Abstract
Introduction and hypothesis
Sacrocolpopexy is effective for apical prolapse repair and is often performed with hysterectomy. It is unknown whether supracervical or total hysterectomy at time of sacrocolpopexy influences prolapse recurrence and mesh complications. The primary objective of this study is to compare reoperations for recurrent prolapse after sacrocolpopexy with either supracervical hysterectomy or total hysterectomy, or without concomitant hysterectomy. We also sought to compare these three groups for the incidence of mesh complications and describe cervical interventions following supracervical hysterectomy.
Methods
A retrospective cohort study of sacrocolpopexy was performed using the MarketScan® Research Database. Women > 18 years who underwent sacrocolpopexy between 2010 to 2014 were identified. Utilizing diagnostic and procedural codes, reoperations for prolapse and mesh complications were identified. Women with < 2 years of follow-up were excluded.
Results
From 2010 to 2014, 3463 women underwent sacrocolpopexy with at least 2 years of follow-up, 910 (26.3%) with supracervical hysterectomy, 1243 (35.9%) with total hysterectomy, and 1310 (37.8%) without hysterectomy. Reoperations for prolapse were similar after supracervical hysterectomy (1.5%), after total hysterectomy (1.1%, p = 0.40), and without hysterectomy (1.5%, p = 0.98). Mesh complications after sacrocolpopexy were similar after supracervical hysterectomy (1.8%), after total hysterectomy (1.5%, p = 0.68), and without hysterectomy (2.8%, p = 0.11). Following supracervical hysterectomy, 0.9% underwent cervical procedures.
Conclusions
When comparing supracervical and total hysterectomy at time of sacrocolpopexy, there were no significant differences in reoperations for recurrent prolapse, reoperations for mesh complications, or mesh complication diagnoses. This study shows that surgeons can be reassured on performing hysterectomy with sacrocolpopexy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse (POP) is common with > 200,000 prolapse repair surgeries performed each year in the USA. As the population ages, the need for prolapse repair surgeries is expected to increase [1, 2]. One option for apical prolapse repair is sacrocolpopexy, which has higher success rates and lower reoperation rates for prolapse recurrence compared to other types of apical prolapse repair procedures [3].
Among women with uterovaginal prolapse, sacrocolpopexy can be performed with or without a concomitant hysterectomy. There is no clear consensus on whether total hysterectomy (TH) or supracervical hysterectomy (SCH) is preferred at the time of sacrocolpopexy [4]. Supracervical hysterectomy can potentially reduce the risk of mesh erosion or exposure [5,6,7]. Others have advocated that total hysterectomy results in better anterior vaginal wall support [7, 8], but the literature has conflicting results. There is also limited and conflicting evidence on recurrent prolapse rates after sacrocolpopexy with supracervical hysterectomy, with total hysterectomy, and without concomitant hysterectomy. Few studies have assessed mesh complications and recurrent prolapse following sacrocolpopexy based on the type of concomitant hysterectomy, and many of these studies are limited by small sample size, short follow-up time, and limited generalizability [6, 7, 9]. Additionally, compared to total hysterectomy, supracervical hysterectomy has the potential to require subsequent cervical interventions for indications such as bleeding, dysplasia, neoplasia, and reoperations. At this time, the rate of cervical interventions following supracervical hysterectomy for prolapse repair is unknown.
Using the IBM MarketScan® Research database, which contains de-identified records of 47 million privately insured US individuals, we sought to compare the reoperations for recurrent prolapse in women undergoing sacrocolpopexy with supracervical hysterectomy, with total hysterectomy, and without concomitant hysterectomy. Based upon a review of the literature, we hypothesized the reoperation rates for recurrent prolapse to be approximately 10% over at least 2 years postoperatively [8] with no significant difference among the three cohorts. We also sought to compare these three groups for the incidence of mesh complications and to describe cervical interventions following supracervical hysterectomy.
Methods
A retrospective cohort study of women undergoing sacrocolpopexy with and without concomitant hysterectomy was performed utilizing the IBM MarketScan® Research database, which contains de-identified records of privately insured patients in the US from over 350 private health insurance organizations. Inpatient and outpatient records were available for more than 47 million enrolled participants. Since the data are de-identified and comply with the requirements of the Health Insurance Portability and Accountability Act (HIPAA), this study was exempt from formal review by the Johns Hopkins University School of Medicine Institutional Review Board. We followed the Strengthening of Reporting of Observational Studies in Epidemiology (STROBE) guidelines [10].
Women 18 to 64 years of age who underwent sacrocolpopexy between 2010 to 2014 were identified using Current Procedural Terminology (CPT) codes. Sacrocolpopexy was defined by two CPT codes: 57425 (laparoscopic) and 57280 (abdominal). CPT codes were also utilized to identify patients who underwent concomitant supracervical hysterectomy and total hysterectomy (Appendix 1). All routes of hysterectomy were included (Supplemental Table 1). There were three cohorts: sacrocolpopexy with supracervical hysterectomy, sacrocolpopexy with total hysterectomy, and sacrocolpopexy without concomitant hysterectomy. For sacrocolpopexy without concomitant hysterectomy, we were unable to identify women who had a prior hysterectomy through the database. Thus, this cohort presumably included women with prior hysterectomy as well as those undergoing uterine-preserving surgery with a sacrohysteropexy. Women enrolled in the database for < 30 days prior to surgery and those with multiple hysterectomy codes were excluded.
The primary outcome was reoperation for prolapse. This was defined as the number of patients undergoing subsequent prolapse repair surgery after sacrocolpopexy. CPT codes were utilized to identify various subsequent prolapse repair surgeries (Appendix 1).
A secondary outcome was mesh complications. We considered the proportion of women in each group with a diagnosis of any mesh complication as well as any intervention for mesh complications. For the diagnosis of any mesh complication, ICD-9 codes were utilized to identify subsequent mesh complications (Appendix 1). For interventions for mesh complications, CPT codes were used to identify patients undergoing mesh revision or removal (Appendix 1).
A third outcome was cervical interventions after supracervical hysterectomy. CPT codes were used to identify cervical procedures following sacrocolpopexy (Appendix 1).
For all outcomes, we excluded outcomes that occurred within a 30-day interval immediately following sacrocolpopexy to minimize duplicate coding errors that could arise from day of surgery and day of hospital discharge. To focus on long-term outcomes, women with < 2 years of follow-up were excluded. All patients were followed until their enrollment in the database was terminated or until the end of 2017, whichever came first.
Patient demographics and clinical variables, including age, year of surgery, health insurance plan, geographic region, rural or urban setting, and history of diabetes, using ICD-9 code 250, were extracted. Women with this diagnosis code at any point prior to their surgery were identified as diabetic. Data on race and ethnicity, body mass index (BMI), smoking status, and operative time were not available. At the time of sacrocolpopexy, patients who underwent a concomitant anti-incontinence procedure, anterior colporrhaphy, posterior colporrhaphy, and enterocele repair were identified utilizing CPT codes.
The incidence rates for the primary and secondary outcomes were calculated within each cohort. Incident rates of each outcome were calculated as the number of patients with the outcome divided by the total person-time at risk in each cohort.
A power calculation was performed with a preliminary data extraction. With an estimated sample size of 2100 eligible patients, and 60% of patients (n = 1260) with follow-up outcome data over 2 years, we estimated we would have 84% power to observe at least a 2% difference in incidence rate in reoperations for prolapse between the cohorts.
Descriptive statistics were calculated with means and standard deviations (SD) or median and interquartile range (IQR) for continuous variables. Categorical variables were calculated as proportions, n (%). Factors of interest were compared between cohorts using chi-square or Fisher’s exact tests for categorical variables, t-tests for normally distributed continuous variables, and Wilcoxon rank-sum tests for continuous variables that were not normally distributed.
Kaplan-Meier curves were used to visually compare outcomes over the follow-up period within each cohort. Cox proportional hazards regression models predicting outcomes of interest were used to compare the relative hazard for reoperations for recurrent prolapse and incidence of mesh complications among the three cohorts. Adjusted hazard ratios (aHR) were reported with a 95% confidence interval (95% CI). For these comparisons, the reference group was sacrocolpopexy with supracervical hysterectomy, adjusting for age and year of surgery. All regression models included terms for age and year of surgery. For reoperations for prolapse and diagnosis of mesh complications, sensitivity analyses were performed limited to women who underwent a concomitant sling procedure.
Two-sided p values, with p < 0.05, were considered statistically significant. Analyses were performed on the Johns Hopkins Joint High Performance Computing Exchange using SAS v9.4 (SAS Institute, Cary, NC).
Results
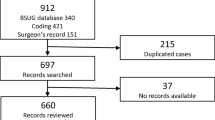
From 2010 to 2014, 8141 women underwent sacrocolpopexy, including 2143 (26.3%) with supracervical hysterectomy, 2863 (35.2%) with total hysterectomy, and 3135 (38.5%) without concomitant hysterectomy (Fig. 1). Of these, 3463 (43%) had at least 2 years of continuous follow-up, including 910 (26.3%) with supracervical hysterectomy, 1243 (35.9%) with total hysterectomy, and 1310 (37.8%) without hysterectomy.
Demographic and clinical characteristics of women who underwent sacrocolpopexy with at least 2 years of follow-up are presented in Table 1. Comparing the three groups, the mean age was highest for sacrocolpopexy without concomitant hysterectomy (54.1 + 6.9 years) and lowest for sacrocolpopexy with total hysterectomy (49 + 8.1 years, p < 0.0001). There were significant geographic differences with higher frequency of sacrocolpopexy with supracervical hysterectomy in the Northeast and higher frequency of sacrocolpopexy with total hysterectomy in the South (p < 0.0001). There was no significant difference in the prevalence of diabetes among the three cohorts (p = 0.55). There were significant differences in concomitant anti-incontinence procedures among the three cohorts, with the highest in sacrocolpopexy with supracervical hysterectomy at 50.8% and lowest with sacrocolpopexy with total hysterectomy at 28.9% (p < 0.0001). We also observed differences in the proportion of women with concomitant prolapse repair procedures, including anterior colporrhaphy, posterior colporrhaphy, enterocele repair, and perineorrhaphy (p = 0.001). The mean follow-up times were 3.9 + 1.5 years for sacrocolpopexy with supracervical hysterectomy, 4.1 + 1.6 years for sacrocolpopexy with total hysterectomy, and 3.9 + 1.4 years for sacrocolpopexy without hysterectomy (p = 0.03).
Reoperations for recurrent prolapse and mesh complications are presented in Table 2. Fourteen women underwent reoperations for prolapse after sacrocolpopexy with supracervical hysterectomy (1.5%), 14 women after sacrocolpopexy with total hysterectomy (1.1%), and 20 women after sacrocolpopexy without concomitant hysterectomy (1.5%). Adjusting for age and year of surgery, compared to sacrocolpopexy with supracervical hysterectomy, there was no difference in reoperations for prolapse with sacrocolpopexy with total hysterectomy [aHR 0.63, 95% CI 0.3–1.3] and sacrocolpopexy without concomitant hysterectomy (aHR 1.0, 95% CI 0.5–2.0). The incidence rate per 1000 person-years for recurrent prolapse was 3.96 in sacrocolpopexy with supracervical hysterectomy, 2.80 with total hysterectomy, and 3.98 without concomitant hysterectomy (Table 3). Kaplan-Meier curves were created for reoperations for recurrent prolapse (Fig. 2), and reoperations occurred as early as 30 days postoperatively in sacrocolpopexy without hysterectomy and as late as 2412 days (6.6 years) postoperatively in sacrocolpopexy without hysterectomy. Sensitivity analyses were performed among only those women who underwent concomitant sling surgery with no difference in reoperations for prolapse and diagnosis for mesh complications (Supplemental Table 2).
Mesh exposure was diagnosed in 16 women after sacrocolpopexy with supracervical hysterectomy (1.8%), 19 women after sacrocolpopexy with total hysterectomy (1.5%), and 37 women after sacrocolpopexy without concomitant hysterectomy (2.8%) (Table 2). Compared to sacrocolpopexy with supracervical hysterectomy, mesh complications were similar after sacrocolpopexy with total hysterectomy (aHR 0.83, 95% CI 0.4–1.6) and without hysterectomy (aHR 1.7, 95% CI 0.9–3.0). The incidence rate per 1000 person-years for mesh complications was 4.53 in sacrocolpopexy with supracervical hysterectomy, 3.81 with total hysterectomy, and 7.36 without concomitant hysterectomy (Table 3). Kaplan-Meier curves were created for reoperations for overall mesh complications (Fig. 3). Mesh complications were seen as early as 70 days postoperatively in sacrocolpopexy without hysterectomy and as late as 1754 days (4.8 years) postoperatively in sacrocolpopexy with supracervical hysterectomy. There were also no differences in sensitivity analyses limited to those with concomitant sling surgery (Supplemental Table 1).
We observed very few reoperations for mesh complications: 5 (0.6%) in sacrocolpopexy with supracervical hysterectomy, 3 (0.2%) in sacrocolpopexy with total hysterectomy, and 13 (1.0%) in sacrocolpopexy without concomitant hysterectomy. Given these small numbers, statistical comparisons were limited. Compared to sacrocolpopexy with supracervical hysterectomy, there was no difference in sacrocolpopexy with total hysterectomy (p = 0.29) and sacrocolpopexy without concomitant hysterectomy (p = 0.25). The incidence rate per 1000 person-years for reoperations for mesh complications was 1.42 in sacrocolpopexy with supracervical hysterectomy, 0.60 with total hysterectomy, and 2.59 without concomitant hysterectomy (Table 3).
After SCP with supracervical hysterectomy, 8 out of 910 patients (0.9%) underwent a cervical procedure. There were four trachelectomies, three cervical amputations, and one cervical excision. Indications for the cervical procedures included genitourinary fistula, prolapse, mesh complications, cervical inflammation, and peritoneal adhesions.
Discussion
Using a national claims database, we found that reoperations for recurrent prolapse and mesh complications are uncommon after sacrocolpopexy, regardless of whether this surgery included supracervical hysterectomy, total hysterectomy, or no hysterectomy. Compared to sacrocolpopexy with supracervical hysterectomy, we observed no difference in the hazard of reoperations for recurrent prolapse or diagnosis of mesh complications among women who underwent sacrocolpopexy with total hysterectomy or sacrocolpopexy without hysterectomy. We also observed that reoperations for mesh complications were uncommon and did not differ among the groups, and the number of subsequent cervical interventions after supracervical hysterectomy was low.
Our findings build on what is already known about reoperations for recurrent prolapse following sacrocolpopexy. One retrospective cohort study with prospective follow-up found no difference in anatomic success rates between laparoscopic supracervical hysterectomy and total vaginal hysterectomy at time of sacrocolpopexy; however, this study had a median follow-up time of 9 months, and only one-third of patients presented for a postoperative follow-up exam [9]. Contrarily, Myers et al. compared recurrent prolapse rates in sacrocolpopexy with supracervical hysterectomy and total hysterectomy in a retrospective cohort of 83 women and found higher rates of stage two prolapse after supracervical hysterectomy; however, when prolapse was defined as at or beyond the level of the hymen, there was no difference [7]. Some surgeons have suggested that leaving the cervix in place can have negative impacts on anterior vaginal wall support and potentially lead to recurrent or de novo anterior vaginal wall prolapse [7]. It has also been speculated that mesh placement on the vagina with a retained cervix may predispose patients to recurrent prolapse since the mesh may not be placed as distally on the anterior vagina [8]. This is not consistent with our study, which found no difference in subsequent anterior colporrhaphy or any type of prolapse repair in sacrocolpopexy with supracervical hysterectomy versus total hysterectomy.
Our results add to the growing literature surrounding mesh complications following sacrocolpopexy, which varies across studies with an estimated range of 2% to 10% [11,12,13,14]. There is conflicting evidence as to whether mesh complications are higher after sacrocolpopexy without concomitant hysterectomy and which type of hysterectomy is associated with better outcomes. We found no significant difference in overall mesh complications or in reoperations for mesh complications between sacrocolpopexy with supracervical and total hysterectomy. Our results are consistent with a single site study, which found no difference in mesh erosion rates between laparoscopic supracervical hysterectomy and transvaginal hysterectomy at time of sacrocolpopexy with a median follow-up of 9 months [9]. Our results are not in concordance with a recent study which found significantly higher rates of mesh complications in total hysterectomy compared to supracervical hysterectomy at the time of robotic-assisted sacrocolpopexy [15]. Using a California database from 2012 to 2018, Dallas et al. found a 3.1% rate of mesh complications in total hysterectomy compared to 0.7% in supracervical hysterectomy with a median follow-up of 3 years. Of note, the Dallas study only evaluated robotic-assisted sacrocolpopexy, whereas our study included all routes of sacrocolpopexy and hysterectomy. A meta-analysis of 27 studies with 1488 women found that concomitant hysterectomy was a risk factor for mesh erosions following sacrocolpopexy [16]. A separate study looking at 2-year outcomes in the Colpopexy and Urinary Reduction (CARE) trial found that concurrent hysterectomy at time of sacrocolpopexy was a risk factor for mesh complications [12], and another study of laparoscopic sacrocolpopexy between 2004 to 2009 found higher rates of mesh erosion in sacrocolpopexy with transvaginal hysterectomy compared to post-hysterectomy sacrocolpopexy [6]. One theory for our higher rates of mesh complications in sacrocolpopexy without concomitant hysterectomy is that women with a prior hysterectomy likely have more scar tissue and adhesions at the vaginal apex, which can impact the dissection and make it more challenging to find the proper tissue planes. Additionally, applying mesh to a scarred area may negatively impact healing, thus predisposing to subsequent mesh complications. Lastly, for this study, we were unable to extract data on prior prolapse repair surgeries, and within this cohort, there may be a greater number of patients with a prior prolapse repair given the older age and inclusion of post-hysterectomy sacrocolpopexy.
Mesh complications appear to increase over time, and therefore studies with longer follow-up detect a higher proportion of this outcome. For example, CARE trial estimated mesh complications may be as high as 10.5% after seven years [17]. Of note, this study had variations in the types of mesh utilized and lightweight meshes were not widely used, which may have contributed to its higher mesh complication rate. A limitation of the present research study is that we were unable to extract data on mesh type. For the past decade, lightweight meshes have been commonly used for sacrocolpopexy, and a prior study showed lower rates of mesh complication with lightweight meshes [18].
We observed cervical interventions following supracervical hysterectomy in 0.9% of women. There is limited literature on cervical interventions following supracervical hysterectomy regardless of concomitant sacrocolpopexy. One study showed that 14.1% of premenopausal patients undergoing supracervical hysterectomy experienced persistent cyclical vaginal bleeding within 1 year postoperatively [19], and another study with 1 year of follow-up showed 20% of premenopausal women undergoing supracervical hysterectomy experienced postoperative vaginal bleeding and 1.2% underwent trachelectomy [20]. This is consistent with a retrospective case-control study showing that the occurrence of trachelectomy following supracervical hysterectomy was 0.9% with most common indication being persistent bleeding [21]. In our study encompassing pre- and postmenopausal women, the frequency of trachelectomy was 0.45% with an overall frequency of 0.9% for all cervical interventions. These numbers can be a valuable and pertinent reference when counseling patients on the type of hysterectomy to perform.
There are several limitations to our study. First, although our study represents data for more than 3000 women followed for 2 to 7.9 years, we acknowledge we had limited statistical power to investigate differences in rare outcomes, such as reoperation for mesh exposure. Additionally, recurrent prolapse and mesh complications can occur many years after sacrocolpopexy, and therefore some events were likely not captured in our study’s follow-up period. Also, this database only includes privately insured women and did not include individuals > 64 years of age, which limits the generalizability of our findings as well as limiting the length of follow-up for those women who achieved an age > 64. Given the nature of the database, we were unable to assess patient-level details such as race and ethnicity, BMI, prolapse stage, prior prolapse treatments, smoking status, and mesh types, all of which can potentially influence the outcomes of interest. Inherent to most databases, this study is subject to coding error, but to reduce this, patients with ambiguous codes, such as duplicate hysterectomy codes, were excluded. As previously mentioned, there are limitations with the sacrocolpopexy without concomitant hysterectomy cohort. Given the mean age of the cohort and time period of the surgeries, we expect the majority in this cohort to be post-hysterectomy sacrocolpopexy since it was more common; however, this cohort can also include sacrohysteropexy. The heterogeneity of this cohort should be considered when interpreting the results. Lastly, there was a statistically significant difference in follow-up time between the cohorts, but the time difference was small.
Strengths of this study include its relatively large sample size, national geographic distribution, broad practice patterns, and long-term follow-up. Both out- and inpatient outcomes were collected, which was important for capturing outcomes encountered in an ambulatory setting. We were also able to extract data for both overall mesh complications, based on ICD codes, and reoperations for mesh complications.
Given the limited and conflicting data in the current literature, the outcomes of this study further enhance the literature and provide more guidance to patients and physicians when choosing a type of hysterectomy at time of sacrocolpopexy. At this time and taken into a broader context of the existing literature, this study shows that surgeons can be reassured on performing a hysterectomy at the time of sacrocolpopexy.
References
Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979-1997. Am J Obstet Gynecol. 2003;188(1):108.
Wu JM, Kawasaki A, Hundley AF, et al. Predicting the number of women who will undergo incontinence and prolapse surgery, 2010 to 2050. Am J Obstet Gynecol. 2011;205(3):230.e1–5.
Maher C, Feiner B, Baessler K, Schmid C. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;4:004014.
Costantini E, Brubaker L, Cervigni M, et al. Sacrocolpopexy for pelvic organ prolapse: evidence-based review and recommendations. Eur J Obstet Gynecol Reprod Biol. 2016;205:60–5.
Osmundsen BC, Clark A, Goldsmith C, Adams K, Denman MA, Edwards R, et al. Mesh erosion in robotic sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2012;18(2):86–8.
Tan-Kim J, Menefee SA, Luber KM, Nager CW, Lukacz ES. Prevalence and risk factors for mesh erosion after laparoscopic-assisted sacrocolpopexy. Int Urogynecol J. 2011;22(2):205–12.
Myers EM, Siff L, Osmundsen B, et al. Differences in recurrent prolapse at 1 year after total vs supracervical hysterectomy and robotic sacrocolpopexy. Int Urogynecol J. 2015;26(4):585–9.
Davidson E, Thomas T, Lampert E, Paraiso M, Ferrando C. Route of hysterectomy during minimally invasive sacrocolpopexy does not affect postoperative outcomes. Int Urogynecol J. 2019;30:649–55.
Nosti PA, Carter CM, Sokol AI, Tefera E, Iglesia CB, Park AJ, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22(3):151–5.
Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1497–9.
Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair: a systematic review. Obstet Gynecol. 2009;113(2 Pt 1):367–73.
Cundiff GW, Varner E, Visco AG, Zyczynski HM, Nager CW, Norton PA, et al. Risk factors for mesh/suture erosion following sacral colpopexy. Am J Obstet Gynecol. 2008;199(6):688.e1–5.
Kohli N, Walsh PM, Roat TW, Karram MM. Mesh erosion after abdominal sacrocolpopexy. Obstet Gynecol. 1998;92(6):999–1004.
Feiner B, Jelovsek JE, Maher C. Efficacy and safety of transvaginal mesh kits in the treatment of prolapse of the vaginal apex: a systematic review. BJOG. 2009;116(1):15–24.
Dallas K, Taich L, Kuhlmann P, et al. Supracervical hysterectomy is protective against mesh complications after robotic-assisted abdominal sacrocolpopexy: a population based cohort study of 12,189 patients. Journal of Urology. 2022;207(3):669–76.
Serati M, Bogani G, Sorice P, et al. Robot-assisted sacrocolpopexy for pelvic organ prolapse: a systematic review and meta-analysis of comparative studies. Eur Urol. 2014;66(2):303–18.
Nygaard I, Brubaker L, Zyczynski HM. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016–24.
Salamon CG, Lewis C, Priestley J, et al. Prospective study of an ultra-lightweight polypropylene Y mesh for robotic sacrocolpopexy. Int Urogynecol J. 2013;24(8):1371–5.
Aleixo GF, Fonseca MCM, Bortolini MAT, et al. Total versus subtotal hysterectomy: systematic review and meta-analysis of intraoperative outcomes and postoperative short-term events. Clin Ther. 2019;41(4):768–89.
Gimbel H, Zobbe V, Andersen BM, et al. Randomized controlled trial of total compared with subtotal hysterectomy with one-year follow up results. BJOG. 2003;110:1088–98.
Tsafrir Z, Aoun J, Papalekas E, et al. Risk factors for trachelectomy following supracervical hysterectomy. Acta Obstetricia et Gynecologica Scandinavica. 2017;96:421–5.
Author information
Authors and Affiliations
Contributions
JY Kikuchi: Project development, Data collection, Manuscript writing
LR Yanek: Data collection and management, Data analysis, Manuscript editing
CCG Chen, S Jacobs, J Blomquist, VL Handa: Project development, Manuscript editing
D Patterson: Project development, Manuscript editing, Other – supervision
Corresponding author
Ethics declarations
Conflicts of interest
CCG Chen serves as a consultant on the Ethicon Educational Summit Advisory Panel. The other authors have no disclosures.
Prior presentations
This research was presented as an oral presentation at the Annual AUGS PFD Week in Phoenix, AZ, on 14 October 2021.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplemental Table 1.
Specific routes of hysterectomy within the supracervical hysterectomy and total hysterectomy cohorts (DOCX 12 kb)
Supplemental Table 2.
Cox proportional hazard regression analyses predicting reoperations for prolapse and total mesh complications with concomitant anti-incontinence procedure. (DOCX 13 kb)
Appendix 1
Appendix 1
Table 4
Rights and permissions
About this article
Cite this article
Kikuchi, J.Y., Yanek, L.R., Handa, V.L. et al. Prolapse and mesh reoperations following sacrocolpopexy: comparing supracervical hysterectomy, total hysterectomy, and no hysterectomy. Int Urogynecol J 34, 135–145 (2023). https://doi.org/10.1007/s00192-022-05263-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05263-w