Abstract
Four lysyl oxidase family genes (LOXL1, LOXL2, LOXL3, and LOXL4), which catalyze cross-linking of collagen and elastin, were considered to be functional candidates for intracranial aneurysms (IA) and were extensively screened for genetic susceptibility in Japanese IA patients. Total RNA was isolated from four paired ruptured IA and superficial temporal artery (STA) tissue and examined by real-time RT-PCR. The expression of LOXL2 in the paired IA and STA tissues was elevated in the IA tissue. A total of 55 single nucleotide polymorphisms (SNPs) of LOXL1-4 were genotyped for an allelic association study in 402 Japanese IA patients and 462 Japanese non-IA controls. Allelic associations were evaluated with the chi-square test and the permutation test especially designed for adjustment of multiple testing. SNPs of LOXL1 and LOXL4 were not significantly associated with IA, while several SNPs of LOXL2 and LOXL3 showed nominally significant associations in IA patients. We detected an empirically significant association with one SNP of LOXL2 in familial IA patients after adjustment for multiple testing [χ 2 = 10.23, empirical P = 0.023, OR (95% CI) = 1.49 (1.17, 1.90)]. Furthermore, multilocus interaction was evaluated by multifactor dimensionality reduction analysis. We found that the SNPs of LOXL2 have an interactive effect with elastin (ELN) and LIM kinase 1 (LIMK1) that have been previously found to be associated with IA. In conclusion, one SNP of LOXL2 showed a significant association with IA individually, and we also detected a gene–gene interaction of LOXL2 with ELN/LIMK1, which may play an important role in susceptibility to IA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rupture of an intracranial aneurysm (IA) (MIM105800) results in a subarachnoid hemorrhage with high morbidity and mortality, representing a major public health concern (Longstreth et al. 1993; Schievink et al. 1995; Inagawa et al. 1995). We have previously reported a genome-wide linkage study of IA in 104 affected Japanese sib pairs in which evidence of linkage at chromosome 5q22-31, 7q11, and 14q22 was detected (Onda et al. 2001). Lysyl oxidase, encoded by LOX located on a suggestive linkage region on chromosome 5q31, is an extracellular copper-containing enzyme that initiates cross-linking of collagen and elastin by oxidative deamination of lysine residues. It plays an essential role in the formation of extracellular matrix and connective tissue (Kagan and Li 2003). LOX is considered to enhance the strength of the blood vessel wall, and was therefore a plausible positional and functional candidate to IA formation. Previously, single nucleotide polymorphisms (SNPs) of LOX were analyzed for allelic and haplotype-based associations in Japanese and Central European IA patients, but no association was detected (Yoneyama et al. 2003; Hofer et al. 2004). Four novel genes encoding LOX-like proteins 1 through 4 (LOXL1, LOXL2, LOXL3, and LOXL4), assigned to chromosome 15q22, 8p21, 2p13, and 10q24, respectively, are categorized to be the “LOX family genes” (Kenyon et al. 1993; Jourdan-Le Saux et al. 1998; Asuncion et al. 2001; Jourdan-Le Saux et al. 2001; Molnar et al. 2003). Products of the LOX family genes have a highly conserved amino acid sequence at the C-terminus and have amine oxidase activity (Molnar et al. 2003). The LOX family genes could also be considered as plausible candidate genes for IA, thus, we evaluated allelic associations between IA patients and SNPs of LOXL1-4. Although we detected multiple SNPs of LOXL2 and LOXL3 that were significantly associated with IA, only one SNP of LOXL2 was associated after adjustment of multiple testing with a permutation test. In addition, using the multifactor dimensionality reduction (MDR) method (Richie et al. 2001) to detect gene–gene interaction among multiple SNPs, we found that SNPs of LOXL2 have an interactive effect with SNPs at an ELN/LIMK1 locus that have been previously found to be, both statistically and functionally, associated with IA (Akagawa et al. 2006). This implicates that the SNPs of LOXL2 could be a genetic risk factor for developing IA and account for a part of the pathogenesis of its formation.
Subjects and methods
Subjects
The Ethical Committees of Tokyo Women’s Medical University, Chiba University, and Tokyo Metropolitan Fuchu Hospital, approved the study protocols, and all participants gave written informed consent. The clinical backgrounds of the Japanese subjects are summarized in Table 1. The DNA samples for the present study were 402 IA patients (mean age: 54.2 ± 10.0 years) and 462 controls (mean age: 63.5 ± 10.0 years), all of which are unrelated to each other. The IA patients included 185 familial IA patients (86 probands from nuclear families that had been used in our linkage study (Onda et al. 2001) and 99 patients with family history of IA) and 217 sporadic IA patients (onset of age < 60 years). The presence of IA was confirmed by conventional angiography, three-dimensional CT angiography, MR angiography, or surgical findings. The control subjects were outpatients with diseases other than IA, at the Department of Neurosurgery of the Tokyo Women’s Medical University, Chiba University, and their nearby affiliated hospitals. The Japanese controls did not harbor an IA as verified by radiological examinations and had no familial history of SAH. All of the controls were over 50 years of age, in order to exclude most unmanifested IAs. Genomic DNA was extracted from peripheral blood or buccal swab according to a standard method.
Real-time RT-PCR analysis of the LOX family genes in paired IA and STA tissues
Four pairs of ruptured IA and superficial temporal artery (STA) specimens were excised from four female patients (aged from 34 to 80 years) who underwent surgical IA clipping at the Department of Neurosurgery of Tokyo Metropolitan Fuchu Hospital. Total RNAs from the arterial specimens were extracted using TRIzol reagent (Invitrogen, Tokyo, Japan) according to the manufacturer’s instructions, and used as a template in first-strand cDNA synthesis with SuperScript III First-Strand Synthesis System (Invitrogen). Real-time PCR was performed using SYBR Premix Ex Taq (Perfect Real Time) (TAKARA BIO, Otsu, Japan) on ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Tokyo, Japan) according to the manufacturer’s instructions. The PCR primers for LOX family genes and GAPDH were designed and synthesized by TAKARA BIO. The primer sequences can be obtained from the authors (see Supplementary Table 1). Relative expression levels for the respective LOX family genes were obtained by normalizing to GAPDH in all specimens. Statistical comparison in the quantitative analysis of the gene expression was performed by the paired t-test; P < 0.05 was considered significant.
SNP selection of LOXL1-4 and genotyping
The SNPs of LOXL1-4 were obtained from the NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/SNP) or the IMS-JST JSNP DATABASE (http://snp.ims.u-tokyo.ac.jp/). SNPs with minor allele frequencies above 10% in an initial genotyping of 12 subjects were selected for further study. The SNPs were genotyped either by using TaqMan SNP Genotyping Assays on ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) or by BigDye Terminator V1.1 Cycle Sequencing on ABI 3700 DNA Analyzer (Applied Biosystems) according to a standard protocol.
Statistical analysis
Differences in allelic frequencies were evaluated using a case–control design with the chi-square test, and the statistical power for association studies (1-β) was calculated using the chi-square test with arcsine approximation (Ambrosius et al. 2004). Haplotype frequencies for multiple loci and pairwise linkage disequilibrium (LD), using the standard definition of D′ and r 2 (Lewontin 1964; Hill and Robertson 1968), were estimated in phase-unknown samples with SNPAlyze V3.2 software (DYNACOM, Mobara, Japan), in which the expectation-maximization (EM) algorithm (Excoffier and Slatkin 1995) is used. For an adjustment of multiple testing, Bonferroni’s correction is known to be too conservative since all SNPs are assumed to be independent, and the false discovery rate (FDR) (Benjamini and Hochberg 1995), in which the most significant association is corrected in the same way as Bonferroni’s correction, may also provide an unreasonably stringent threshold at least for the most significantly associated SNP. Thus, we applied a permutation test as proposed by Churchill and Doerge (1994) to determine empirical P-values. The individuals in an experimental dataset were labeled 1–n and each was scored at m SNPs. An affection status was also associated with each individual. In the permutation test, the affection statuses were shuffled N times among the n individuals to create permuted datasets that had only random genotype–phenotype associations and that were representative sample from an appropriate null distribution. A test statistic was computed at each of the SNPs and only its maximum value was recorded for each of the N permuted dataset. An empirical threshold value was obtained by computing the (1-α) percentile from the N maximum test statistics.
MDR for multi-locus interaction
In order to explore gene–gene interaction between the LOX family genes and ELN/LIMK1 that had previously been identified as susceptibility genes (Akagawa et al. 2006), we performed MDR analysis (Richie et al. 2001). This method is based on cross-validation (CV) procedure, in which the dataset is divided into 10 equal parts, and the MDR model is developed using 9/10 of the data (training set). Each of the multilocus genotypes is categorized, depending on the case and control count, as either high- or low-risk groups and then the remaining 1/10 (testing set) is used to predict the affection status. The proportion of subjects whose affection status was correctly predicted is defined as testing balanced accuracy (TBA), and the number of times a particular model is identified as the best one is defined as CV consistency, and the two indexes are used to evaluate the goodness-of-fit of models. The procedure is repeated a total of 100 times, and TBA and CV consistencies were averaged for all combinations of SNPs.
Results
Quantitative expression analyses of LOX family genes in paired IA and STA tissues
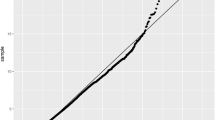
Real-time RT-PCR was performed to examine gene expression patterns in IA tissue compared with STA tissue (paired sample from the same individual) (Fig. 1). Consistently elevated expressions of LOX (3.9-fold, P = 0.059) and LOXL2 (5.1-fold, P = 0.011) were observed in IA tissues in comparison with STA tissues. The expressions of LOXL1, LOXL3, and LOXL4 were not significantly different. These results indicate a possible involvement of LOX and LOXL2 in the pathophysiology of IA formation.
Comparisons of expression levels of the LOX family genes in paired IA and STA tissues from the same IA patients. Expression levels of the LOX family genes relative to GAPDH (Y-axis) were evaluated by real-time RT-PCR analyses in four pairs of IA (closed circle) and STA (open circle) specimens that were surgically excised from four patients with IA(s). The differences in expression between IA tissues and the corresponding STA tissues were analyzed by paired t-test. The P-value obtained is shown in each panel. The numeral (1–4) in the panel represents patient’s identification number. In each of paired IA and STA tissues, the increase in gene expression in IA tissue was obtained in comparison with the corresponding STA tissue, and the average fold-increases of the LOX family genes are shown in the upper part of the panels
Allelic association study and linkage disequilibrium of LOXL1-4
Forty-nine SNPs, comprising 25, 11, 6, and 7 SNPs of LOXL1, LOXL2, LOXL3, and LOXL4, respectively, were first genotyped in 96 familial IA patients and 96 non-IA controls. The results of the screening were evaluated by the simple chi-square test as summarized in Table 2. Two SNPs of LOXL2, Exon5(34923)G/A (denoted as SNP7, see Table 2) and Intron6(41002)G/C (SNP8), and two SNPs of LOXL3, 3′FLR(20827)C/T (SNP5) and 3′FLR(21095)C/T (SNP6), both located next to each other, showed nominally significant association with IA (P < 0.05).
The physical and LD maps are shown in Fig. 2. LD patterns of the controls were depicted in the figure and essentially the same patterns were observed with the patients. All of the SNPs in either LOXL1 or LOXL3 were in near complete LD (D′ > 0.7). In LOXL2, there was a structured LD block (D′ > 0.7) from SNP1 to SNP6 and a scattered LD block from SNP7 to SNP16. In LOXL4, a LD block (D′ > 0.7, r 2 > 0.7) from SNP3 to SNP7, which includes the middle to 3′-flanking region, was observed.
Linkage disequilibrium pattern of LOXL1-4. The gene structure together with the position of SNPs is shown for each gene. Pairwise LD coefficient, D′ and r 2, were determined and expressed as a block structure. In the schematic block, dark shaded boxes indicate pairwise LD of D′ or r 2 > 0.9 and light gray D′ or r 2 = 0.7–0.9. Blank boxes represent D′ or r 2 < 0.7
All of the subjects including the individuals in the first screening were then subjected to genotyping of the significant SNPs of LOXL2 and LOXL3 (Table 3). In LOXL3, significant association was replicated only in SNP5, which is located on the 3′ flanking region of the gene, with the familial patients (χ 2 = 5.33, df = 1, and P = 0.021). As the functional impact of SNPs outside of LOXL3 is not easily evaluated and the evidence of association had disappeared after adjustment for multiple testing, subsequent studies focused on SNPs of LOXL2. We resequenced LOXL2 and identified six additional known SNPs. Among a total of 17 SNPs of LOXL2 we detected a nominally significant allelic association with SNP7 in all IA patients [χ 2 = 7.00, df = 1, P = 0.008, and OR (95% CI) = 1.29 (1.07, 1.56)]. It is synonymous and located on exon 5. Exon 5(34869)A/G (SNP6b), Exon5(34938)G/A (SNP7a), and Intron9(52273)C/T (SNP9) also showed weak but nominally significant associations (Table 3). To avoid false positive results, we applied the permutation test for an adjustment of multiple testing (Churchill and Doerge 1994). However, except for the SNP7 with the most significant association in the familial IA patients [χ 2 = 10.23, empirical P = 0.023, and OR (95% CI) = 1.49 (1.17, 1.90)], none of the associations met the empirical significance level according to the permutation test (Table 3). SNP7 also showed a significant association with IA under the genotypic models, especially under the recessive model for the major allele in the familial patients (χ 2 = 13.74, df = 1, P = 2.1 × 10−4) (see Supplementary Tables 2, 3). None of the SNPs in the control samples showed deviation from Hardy–Weinberg’s equilibrium (data not shown). The statistical power for the association study with this dataset was >0.98, with difference in allele frequencies between the cases and controls of 0.1.
Haplotype-based association study of LOXL2
The three SNPs, SNP6b, SNP7, and SNP7a which were located on exon 5 of LOXL2, showed significant association (P < 0.05) in all cases of IA. Because these three SNPs are in tight LD with each other (D′ > 0.9), the SNPs were selected to construct haplotypes for a haplotype-based association study. Three major haplotypes and their estimated frequencies are shown in Table 4 for IA patients and their controls. Bonferroni’s correction was used with the number of the haplotypes, since they were independent from one another. The most common haplotype, Hap2, was over-represented with statistical significance both in total (χ 2 = 7.48, df = 1, nominal P = 0.006, and corrected P = 0.018) and familial IA patients (χ 2 = 10.69, df = 1, nominal P = 0.001, and corrected P = 0.003). In contrast, Hap1 was more frequent in the controls than in the familial IA patients (χ 2 = 6.46, df = 1, nominal P = 0.011, and corrected P = 0.033), which indicates that Hap1 is a protective haplotype. The haplotype-based association showed that Hap2 could be regarded as an at-risk haplotype and that the SNP7 constitutes a tag-SNP for Hap2.
MDR analysis
Subsequently, we performed MDR analysis to explore gene–gene interaction between LOXL2 and ELN/LIMK1 that had previously been identified as susceptibility genes (Table 5). The 3′-UTR (+502) and 3′-UTR (+659) SNPs of ELN, the Pro.(−961), Pro.(−428) and Pro.(−187) SNPs of LIMK1 that showed significant associations with IA in the previous study (Akagawa et al. 2006) were tested with SNP6b, SNP7, SNP7a, SNP8, and SNP9 of LOXL2. In the MDR analysis, SNPs in strong LD evaluated by r 2 statistics with each other tend to result in unreasonably low-CV consistency because the SNPs have an equal chance to be selected and one SNP may falsely be selected instead of the highly associated SNP (Heidema et al. 2006). Thus, it would be appropriate to select only the most significantly associated SNP among SNPs in strong LD. Also, we removed SNPs with low minor allele frequency (<0.05). Eventually, we selected six SNPs, i.e., 3′-UTR (+502) and 3′-UTR (+659) SNPs of ELN, Pro. (−428) and Pro. (−187) SNPs of LIMK1, and SNP6b and SNP7 of LOXL2, and one more LOXL2 SNP, Exon10(58511)A/C (SNP10), was also added because it was non-synonymous. Table 5 shows that the 5-SNP (3′-UTR (+502) SNP of ELN, Pro. (−428) SNP of LIMK1, SNP6b, SNP7, and SNP10 of LOXL2) model was supported (TBR = 0.5988 and P < 0.001), which indicates that although LOXL2 itself does not have such a strong effect on susceptibility to IA, it has an interactive and significant effect with ELN/LIMK1.
Discussion
Since the first genome-wide linkage study of IA with affected siblings (Onda et al. 2001), several genome-wide and candidate locus linkage studies have been reported (Olson et al. 2002; Yamada et al. 2003; Farnham et al. 2004; Roos et al. 2004; van der Voet et al. 2004; Yamada et al. 2004; Nahed et al. 2005), further reports have been summarized by Krischek and Inoue (2006). The linkage results of these studies, however, have not been consistent presumably reflecting the heterogeneous nature of the disease (Yamada et al. 2003, Krischek et al. 2006). As widely accepted, association study with candidate genes is an alternative approach to identify genetic susceptibility of complex diseases. Thus far, candidate genes encoding components of extracellular matrix and connective tissue or involved in vascular remodeling have been studied for IA in distinct populations. Significant associations were reported in genes such as ELN/LIMK1, collagen 1A2, endoglin, and angiotensin converting enzyme (Takenaka et al. 1999; Onda et al. 2001; Ruigrok et al. 2004; Slowik et al. 2004; Yoneyama et al. 2004; Akagawa et al. 2006). In the present study, the LOX family genes, LOXL1-4, were extensively and systematically investigated. They are plausible functional candidates since lysyl oxidase activities that cross-link collagen and elastin are essential for mechanical stability and maintenance of vascular structure. The LOX family gene-products appear to have not only common lysyl oxidase activities but also diverse biological functions including tumor suppression, cell adhesion, and senescence (Saito et al. 1997; Csiszar 2001). However, functional assignments of the family members remain largely undetermined. Mice lacking Lox showed perinatal lethality despite the redundancy of lysyl oxidase activity suggesting a critical role of the gene. In addition, saccular aneurysms in the thoracic aorta were observed in the Lox deficient mice suggesting an important role of the gene in aneurysm formation, possibly by reducing the strength of the vascular structure (Maki et al. 2002). Distinct functional significance was shown in mice lacking Loxl1 that were viable and showed a specific role of LOXL1 in elastogenesis (Liu et al. 2004). LOXL2 is highly expressed in metastatic breast cancer-derived cell lines (Akiri et al. 2003) and it was shown that LOXL2 interacts and cooperates with snail, a transcription factor, to down-regulate E-cadherin expression (Peinado et al. 2005) that might play a role in tumor progression.
In the present study, 55 SNPs in the LOX family genes were examined for allelic association with Japanese IA patients. Significant association was identified with SNP7 of LOXL2 in the familial IA cases (nominal P = 0.001 and empirical P = 0.023) (Table 3). Because SNP7, located in the center of exon 5 of LOXL2, is synonymous, we first examined the possibility whether SNP7 functions as a splicing enhancer but obtained negative results with human umbilical artery smooth muscle cells (data not shown). A possibility remains that an unknown SNP in tight LD with SNP7 is a bonafide causality to IA formation.
As we previously reported that SNPs in the ELN/LIMK1 locus were associated with IA (Akagawa et al. 2006), we tested possible gene–gene interaction between LOXL2 and ELN/LIMK1. Multilocus analysis was performed by using MDR and we found that SNP6b, SNP7, and SNP10 of LOXL2 have a statistically interactive effect with SNPs of ELN/LIMK1. In addition to the evidence of genetic association, LOXL2 showed a significantly elevated expression in IA tissue (5.1-fold increase) (Fig. 1), whereas LOXL1, LOXL3, and LOXL4 did not. These results reinforce the possibility that LOXL2 plays an important role in IA formation. Elevated levels of LOXL2 could be explained by a compensation mechanism to strengthen the vascular wall of aneurysm and also indicate an important role of LOXL2 in aneurysmal formation. It was reported that LOX and LOXL2 target collagen molecules to cross-link while LOXL1 preferentially targets elastin as a substrate (Liu et al. 2004; Vadas et al. 2005). Thereby, a physiological interaction between LOXL2 and ELN/LIMK1 is still unclear and further investigation is evidently needed.
In general, inconsistent results are often yielded for linkage and association studies, presumably because of genetic heterogeneity, sample size, and the different ways of determining a significance level, etc. Thus, it is necessary to perform genetic analyses more carefully, e.g., by setting an integrated and appropriate significance level or by confirming an association in several populations, and to improve the availability of statistical methods. Also the MDR method is not the ultimate solution for identifying gene–gene interactions, it still has some issues that need to be solved; e.g., how to overcome the fact that SNPs in extremely strong LD can lead to inadequate results, and how to find out in which way each of SNPs included in the model contributes to susceptibility. Thus, more sophisticated statistical methods to detect gene–gene interaction will also become increasingly important for understanding the complicated mechanisms of human common complex diseases.
In conclusion, SNP7 (rs1010156) of LOXL2 showed an empirically significant association with IA, and this SNP together with SNP6b (rs2294127) and SNP10 (rs1063582), of LOXL2 showed a significantly interactive effect with ELN and LIMK1. Currently, the functional impact of SNP7 of LOXL2 is uncertain. However, elevated expression of LOXL2 in IA tissues suggests that the protein plays an important role in IA formation.
References
Akagawa H, Tajima A, Sakamoto Y, Krischek B, Yoneyama T, Kasuya H, Onda H, Hori T, Kubota M, Machida T, Saeki N, Hata A, Hashiguchi K, Kimura E, Kim CJ, Yang TK, Lee JY, Kimm K, Inoue I (2006) A haplotype spanning two genes, ELN and LIMK1, decreases their transcripts and confers susceptibility to intracranial aneurysms. Hum Mol Genet 15:1722–1734
Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M, Neufeld G (2003) Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res 63:1657–1666
Ambrosius WT, Lange EM, Langefeld CD (2004) Power for genetic association studies with random allele frequencies and genotype distributions. Am J Hum Genet 74:683–693
Asuncion L, Fogelgren B, Fong KS, Fong SF, Kim Y, Csiszar K (2001) A novel human lysyl oxidase-like gene (LOXL4) on chromosome 10q24 has an altered scavenger receptor cysteine rich domain. Matrix Biol 20:487–491
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J RStat Soc B 57:289–300
Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138:963–971
Csiszar K (2001) Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol 70:1–32
Excoffier L, Slatkin M (1995) Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol Biol Evol 12:921–927
Farnham JM, Camp NJ, Neuhausen SL, Tsuruda J, Parker D, MacDonald J, Cannon-Albright LA (2004) Confirmation of chromosome 7q11 locus for predisposition to intracranial aneurysm. Hum Genet 114:250–255
Heidema AG, Boer JM, Nagelkerke N, Mariman EC, van der A DL, Feskens EJ (2006) The challenge for genetic epidemiologists: how to analyze large numbers of SNPs in relation to complex diseases. BMC Genet 7:23
Hill WG, Robertson A (1968) Linkage disequilibrium in finite populations. Theor Appl Genet 38:226–231
Hofer A, Ozkan S, Hermans M, Kubassek N, Sitzer M, Burtscher J, Knopp U, Schoch B, Wanke I, Huebner F, Raabe A, Steinmetz H, Auburger G (2004) Mutations in the lysyl oxidase gene not associated with intracranial aneurysm in Central European families. Cerebrovasc Dis 18:189–193
Inagawa T, Tokuda Y, Ohbayashi N, Takaya M, Moritake K (1995) Study of aneurysmal subarachnoid hemorrhage in Izumo City, Japan. Stroke 26:761–766
Jourdan-Le Saux C, Le Saux O, Donlon T, Boyd CD, Csiszar K (1998) The human lysyl oxidase-related gene (LOXL2) maps between markers D8S280 and D8S278 on chromosome 8p21.2-p21.3. Genomics 51:305–307
Jourdan-Le Saux C, Tomsche A, Ujfalusi A, Jia L, Csiszar K (2001) Central nervous system, uterus, heart, and leukocyte expression of the LOXL3 gene, encoding a novel lysyl oxidase-like protein. Genomics 74:211–218
Kagan HM, Li W (2003) Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem 88:660–672
Kenyon K, Modi WS, Contene S, Friedman RM (1993) A novel human cDNA with a predicted protein similar to lysyl oxidase maps to chromosome 15q24-q25. J Biol Chem 268:18435–18437
Krischek B, Inoue I (2006) The genetics of intracranial aneurysms. J Hum Genet 51:587–594
Krischek B, Narita A, Tajima A, Akagawa H, Kasuya H, Hori T, Yoneyama T, Inoue I (2006) Is there any evidence for linkage on chromosome 17cen in affected Japanese sib-pairs with an intracranial aneurysm? J Hum Genet 51:491–494
Lewontin RC (1964) The interaction of selection and linkage. I. General considerations; heterotic models. Genetics 49:49–67
Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T (2004) Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet 36:178–182
Longstreth WT Jr, Nelson LM, Koepsell TD, van Belle G (1993) Clinical course of spontaneous subarachnoid hemorrhage: a population-based study in King County, Washington. Neurology 43:712–718
Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R (2002) Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106:2503–2509
Molnar J, Fong KS, He QP, Hayashi K, Kim Y, Fong SF, Fogelgren B, Szauter KM, Mink M, Csiszar K (2003) Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim Biophys Acta 1647:220–224
Nahed BV, Seker A, Guclu B, Ozturk AK, Finberg K, Hawkins AA, DiLuna ML, State M, Lifton RP, Gunel M (2005) Mapping a Mendelian form of intracranial aneurysm to 1p34.3-p36.13. Am J Hum Genet 76:172–179
Olson JM, Vongpunsawad S, Kuivaniemi H, Ronkainen A, Hernesniemi J, Ryynanen M, Kim LL, Tromp G (2002) Search for intracranial aneurysm susceptibility gene(s) using Finnish families. BMC Med Genet 3:7
Onda H, Kasuya H, Yoneyama T, Takakura K, Hori T, Takeda J, Nakajima T, Inoue I (2001) Genome-wide-linkage and haplotype-association studies map intracranial aneurysm to chromosome 7q11. Am J Hum Genet 69:804–819
Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F (2005) A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J 24:3446–3458
Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF, Moore JH (2001) Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet 69:138–147
Roos YB, Pals G, Struycken PM, Rinkel GJ, Limburg M, Pronk JC, van den Berg JS, Luijten JA, Pearson PL, Vermeulen M, Westerveld A (2004) Genome-wide linkage in a large Dutch consanguineous family maps a locus for intracranial aneurysms to chromosome 2p13. Stroke 35:2276–2281
Ruigrok YM, Seitz U, Wolterink S, Rinkel GJ, Wijmenga C, Urban Z (2004) Association of polymorphisms and haplotypes in the elastin gene in Dutch patients with sporadic aneurysmal subarachnoid hemorrhage. Stroke 35:2064–2068
Saito H, Papaconstantinou J, Sato H, Goldstein S (1997) Regulation of a novel gene encoding a lysyl oxidase-related protein in cellular adhesion and senescence. J Biol Chem 272:8157–8160
Schievink WI, Wijdicks EF, Parisi JE, Piepgras DG, Whisnant JP (1995) Sudden death from aneurysmal subarachnoid hemorrhage. Neurology 45:871–874
Slowik A, Borratynska A, Pera J, Betlej M, Dziedzic T, Krzyszkowski T, Czepko R, Figlewicz DA, Szczudlik A (2004) II genotype of the angiotensin-converting enzyme gene increases the risk for subarachnoid hemorrhage from ruptured aneurysm. Stroke 35:1594–1597
Takenaka K, Sakai H, Yamakawa H, Yoshimura S, Kumagai M, Yamakawa H, Nakashima S, Nozawa Y, Sakai N (1999) Polymorphism of the endoglin gene in patients with intracranial saccular aneurysms. J Neurosurg 90:935–938
Vadas Z, Kessler O, Akiri G, Gengrinovitch S, Kagan HM, Baruch Y, Izhak OB, Neufeld G (2005) Abnormal deposition of collagen around hepatocytes in Wilson’s disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase-like protein-2. J Hepatol 43:499–507
van der Voet M, Olson JM, Kuivaniemi H, Dudek DM, Skunca M, Ronkainen A, Niemela M, Jaaskelainen J, Hernesniemi J, Helin K, Leinonen E, Biswas M, Tromp G (2004) Intracranial aneurysms in Finnish families: confirmation of linkage and refinement of the interval to chromosome 19q13.3. Am J Hum Genet 74:564–571
Yamada S, Utsunomiya M, Inoue K, Nozaki K, Miyamoto S, Hashimoto N, Takenaka K, Yoshinaga T, Koizumi A (2003) Absence of linkage of familial intracranial aneurysms to 7q11 in highly aggregated Japanese families. Stroke 34:892–900
Yamada S, Utsunomiya M, Inoue K, Nozaki K, Inoue S, Takenaka K, Hashimoto N, Koizumi A (2004) Genome-wide scan for Japanese familial intracranial aneurysms: linkage to several chromosomal regions. Circulation 110:3727–3733
Yoneyama T, Kasuya H, Onda H, Akagawa H, Jinnai N, Nakajima T, Hori T, Inoue I (2003) Association of positional and functional candidate genes FGF1, FBN2, and LOX on 5q31 with intracranial aneurysm. J Hum Genet 48:309–314
Yoneyama T, Kasuya H, Onda H, Akagawa H, Hashiguchi K, Nakajima T, Hori T, Inoue I (2004) Collagen type I alpha2 (COL1a2) is the susceptible gene for intracranial aneurysms. Stroke 35:443–448
Acknowledgments
We thank the DNA donors and the supporting medical staff for making this study possible. This work was supported in part by the Grant-in-Aid for scientific research on Priority Area “Applied Genomics” from the Japanese Ministry of Education, Science, Sports, and Culture (I.I) and the Personalized Medicine Project of RIKEN (II).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hiroyuki Akagawa and Akira Narita contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akagawa, H., Narita, A., Yamada, H. et al. Systematic screening of lysyl oxidase-like (LOXL) family genes demonstrates that LOXL2 is a susceptibility gene to intracranial aneurysms. Hum Genet 121, 377–387 (2007). https://doi.org/10.1007/s00439-007-0333-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-007-0333-3