
- OC:
-
Osteocalcin
- DRIs:
-
Dietary reference intakes
- AI:
-
Adequate intake
- ucOC:
-
Undercarboxylated OC
- BMD:
-
Bone mineral density
- TC:
-
Total cholesterol
- TG:
-
Triacylglycerol
- HDL:
-
High density lipoprotein
- LDL:
-
Low density lipoprotein
- E2 :
-
17β-Estradiol
- BS-ALP:
-
Bone-specific alkaline phosphatase
- GlaOC:
-
γ-Carboxylated OC
- DPD:
-
Deoxypiridinoline
- 25[OH]D:
-
25-Hydroxyvitamin D
References
Vermeer C (1990) Gamma-carboxyglutamate-containing proteins and the vitamin-K-dependent carboxylase. Biochem J 266:625–636
Adams J, Pepping J (2005) Vitamin K in the treatment and prevention of osteoporosis and arterial calcification. Am J Health Syst Pharm 62:1574–1581
Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K (2008) Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem 283:11270–11279
Plaza SM, Lamson DW (2005) Vitamin K2 in bone metabolism and osteoporosis. Altern Med Rev 10:24–35
Ozuru R, Sugimoto T, Yamaguchi T, Chihara K (2002) Time-dependent effects of vitamin K2 (menatetrenone) on bone metabolism in postmenopausal women. Endocr J 49:363–370
Iwamoto I, Kosha S, Noguchi S, Murakami M, Fujino T, Douchi T, Nagata Y (1999) A longitudinal study of the effect of vitamin K2 on bone mineral density in postmenopausal women a comparative study with vitamin D3 and estrogen–progestin therapy. Maturitas 31:161–164
Ishida Y, Kawai S (2004) Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, alfacalcidol, and vitamin K in postmenopausal women with osteoporosis: the Yamaguchi Osteoporosis Prevention Study. Am J Med 117:549–555
Shiraki M, Shiraki Y, Aoki C, Miura M (2000) Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res 15:515–521
Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ (2006) Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med 166:1256–1261
Binkley NC, Krueger DC, Kawahara TN, Engelke JA, Chappell RJ, Suttie JW (2002) A high phylloquinone intake is required to achieve maximal osteocalcin gamma-carboxylation. Am J Clin Nutr 76:1055–1060
Fang Y, Hu C, Tao X, Wan Y, Tao F (2012) Effect of vitamin K on bone mineral density: a meta-analysis of randomized controlled trials. J Bone Miner Metab 30:60–68
Koitaya N, Ezaki J, Nishimuta M, Yamauchi J, Hashizume E, Morishita K, Miyachi M, Sasaki S, Ishimi Y (2009) Effect of low dose of vitamin K2 (MK-4) supplementation on bio-indices in postmenopausal Japanese women. J Nutr Sci Vitaminol 55:15–21
Ministry of Health, Labour, and Welfare, Japan (2010) Dietary reference intakes for Japanese. Daiichi Shuppan, Tokyo
Booth SL, Martini L, Peterson JW, Saltzman E, Dallal GE, Wood RJ (2003) Dietary phylloquinone depletion and repletion in older women. J Nutr 133:2565–2569
Kaneki M, Hodges SJ, Hosoi T, Fujiwara S, Lyons A, Crean SJ, Ishida N, Nakagawa M, Takechi M, Sano Y, Mizuno Y, Hoshino S, Miyao M, Inoue S, Horiki K, Shiraki M, Ouchi Y, Orimo H (2001) Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip-fracture risk. Nutrition 17:315–321
Tsugawa N, Shiraki M, Suhara Y, Kamano M, Ozaki R, Tanaka K, Okano T (2008) Low plasma phylloquinone concentration is associated with high incidence of vertebral fracture in Japanese women. J Bone Miner Metab 26:79–85
Langenberg JP, Tjaden UR (1984) Improved method for the determination of vitamin K1 epoxide in human plasma with electrofluorimetric reaction detection. J Chromatogr 289:377–385
Kagawa Y (2005) Standard tables of food composition in Japan. 5th revised edition. Kagawa Education Institute of Nutrition, Tokyo
Tsugawa N, Shiraki M, Suhara Y, Kamano M, Tanaka K, Okano T (2006) Vitamin K status of healthy Japanese women: age-related vitamin K requirement for γ-carboxylation of osteocalcin. Am J Clin Nutr 83:380–386
Knapen MH, Nieuwenhuijzen Kruseman AC, Wouters RS, Vermeer C (1998) Correlation of serum osteocalcin fractions with bone mineral density in women during the first 10 years after menopause. Calcif Tissue Int 63:375–379
Sokoll LJ, Sadowski JA (1996) Comparison of biochemical indexes for assessing vitamin K nutritional status in a healthy adult population. Am J Clin Nutr 63:566–573
Szulc P, Chapuy MC, Meunier PJ, Delmas PD (1993) Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest 91:17–74
Knapen MH, Jie KS, Hamulyak K, Vermeer C (1993) Vitamin K-induced changes in markers for osteoblast activity and urinary calcium loss. Calcif Tissue Int 53:81–85
Szulc P, Arlot M, Chapuy MC, Duboeuf F, Meunier PJ, Delmas PD (1994) Serum undercarboxylated osteocalcin correlates with hip bone mineral density in elderly women. J Bone Miner Res 9:1591–1595
Takahashi M, Naitou K, Ohishi T, Kushida K, Miura M (2001) Effect of vitamin K and/or D on undercarboxylated and intact osteocalcin in osteoporotic patients with vertebral or hip fractures. Clin Endocrinol 54:219–224
Knapen MH, Schurgers LJ, Vermeer C (2007) Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int 18:963–972
Shiraki M, Aoki C, Yamazaki N, Ito Y, Tsugawa N, Suhara Y, Okano T (2007) Clinical assessment of undercarboxylated osteocalcin measurement in serum using an electrochemiluminescence immunoassay: establishments of cut-off values to determine vitamin K insufficiency in bone and to predict fracture leading to clinical use of vitamin K2. Jpn J Med Pharm Sci 57:537–546
Bügel S (2008) Vitamin K and bone health in adult humans. Vitam Horm 78:393–416
Wang X, Shen X, Li X, Agrawal CM (2002) Age-related changes in the collagen network and toughness of bone. Bone (NY) 31:1–7
Saito M, Marumo K (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 21:195–214
Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, Inoue S (2006) Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J Biol Chem 281:16927–16934
Vervoort LM, Ronden JE, Thijssen HH (1997) The potent antioxidant activity of the vitamin K cycle in microsomal lipid peroxidation. Biochem Pharmacol 54:871–876
Roux C, Arabi A, Porcher R, Garnero P (2003) Serum leptin as a determinant of bone resorption in healthy postmenopausal women. Bone (NY) 33:847–852
Zhang H, Xie H, Zhao Q, Xie GQ, Wu XP, Liao EY, Luo XH (2010) Relationships between serum adiponectin, apelin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in post-menopausal Chinese women. J Endocrinol Invest 33:707–711
Zhang Y, Zhou P, Kimondo JW (2012) Adiponectin and osteocalcin: relation to insulin sensitivity. Biochem Cell Biol 90:613–620
Orimo H, Nakamura T, Hosoi T, Iki M, Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, Soen S, Nishizawa Y, Hagino H, Fukunaga M, Fujiwara S (2012) Japanese 2011 guidelines for prevention and treatment of osteoporosis: executive summary. Arch Osteoporos 7:3–20
Acknowledgments
We gratefully acknowledge the dedicated women who participated in this study. This study was supported by a grant from the National Institute of Health and Nutrition, Ministry of Health, Labor and Welfare, Japan and KYOWA HAKKO BIO. Co., Ltd.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Koitaya, N., Sekiguchi, M., Tousen, Y. et al. Low-dose vitamin K2 (MK-4) supplementation for 12 months improves bone metabolism and prevents forearm bone loss in postmenopausal Japanese women. J Bone Miner Metab 32, 142–150 (2014). https://doi.org/10.1007/s00774-013-0472-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-013-0472-7