Abstract
Purpose
The role of the anterior cruciate ligament (ACL) in knee biomechanics in vivo and under weight-bearing is still unclear. The purpose of this study was to compare the tibiofemoral kinematics of ACL-deficient knees to healthy contralateral ones during the execution of weight-bearing activities.
Methods
Eight patients with isolated ACL injury and healthy contralateral knees were included in the study. Patients were asked to perform a single step forward and a single leg squat first with the injured knee and then with the contralateral one. Knee motion was determined using a validated model-based tracking process that matched subject-specific MRI bone models to dynamic biplane radiographic images, under the principles of Roentgen stereophotogrammetric analysis (RSA). Data processing was performed in a specific software developed in Matlab.
Results
Statistically significant differences (p < 0.05) were found for single leg squat along the frontal plane: ACL-deficient knees showed a more varus angle, especially at the highest knee flexion angles (40°–50° on average), compared to the contralateral knees. Furthermore, ACL-deficient knees showed tibial medialization along the entire task, while contralateral knees were always laterally aligned. This difference became statistically relevant (p < 0.05) for knee flexion angles included between 0° and about 30°.
Conclusion
ACL-deficient knees showed an abnormal tibial medialization and increased varus angle during single leg squat when compared to the contralateral knees. These biomechanical anomalies could cause a different force distribution on tibial plateau, explaining the higher risk of early osteoarthritis in ACL deficiency. The clinical relevance of this study is that also safe activities used in ACL rehabilitation protocols are significantly altered in ACL deficiency.
Level of evidence
III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of anterior cruciate ligament (ACL) in knee kinematics has been largely investigated. ACL function as a primary restrain of the anterior tibial displacement in static conditions is widely accepted, like its probable role in acting like a secondary restraint of internal tibial rotation [1, 5, 8,9,10,11, 18,19,20, 25, 32]. The relevance of biomechanical studies and the importance of their constant technological improvement derive from the necessity of a better comprehension of mechanisms that lead to an improved risk of osteoarthritis in patients affected by ACL deficiency [1, 2, 5, 6, 9, 10, 14, 19, 24, 34].
In particular, the comprehension of how the lack of ACL modifies knee biomechanics not only in vivo and dynamically, but also under weight-bearing conditions, is crucial to gain information as close as possible to what happens in daily life motion.
Motion capture tools such as video analysis and radiostereometry are valuable tools to understand better the biomechanics of the knee during common movements of daily and sport activities [1, 5, 8,9,10, 15, 19, 27, 34]. The main limits of these methods are related to their accuracy, because reconstruction of joint kinematics is based on skin sensors, which are affected by relevant artifacts. Double fluoroscopy overcomes the previous problem, because it allows studying directly bone movements through radiographs’ exposition of patients executing motor tasks [3, 4, 6, 14, 30, 34]. In this scenario, joint biomechanical anomalies following distinct pathologies could be investigated in a more accurate way, thanks to dynamic Roentgen stereophotogrammetric analysis (RSA) [3, 4]. Biomechanical differences between the anterior cruciate ligament-deficient (ACLD) knees and contralateral of the same subjects could be identified using a biplane radiographic system. In the present study, gait and single leg squat were analyzed, since the first one is a basic activity of daily living and the second one is a more demanding motor task, but safe and easy to perform for the patients [18, 32].
The aim of the present study was to identify knee biomechanical anomalies following ACL rupture, during the execution of in vivo under weight-bearing activities, to investigate the mechanisms that lead to improved risk of osteoarthritis in ACL deficiency.
It was hypothesized that knee tibiofemoral kinematics is altered after ACL tear and that the alteration probably does not involve only anterior posterior laxity or internal–external rotation, but also flexion–extension and medio-lateral tibial alignment, as previously reported by other investigators [1, 5, 9, 15, 19, 20].
The clinical relevance of this work is that proving a significant impairment and altered patterns in gait kinematics could support a wider recourse to surgery, because walking is a basilar activity and its constant alteration could influence knee degeneration more than sport activities, which most of the people do occasionally. Moreover, an altered knee kinematics in single leg squat could confirm the necessity of surgery for athletes.
Materials and methods
All the patients involved in this research study signed informed consent forms. This study obtained the approval from the Institutional Review Board (IRB) of Rizzoli Orthopaedic Institute (ID: 40/CE/US/ml—Clinical Trial Gov ID: NCT02323386). This study represents the secondary analysis of data collected from a prospective study, aimed to evaluate the outcome of ACL reconstruction. Based on the original study protocol, 62 patients were included and assessed preoperatively with 1.5 T MRI analysis and dynamic RSA of injured and contralateral knee.
The inclusion criteria for the original study were:
-
Age 16–50 years.
-
Complete, traumatic and unilateral ACL injury.
-
No previous knee ligament reconstruction or repair.
-
No concomitant posterior cruciate ligament, postero-lateral corner, lateral collateral ligament or medial collateral ligament lesion.
-
Absence of mild or advanced knee osteoarthritis (Kellgren–Lawrence III–IV).
For the purpose of the present study, the inclusion criteria were:
-
Isolated ACL tear.
-
No injury of contralateral knee.
Exclusion criteria were:
-
Concomitant other ligamentous or meniscal injuries.
-
Incomplete kinematic data.
-
Unwillingness to take part in the study.
From the 62 patients of the initial cohort, 10 patients underwent dynamic RSA of the contralateral knee. Two more patients were then excluded because of incomplete kinematic data. Overall, eight patients (5 men, 3 women, 30 ± 12 years old) matched the inclusion criteria and were included in the study.
Motor tasks
The patients were asked to perform two motor tasks: a single step and a single leg squat. The tasks were performed with the ACLD limb and subsequently with the contralateral one. Patients were asked to perform the tasks according to their possibilities. The investigators carefully checked the initial position of the foot to limit the bias caused by internal–external alignment: the foot had to be aligned with the ideal antero-posterior axis of the knee, thus pointing forward. The acquisition was performed in a specialized radiographic room. The tasks were performed three times per limb, the first two to gain comfort with the experimental setup (no X-ray exposure) and the third one for data acquisition (X-ray exposure).
Data acquisition
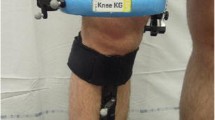
The data were collected using a radiographic setup for dynamic RSA. The device used (BI-STAND DRX 2) was developed in our institute, in collaboration with ASSING (ASSING Group, Rome, Italy). The specifics of the RSA radiographic setup were analogous to the ones already published in previous articles from the same study group [3, 4] (Fig. 1a).
Radiological setup of the RSA device, where patients performed motor tasks. The orthogonal arrangement of flat panels and X-ray tubes allows a 3D reconstruction of bone movements (a); virtual reconstruction of a motor task in the RSA software, where mathematical data describing tibio-femoral kinematics were extrapolated (b)
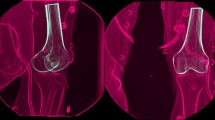
Bone models of tibia and femur were obtained from a 1.5 T MRI of either the affected or the contralateral knee. When MRI images of the contralateral knees were not available, the models were derived from a process of mirroring of the ones of the affected knee and of their correspondent reference systems. The radiographic images were processed in a dedicated software in Matlab® (R2016a, MathWorks Inc., Natik, MA, USA) developed at our institute, applying algorithms related to the Model-Based Dynamic RSA. A 3D virtual environment was used for semi-automatic segmentation of bone contours on radiographic images and, subsequently, to place the bone models according to the contours (Fig. 1b).
The dynamic RSA was validated before to start the clinical study. The validation protocol was based on radiograph computer simulations of the radiological setup and images, with different quality and noise level. The accuracy of the radiological scene reconstruction and of the model position was assessed according to the ISO-5725 regulation [16]. The global accuracy of model positioning and orientation, evaluated in terms of “trueness ± precision”, resulted to be sub-millimetric, respectively, 0.22 ± 0.46 mm and 0.26° ± 0.2°. Kinematics data are presented as mean ± standard error over the percentage of the task. Figure 2 shows the reference systems of the tibial and femoral models in the RSA software. The kinematical quantitative data for each patient, in 6 degrees of freedom, were calculated using the Grood and Suntay decomposition [13].
Since it was impossible to standardize the time elapsed to perform the motor task by each patient, we normalized the data on the percentage of the task (% task), based on specific moments to determine the beginning, the middle and the end (Table 1). Regarding the gait, only the stance phase was taken into account.
Statistical analysis
The kinematic data were processed using Matlab. The paired t test was used to compare the data of the ACLD and contralateral knees along each frame of the entire motor task for all the parameters. Differences were considered statistically significant for p < 0.05.
An a priori power analysis was conducted, based on previous studies using fluoroscopic technique to evaluate knee kinematics in ACLD conditions [6, 30, 31]. Considering a medio-lateral translation of 2.51 ± 1.30 mm for ACLD knee and of 0.89 ± 1.47 mm for contralateral knee, to achieve a power of 0.8 and an alpha level of 0.05, the minimum number of patients required was set to seven.
Results
Frontal plane
Regarding the joint angles and translations on the frontal plane, there were statistically significant differences between ACLD and contralateral knee (p < 0.05) (Table 2). In particular, varus–valgus rotations were statistically different from the 50% to the 80% of the squat (Fig. 3b): ACLD knee showed, on average, a more varus rotation compared to the contralateral knee. Furthermore, medio-lateral translations showed a more medial tibial alignment for ACLD knees with respect to the frontal plane. This trend was present both in the squat and in the step (Figs. 3a, 4): in the squat, the difference was statistically significant from 0 to 35% and from 65 to 100% of the task; no statistical differences were found in the step.
Medio-lateral translations (mean ± SEM) of the tibia with respect to the femur during single leg squat; notice that significant differences were found from 0 to 35% and from 75 to 100% of the motor task (that correspond to an average flexion value from 0° to 30°) (a). Varus–valgus rotations (mean ± SEM) of the tibia with respect to the femur during single leg squat; notice that significant differences were found from 50 to 80% of the motor task (b)
Sagittal and transverse plane
Regarding sagittal and transverse plane joint angles and translation, no statistical differences were found between ACLD and contralateral knee kinematics along the entire percentage of both motor tasks (n.s.).
Discussion
The main findings of the present study were:
-
Statistically significant differences were found in medio-lateral translations between ACL-deficient and contralateral knees during single leg squat from 0 to 35% and from 75 to 100% of the motor task (that correspond to an average flexion value from 0° to 30°).
-
During single leg squat, significant differences were found in varus–valgus angle from 50 to 80% of motor task.
-
No differences were observed between afflicted and contralateral knee during the stance phase of the gait.
The influence of ACL deficiency on knee kinematics is a hot topic in recent orthopedic researches, due to the correlation altered biomechanics is supposed to have with increased risk of early osteoarthritis [1, 2, 5, 6, 9, 10, 14, 19, 24, 34]. To the best of our knowledge, this is one of the first studies aimed to analyze, with an advanced and highly accurate technology, the translations and rotations of ACLD and contralateral knee joint in vivo and under weight-bearing conditions. On purpose, two tasks that differed in terms of closed (squat) and open (step) kinetic chain were analyzed. On the one hand, gait is one of the commonest daily activities, easily performed by ACLD patients too. On the other hand, the squat was chosen since it is more demanding but, at the same time, safe to perform [18, 32].
Other investigators have already observed the concept of tibial medialization (Fig. 5) after ACL injury, inferring this is due to the oblique orientation of ACL. Li et al. [19], analyzed single leg weight-bearing lunge through double fluoroscopy and found a significant lateral shift of tibio-femoral cartilage contact points, both in the medial (between 0° and 60° of flexion) and the lateral compartment of the tibia (between 15° and 30° of flexion). This finding was reproduced also in a cadaveric study [20], where the application of different loading conditions in specimens with ACLD knee led to a significant tibial medialization between 15° and 30° of flexion. Furthermore, DeFrate et al. [5] found a greater tibia medialization in ACLD knees from 0° to 90° of flexion during the execution of a quasi-static lunge. These results are in accordance with the findings of the present study, since a significant tibial medialization was observed in correspondence to a knee range of flexion between 0° and 30°. This abnormal position could explain the high incidence of osteoarthritis on the medial femoral condyle and anterior tibial spine in chronic ACL deficiency [7, 24]: medial shift of the tibia could reduce the distance between these two knee structures, leading to an altered force distribution on their surfaces [19].
The contribution of ACL in varus–valgus laxity is also a controversial topic [12, 23, 30, 33]. In the present study, ACLD knees were found significantly more varus than the contralateral ones in the first degrees of the re-extension phase of the squat, after they reach the maximum flexion. A crucial role of ACL in frontal plane knee rotations can therefore be supposed. Previous literature studies drew the same conclusion. Yamazaky et al. [33] demonstrated ACL injured limbs had a more knee varus than uninjured of about 5° at the maximum flexion angle of a single leg squat, using an electromagnetic device. In another study [30], performed with fluoroscopy, knees after ACL reconstruction were shown to be more varus than contralateral during downhill running. This aspect could endorse the surgical techniques’ inability to restore physiologic knee varus–valgus after ACL tear. Lastly, there is the recent concept of valgus collapse as a frequent mechanism involved in ACL non-contact injury [26], which could bring to suppose knee valgus as a position of discomfort for patients simulating the ligament rupture biomechanics. ACL-injured patients could probably maintain an easier balance keeping a more varus position [33].
Differently than expected, no differences were found either in tibial anterior–posterior translation or in knee internal–external rotation. Closed kinetic chain exercises like squat are considered safer than open kinetic chain ones in ACL injury rehabilitation programs, especially when patients need to increase muscle activity, because they are supposed to cause less ligament strain [21]. For this reason, squat exercises have a role in ACL deficiency rehabilitation: the high muscular co-activation of quadriceps and hamstrings provides a greater anterior–posterior tibial stability [18, 32]. This consideration could justify the absence of differences in tibial position in anterior–posterior knee laxity and in internal–external rotation in our data. Moreover, some previous studies described a higher tibial internal rotation in ACLD knees, but for motor task different from the squat [5, 10].
In step, we did not found any statistical difference between ACLD knee kinematics and contralateral one. These results are partially in contrast with literature: several studies [9, 15] identified anomalies in knee flexion–extension during walking, but showed neither significantly more anterior tibial translation nor an increased antero-posterior laxity range. Gao et al. [9] described an increased tendency of the ACLD knees to remain in flexion at the end of the stance phase of the gait, while Hurd and Snyder-Mackler [15] described a “joint stiffness strategy” as a combination of reduced peak knee flexion and lack of extension during the mid-stance. The main thesis for this altered knee flexion pattern relies on abnormal muscle activation in patients with ACL tear, aimed to better control knee anterior–posterior laxity. Indeed, many studies based on electromyography highlighted differences in activation of quadriceps and hamstrings after ACL injury, even if there is no consensus regarding the adaptation mechanism [15, 27, 28].
In the present study, no flexion–extension anomalies were identified. The step was executed at a low speed and usually with small step length. Previous investigators demonstrated that small spatiotemporal parameters influence knee flexion during stance, thus resulting in a stiff knee strategy [22, 29] and an almost full extension, similar to our results.
In brief, the findings of the present study could indicate the role of ACL in knee biomechanics: in vivo and under weight-bearing conditions, the ACL could decisively contribute to medio-lateral tibial alignment and knee varus–valgus. So far, the ACL reconstruction techniques have focused on the restoration of anterior–posterior and internal–external rotation knee stability, without considering the anomalies on frontal plane. Actually, previous studies reported that ACL reconstruction does not restore these parameters [6, 30]. According to the present study, surgeons should observe ACL injury from a wider perspective, thus considering also ACLD knee motion anomalies in the frontal plane, to develop reconstruction techniques aimed to reproduce physiological knee stability.
The present study has several limitations. First, due to the controlled nature of the tasks (especially the step), the small sample size could have affected the statistical analysis and probably failed to reveal other differences between the two groups. However, it was possible to demonstrate some consistent trends. A second intrinsic limitation linked to the sample size relied upon the high intra-subject knee motion variability. The choice to acquire, under radiograph exposure, only one repetition per task was made due to ethical reasons. This issue was minimized through a direct comparison of healthy and unhealthy limbs of the same patients.
The other two considerations include the selection of patients based on time from injury and the choice of contralateral limbs as gold standard. When debating on ACL-deficient knee biomechanics, the time from injury is crucial, because patients may progressively develop muscular asymmetries to stabilize the joint [34]. Nevertheless, the present study was mainly focused on how the injury affected the biomechanics and not on how rehabilitation could restore knee stability. The contralateral knees might not reproduce a normal knee kinematics [17]. Anyhow, obtaining a pool of healthy controls would have been highly unethical due to radiograph exposure; furthermore, the evaluation of contralateral knees as controls is typical of nearly all the fluoroscopic studies.
Lastly, the choice of the tasks was related to the actual radiographic setup: due to the limited spaces and the obstacles represented by the medical devices around, it would have been unsafe and impossible to analyze high-dynamics tasks, such as jumps or cut maneuvers. These last tasks could have stressed the knee joint more, and maybe underlined further differences from the contralateral. A future setup development will permit acquiring more complex and stressing tasks.
Conclusion
ACL-deficient knees showed an abnormal tibial medialization and increased varus angle compared to the contralateral knees. These biomechanical anomalies may lead to different force distributions on the tibial plateau, explaining the higher risk of early osteoarthritis in ACL deficiency. Clinicians should take into account the influence of ACL tear on frontal plane knee kinematics in movement commonly used in ACL rehabilitation protocols.
Abbreviations
- ACL:
-
Anterior cruciate ligament
- ACLD:
-
Anterior cruciate ligament deficient
- RSA:
-
Roentgen stereophotogrammetric analysis
References
Andriacchi TP, Dyrby CO (2005) Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J Biomech 38:293–298
Andriacchi TP, Koo S, Scanlan SF (2009) Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Jt Surg Am 91(Suppl 1):95–101
Bontempi M, Roberti di Sarsina T, Marcheggiani Muccioli GM, Pizza N, Cardinale U, Bragonzoni L, Zaffagnini S (2019) J-curve design total knee arthroplasty: the posterior stabilized shows wider medial pivot compared to the cruciate retaining during chair raising. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-019-05645-6
Bragonzoni L, Marcheggiani Muccioli GM, Bontempi M, Roberti di Sarsina T, Cardinale U, Alesi D, Iacono F, Neri MP, Zaffagnini S (2019) New design total knee arthroplasty shows medial pivoting movement under weight-bearing conditions. Knee Surg Sports Traumatol Arthrosc 27:1049–1056
DeFrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G (2006) The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sports Med 34:1240–1246
Deneweth JM, Bey MJ, McLean SG, Lock TR, Kolowich PA, Tashman S (2010) Tibiofemoral joint kinematics of the anterior cruciate ligament-reconstructed knee during a single-legged hop landing. Am J Sports Med 38:1820–1828
Fairclough JA, Graham GP, Dent CM (1990) Radiological sign of chronic anterior cruciate ligament deficiency. Injury 21:401–402
Frank RM, Lundberg H, Wimmer MA, Forsythe B, Bach BR, Verma NN, Cole BJ (2016) Hamstring activity in the anterior cruciate ligament injured patient: injury implications and comparison with quadriceps activity. Arthrosc J Arthrosc Relat Surg 32:1651–1659
Gao B, Zheng NN (2010) Alterations in three-dimensional joint kinematics of anterior cruciate ligament-deficient and -reconstructed knees during walking. Clin Biomech Bristol Avon 25:222–229
Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N (2003) Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med 31:75–79
Grassi A, Signorelli C, Lucidi GA, Raggi F, Macchiarola L, Roberti Di Sarsina T, Muccioli GMM, Filardo G, Zaffagnini S (2019) ACL reconstruction with lateral plasty reduces translational and rotatory laxity compared to anatomical single bundle and non-anatomical double bundle surgery: an in vivo kinematic evaluation with navigation system. Clin Biomech Bristol Avon 69:1–8
Grood ES, Noyes FR, Butler DL, Suntay WJ (1981) Ligamentous and capsular restraints preventing straight medial and lateral laxity in intact human cadaver knees. J Bone Jt Surg Am 63:1257–1269
Grood ES, Suntay WJ (1983) A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng 105:136–144
Hoshino Y, Fu FH, Irrgang JJ, Tashman S (2013) Can joint contact dynamics be restored by anterior cruciate ligament reconstruction? Clin Orthop Relat Res 471:2924–2931
Hurd WJ, Snyder-Mackler L (2007) Knee instability after acute ACL rupture affects movement patterns during the mid-stance phase of gait. J Orthop Res 25:1369–1377
ISO 5725-1:1994(en), Accuracy (trueness and precision) of measurement methods and results—part 1: General principles and definitions. https://www.iso.org/obp/ui/#iso:std:iso:5725:-1:en. Accessed 22 Nov 2019
Kozanek M, Van de Velde SK, Gill TJ, Li G (2008) The contralateral knee joint in cruciate ligament deficiency. Am J Sports Med 36:2151–2157
Kvist J, Gillquist J (2001) Sagittal plane knee translation and electromyographic activity during closed and open kinetic chain exercises in anterior cruciate ligament-deficient patients and control subjects. Am J Sports Med 29:72–82
Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ (2006) Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Jt Surg Am 88:1826–1834
Li G, Papannagari R, DeFrate LE, Yoo JD, Park SE, Gill TJ (2007) The effects of ACL deficiency on mediolateral translation and varus–valgus rotation. Acta Orthop 78:355–360
Luque-Seron JA, Medina-Porqueres I (2016) Anterior cruciate ligament strain in vivo: a systematic review. Sports Health 8:451–455
Mannering N, Young T, Spelman T, Choong PF (2017) Three-dimensional knee kinematic analysis during treadmill gait: slow imposed speed versus normal self-selected speed. Bone Jt Res 6:514–521
Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GA, Slauterbeck JL (1995) Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res 13:930–935
Murrell GA, Maddali S, Horovitz L, Oakley SP, Warren RF (2001) The effects of time course after anterior cruciate ligament injury in correlation with meniscal and cartilage loss. Am J Sports Med 29:9–14
Musahl V, Seil R, Zaffagnini S, Tashman S, Karlsson J (2012) The role of static and dynamic rotatory laxity testing in evaluating ACL injury. Knee Surg Sports Traumatol Arthrosc 20:603–612
Quatman CE, Kiapour AM, Demetropoulos CK, Kiapour A, Wordeman SC, Levine JW, Goel VK, Hewett TE (2014) Preferential loading of the ACL compared with the MCL during landing: a novel in sim approach yields the multiplanar mechanism of dynamic valgus during ACL injuries. Am J Sports Med 42:177–186
Shanbehzadeh S, Mohseni Bandpei MA, Ehsani F (2017) Knee muscle activity during gait in patients with anterior cruciate ligament injury: a systematic review of electromyographic studies. Knee Surg Sports Traumatol Arthrosc 25:1432–1442
Sonesson S, Kvist J (2017) Dynamic and static tibial translation in patients with anterior cruciate ligament deficiency initially treated with a structured rehabilitation protocol. Knee Surg Sports Traumatol Arthrosc 25:2337–2346
Stansfield B, Hawkins K, Adams S, Church D (2018) Spatiotemporal and kinematic characteristics of gait initiation across a wide speed range. Gait Posture 61:331–338
Tashman S, Collon D, Anderson K, Kolowich P, Anderst W (2004) Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med 32:975–983
Tashman S, Kolowich P, Collon D, Anderson K, Anderst W (2007) Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res 454:66–73
Wilk KE, Escamilla RF, Fleisig GS, Barrentine SW, Andrews JR, Boyd ML (1996) A comparison of tibiofemoral joint forces and electromyographic activity during open and closed kinetic chain exercises. Am J Sports Med 24:518–527
Yamazaki J, Muneta T, Ju YJ, Sekiya I (2010) Differences in kinematics of single leg squatting between anterior cruciate ligament-injured patients and healthy controls. Knee Surg Sports Traumatol Arthrosc 18:56–63
Yang C, Tashiro Y, Lynch A, Fu F, Anderst W (2018) Kinematics and arthrokinematics in the chronic ACL-deficient knee are altered even in the absence of instability symptoms. Knee Surg Sports Traumatol Arthrosc 26:1406–1413
Funding
This work was supported by Italian Ministry of Health, Progetto RF Ministero della Salute (Grant number 2010-2312173).
Author information
Authors and Affiliations
Contributions
PA analyzed patients’ kinematics data, participated in study design and drafted the manuscript, SDP contributed in methods development, helped to draft the manuscript and performed statistical analysis, AG took care of clinical part of the study and helped to draft the manuscript, EP participated in kinematics data analysis, MB developed the software used to extrapolate data of present study, LB and SZ participated in study design, coordinated activities and helped to draft the manuscript. All the authors read the final manuscript and approved it.
Corresponding author
Ethics declarations
Conflict of interest
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Ethical approval
This study obtained the approval from Institutional Review Board (IRB) of Rizzoli Orthopaedic Institute (ID: 40/CE/US/ml—Clinical Trial Gov ID: NCT02323386.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Agostinone, P., Di Paolo, S., Grassi, A. et al. ACL deficiency influences medio-lateral tibial alignment and knee varus–valgus during in vivo activities. Knee Surg Sports Traumatol Arthrosc 29, 389–397 (2021). https://doi.org/10.1007/s00167-020-05979-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-020-05979-6