Abstract
Introduction and hypothesis
To prospectively evaluate the use of a particular polypropylene Y mesh for robotic sacrocolpopexy.
Methods
This was a prospective study of 120 patients who underwent robotic sacrocolpopexy. We compared preoperative and 12-month postoperative objective and subjective assessments via the Pelvic Organ Prolapse Quantification (POP-Q), the Pelvic Floor Distress Inventory, Short Form 20 (PFDI-20); the Pelvic Floor Impact Questionnaire, Short Form 7 (PFIQ-7); and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire 12 (PISQ-12). Objective “anatomical success” was defined as POP-Q stage 0 or 1 at all postoperative intervals. We further defined “clinical cure” by simultaneously considering POP-Q points and subjective measures. To be considered a “clinical cure,” a given patient had to have all POP-Q points ≤0, apical POP-Q point C ≤5, no reported pelvic organ prolapse symptoms on the PFDI-20, and no reoperation for prolapse at all postoperative intervals.
Results
Of the 120 patients, 118 patients completed the 1-year follow-up. The objective “anatomical success” rate was 89 % and the “clinical cure” rate was 94 %. The PFDI-20 mean score improved from 100.4 at baseline to 21.0 at 12 months (p < 0.0001); PFIQ-7 scores improved from 61.6 to 8.0 (p < 0.0001); and PISQ-12 scores improved from 35.7 to 38.6 (p < 0.0009). No mesh erosions or mesh-related complications occurred.
Conclusion
The use of this ultra-lightweight Y mesh for sacrocolpopexy, eliminated the mesh-related complications in the first postoperative year, and provided significant improvement in subjective and objective outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sacrocolpopexy surgical procedure involves the placement of a bridge of graft material to attach the prolapsed vagina to the anterior longitudinal ligament of the sacrum. The use of mesh was initially advocated by Lane in 1962 to overcome excessive tension on the vagina [1]. Given its efficacy, durability, and reproducibility, the operation was widely adopted and dubbed the “gold standard prolapse operation” at the turn of the second millennium [2]. While the basics of the surgery have remained the same, advances have been made in surgical access point and in the material used. Many different graft materials have been used over the past half century, yet none has emerged as the “perfect material”; thus, the quest for the ideal graft continues [2, 3]. Currently, type-1 polypropylene mesh is the most widely used material for sacrocolpopexy. Not all type-I polypropylene mesh products are the same. As Dr Ostergard explained in his 2010 commentary, there are many factors involved in the ultimate fate of the implanted polypropylene mesh [4]. Such mesh-related factors include: density, filament size, elasticity, pore size, surface area, and overall “mesh load.” Even when type-1 polypropylene mesh is used, graft-related complications such as exposure, pain, and dyspareunia remain the most common complications associated with the sacrocolpopexy procedure.
Recently, biomechanical engineers working with type-1 polypropylene have focused on reducing overall “mesh load” while maintaining durability. These efforts seem to stem from a belief that—when it comes to synthetic graft material to support the vagina—lighter is better. However, simply handling these ultra-lightweight grafts can leave a surgeon asking, “How light is too light?” Such questions can only be answered through clinical research.
One ultra-lightweight mesh, Restorelle Y Smartmesh™ (technology-density 18.69 g/m2, Coloplast A/S, Humlebæk, Denmark), has been widely adopted for sacrocolpopexy; yet no long-term prospective studies have been published regarding its efficacy.
The FDA safety communications regarding the use of mesh in prolapse repair and the ensuing debate is a clear reminder that any new product seeking to be a “game changer” should be subject to a clinical trial [5, 6]. Therefore, our study was designed to prospectively evaluate the use of “Restorelle Y” ultra-lightweight polypropylene Y mesh for robotic-assisted laparoscopic sacrocolpopexy.
Materials and methods
This single-arm prospective study was approved by the Atlantic Health System institutional review board (R09-06-005) and was listed on the clinical trials.gov web site (identifier NCT01320618). During the 11-month study period, all women with stage II or greater apical prolapse scheduled to undergo a robotic sacrocolpopexy were included in the study.
All outcome measures were collected by our clinical research nurse at baseline, 6 months, and 1 year postoperatively. Anatomical measures were obtained using the Pelvic Organ Prolapse Quantification (POP-Q) system [7]. Pelvic floor disorder symptoms and impact measures included the Incontinence Severity Index, the Pelvic Floor Distress Inventory, Short Form (PFDI-20), the Pelvic Floor Impact Questionnaire-Short Form (PFIQ-7), the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), and the Surgical Satisfaction Questionnaire (SSQ-8) [8–11]. Postoperative vaginal examinations specifically noted whether the mesh or its edges were palpable through the vaginal wall in the absence of mesh exposure. Demographic data included age, body mass index, ethnicity, prior hysterectomy or prolapse surgery, and smoking and menopausal status. Operative data were collected prospectively including: total operative time, blood loss, concomitant supracervical hysterectomy, concomitant suburethral sling or perineorrhaphy, length of stay, hospital readmissions, and blood transfusions. Total operative time was defined as the time between abdominal skin incisions to skin closure. Wound infections/separation/hernia, febrile illnesses, laparotomy conversions, intraoperative complications, and mesh-related complications were all reported as adverse events.
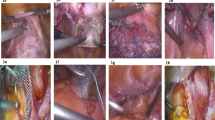
Each robotic sacrocolpopexy was performed using the daVinci Surgical System (intuitive Surgical, Sunnyvale, CA, USA) following a previously described technique [12]. A robotic supracervical hysterectomy was performed if a uterus was present, and then the cervix was grasped with a robotic tenaculum, which in turn was used to manipulate the vagina. This technique allowed full dissection and suturing without any vaginal instrumentation. In post-hysterectomy cases, a Lucite vaginal probe was held in the vagina during dissection and suturing. The vesicovaginal space was sharply developed to within 1 cm from the bladder neck, and the rectovaginal space was developed to the level of the perineal body. This technique provided 4–6 cm of anterior coverage and 8–10 cm of posterior coverage, with each dissection pane measuring 4–6 cm wide. The mesh was sutured to the cervix and vagina using polytetrafluroethylene (CV4 Gore-Tex suture on TH-26 needle; Gore Medical Products Division, Flagstaff, AZ, USA). The proximal end of the Y mesh was attached to the anterior longitudinal ligament at the level of the sacral promontory using two sutures of zero-gauge braided polyester (Ethibond on SH needle, Ethicon, San Antonio, TX, USA). Appropriate tensioning was determined by a vaginal examination at the time of attachment ensuring adequate correction of the prolapse without undue strain. The peritoneum was approximated over the mesh using zero-gauge poliglecaprone (Monocryl on CT1 needle; Ethicon, San Antonio, TX, USA). Suburethral sling and perineorrhaphy were the only concomitant procedures performed at the primary surgeon’s discretion.
The primary outcome measure was “anatomical success” using the NIH definition of cure (POP-Q stage 0 or 1) [13]. In addition, we defined “clinical cure“ as a combination of objective and subjective measures requiring the following four criteria: all POP-Q points ≤0; apical POP-Q point C ≤5; the absence of pelvic organ prolapse symptoms as reported on the PFDI-20 question 3 (do you usually have a bulge or something falling out that you can see or feel in the vaginal area?); no prolapse reoperation during the study period.
Statistical analysis was performed using SAS 9.2 (SAS, Cary, NC, USA). The analysis of the primary outcome was performed using Wilcoxon signed rank test and paired t test. Additionally, Chi-squared and Fisher’s exact test were used with the alpha value set at 0.05.
Results
A total of 120 consecutive patients underwent a robotic-assisted laparoscopic sacrocolpopexy at our institution from June 2009 till May 2010 using the Restorelle™ Y mesh. Of these 120 patients, 118 (98.3 %) completed the 1-year postoperative follow-up. Table 1 outlines the demographic data for the study group. The mean total operating time was 161 ± 29 min and mean blood loss was 49 ml (25–100 ml). Concomitant procedures included 88 patients (73.3 %) who underwent a supracervical hysterectomy, 85 patients (70 %) who received a suburethral sling, and 27 (22 %) who had a perineorrhaphy. There were no cystotomies no bowel injuries, no conversions to laparotomies, and no blood transfusions. All patients were discharged home on postoperative day 1 and did not experience any wound-related complications or infectious morbidities. One patient was readmitted on postoperative day 3 for a postoperative ileus that resolved with conservative measures. There were no sacrocolpopexy mesh-related complications, no exposures or erosions, and no reoperations due to the mesh. Additionally, the mesh was not palpable on any of the postoperative vaginal examinations; in other terms, the examiner was never able to guess the specific limits of the vaginal area covered by the mesh.
As to the primary outcome of the study, the “anatomical success” rate was 89 %. Using the alternative combined definition of cure, 94 % of patients met all four criteria for “clinical cure”: Tables 2 and 3 details the pre- and postoperative comparisons for POP-Q measurements. There was a highly significant improvement in the anterior, posterior, and apical compartments. A similar improvement was noted for the subjective measures including the PFDI-20, the PFIQ-7, the PISQ-12, and the Incontinence Severity Index (Table 4). Patients responded favorably to the surgical satisfaction questionnaire (SSQ-8) with a 97 % satisfaction rate.
During the 12-month postoperative follow-up, 5 patients (4.1 %) required a suburethral sling insertion for new onset stress incontinence and 1 patient required reoperation for prolapse. Overall, urinary symptoms were vastly improved as demonstrated by the significant improvement in the Incontinence Severity Index (2.2 vs 1.1; p < 0.0001) and UDI-6 (37.3 vs 9.1 p < 0.0001). One patient (0.8 %) developed new onset frequency/urgency, which responded well to conservative measures. Another patient continued to experience incomplete bladder emptying and required revision of a previously placed suburethral sling.
Preoperatively, 77 patients (64 %) were sexually active. Postoperatively 75 patients (63 %) were sexually active. During the study period, 7 women ceased sexual intercourse and 5 women became sexually active. The majority of women who discontinued sexual intercourse did so because of partner- or couple-related issues and none of them complained of dyspareunia. Similarly, none of the women who became sexually active complained of painful intercourse. At baseline, 9 women complained of pain during sexual intercourse; of these 9 women, 2 continued to have this complaint at 1 year postoperatively. Only 2 patients developed new onset dyspareunia after sacrocolpopexy for a rate of 2.6 %. Finally, preoperatively, 39 of the sexually active women (51 %) responded by “sometimes,” “usually,” or “always” to PISQ question 8 (do you avoid sexual intercourse because of bulging in the vagina?) as opposed to none (0 %) at 1 year after sacrocolpopexy.
Discussion
The use of an ultra-lightweight polypropylene Y mesh produced significant anatomical and functional improvements. These results compare favorably with previous reports for open, laparoscopic, and robotic sacrocolpopexies [14–16]. As expected, the robotic-assisted laparoscopic approach was associated with a very low morbidity rate. Recent studies suggest that total hysterectomy at the time of sacrocolpopexy is associated with a higher mesh exposure rate and that is why we only performed supracervical hysterectomies [17]. However, even studies in which a total hysterectomy was avoided at the time of sacrocolpopexy still reported mesh-related complications [15, 17, 18]. The lack of mesh-related complications within the first postoperative year is a remarkable finding, suggesting a potential qualitative difference in the way in which the lighter mesh interacts with the surrounding tissue. Additionally, our clinical research nurse was unable to palpate the borders of the implanted mesh during any of her examinations. While somewhat subjective, this finding remains compelling. It is not clear, though, whether this feature would translate into any direct clinical benefit. Similar to other sexual function studies after sacrocolpopexy, the PISQ-12 sexual function scores significantly improved at 1 year. The rate of new onset dyspareunia was very small (2.6 %), the mesh was not exposed in these 2 women, nor was it palpable. Both patients underwent a concomitant supracervical hysterectomy and suburethral sling and 1 patient had a perineorrhaphy at the time of sacrocolpopexy. The perineorrhaphy patient responded well to trigger point injection into her levator muscle and to vaginal estrogen/massage therapy. The other patient only required vaginal estrogen.
We only performed a suburethral sling on patients who demonstrated stress urinary incontinence on preoperative urodynamic studies and at the discretion of the operating surgeon, hence the need for a sling placement in 5 women who developed post-operative new onset/worsening stress incontinence.
The prospective nature of our study, the inclusion of all consecutive patients and the high rate of follow-up strengthen the value of our results. Outcome measures including postoperative examinations were collected by our clinical research nurse (not the operating surgeon). Additionally, there was consistent use of suture material and surgical technique throughout the study period. The robotic sacrocolpopexy was performed by two experienced fellowship trained urogynecologists who were well beyond their learning curve and consistently performing over 6 (range 6 to 10) procedures per month, which could limit the generalizability of these findings. The lack of a comparative arm, randomization, and blinding were the major limitations of the study.
Although comparative studies are preferable, well-conducted prospective single-arm trials are of great value in the study of implantable devices. As new pelvic floor implants make their way to market they should be subjected to vigorous prospective safety and efficacy trials. At the very least, studies like ours establish much-needed baseline data that can later be used to construct randomized clinical trials.
Finally, based on this study, the use of this ultra-lightweight Y mesh eliminated the mesh-related complications in the first postoperative year without sacrificing the efficacy of the sacrocolpopexy. Future studies with longer follow-up are required to confirm these promising 1-year results.
References
Lane FE (1962) Repair of posthysterectomy vaginal-vault prolapse. Obstet Gynecol 20:72–77
Nygaard IE, McCreery R, Brubaker L, Connolly A, Cundiff G, Weber AM et al (2004) Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol 104:805–823
Culligan PJ, Blackwell L, Goldsmith LJ, Graham CA, Rogers A, Heit MH (2005) A randomized controlled trial comparing fascia lata and synthetic mesh for sacral colpopexy. Obstet Gynecol 106:29–37
Ostergard DR (2010) Polypropylene mesh grafts in gynecology. Obstet Gynecol 116:962–966
U. S. Food and Drug Administration (2008) FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm Accessed 26 August 2012
U.S. Food and Drug Administration (2011) FDA safety communication: UPDATE on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm Accessed 26 August 2012
Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Klarskov P et al (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175:10–17
Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H (1993) Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health 47:497–499
Barber MD, Walters MD, Bump RC (2005) Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol 193:103–113
Rogers RG, Coates KW, Kammerer-Doak D, Khalsa S, Qualls C (2003) A short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Int Urogynecol J Pelvic Floor Dysfunct 14:164–168, discussion 168
Murphy M, Sternschuss G, Haff R, van Raalte H, Saltz S, Lucente V (2008) Quality of life and surgical satisfaction after vaginal reconstructive vs obliterative surgery for the treatment of advanced pelvic organ prolapse. Am J Obstet Gynecol 198(5):573.e1–573.e7
Salamon C, Shariati A, Culligan PJ (2010) Optimizing efficiency with robot-assisted laparoscopic sacrocolpopexy. Female Patient 35(4):33–38
Weber AM, Abrams P, Brubaker L, Davis G, Dmochowski RR et al (2001) The standardization of terminology for researchers in female pelvic floor disorders. Int Urogynecol J Pelvic Floor Dysfunct 12:178–186
Brubaker L, Nygaard IE, Richter HE, Visco A, Weber AM, Cundiff G et al (2008) Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol 112:49–55
Paraiso MFR, Jelovsek JE, Frick A, Chen CCG, Barber MD (2011) Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: a randomized controlled trial. Obstet Gynecol 118:1005–1013
Salamon CG, Culligan PJ (2012) Subjective and objective outcomes 1 year after robotic-assisted laparoscopic sacrocolpopexy. J Robotic Surg 88:245–246
Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE (2009) Complication and reoperation rates after apical vaginal prolapse surgical repair a systematic review. Obstet Gynecol 113:367–373
Tan-Kim J, Menefee SA, Luber KM, Nager CW, Lukacz ES (2011) Prevalence and risk factors for mesh erosion after laparoscopic-assisted sacrocolpopexy. Int Urogynecol J Pelvic Floor Dysfunct 22(2):205–212
Conflict of interest
The study was funded through an unrestricted grant from Coloplast A/S, Humlebæk, Denmark.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salamon, C.G., Lewis, C., Priestley, J. et al. Prospective study of an ultra-lightweight polypropylene Y mesh for robotic sacrocolpopexy. Int Urogynecol J 24, 1371–1375 (2013). https://doi.org/10.1007/s00192-012-2021-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-2021-7