Abstract
Purpose
While many studies about anterior-cruciate-ligament-deficient (ACLD) patients have demonstrated functional adaptations to protect the knee joint, an increasing number of patients undergo ACL reconstruction (ACLR) surgery in order to return to their desired level of activity. The purpose of this study was to compare 3D kinematic patterns between individuals having undergone ACLR with their healthy contralateral knee and a control group.
Methods
Three-dimensional kinematic data were obtained from 15 patients pre- and post-ACLR, 15 contralateral knees and 15 healthy controls. Data were recorded during treadmill walking at self-selected speed. Flexion/extension, external/internal tibial rotation, adduction/abduction and anterior/posterior tibial translation were compared between groups.
Results
ACLR knees showed a significantly higher knee-joint extension during the entire stance phase compared with ACLD knees. However, ACLR knees still showed a deficit of extension compared with healthy control knees. In the axial plane, there was no significant difference in pre- and postoperative kinematic data. Significant difference was achieved between ACLR knees and healthy control knees, specifically between 28 and 34 % and 44 and 54 % of the gait cycle. There was no significant difference in anterior–posterior translation or coronal plane between groups.
Conlusion
Following ACL reconstruction, patients have better clinical and kinematic parameters. Despite improvements, knee kinematics during gait in the ACLR group differed from the control group. These kinematic changes could lead to abnormal loading in the knee joint and initiate the process for future chondral degeneration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alterations in biomechanical features of the knee during walking following ACL reconstruction (ACLR) in patients with ACL-deficient (ACLD) knees have been evaluated in different studies. While many studies demonstrated functional adaptations to protect the knee joint, an increasing number of patients undergo ACLR surgery in order to return to their desired level of activity.
Many studies reported good clinical outcomes following ACLR. However, long-term patient follow-up studies reported a high incidence of degenerative changes [1], abnormal knee laxity [2], the need for revision surgery [3] and anterior knee pain [4]. The precise mechanism contributing to these postoperative complications are unknown.
Abnormal knee kinematics is thought to be one of the possible reasons for long-term development of degenerative changes after ACLR. It is therefore interesting to study the kinematics associated with this type of surgery. However, even though several devices are available to assess knee-joint kinematics [5–7], 3D biomechanical changes caused by ACL injury and the effect of ACLR in knee kinematics are still not clearly understood. Thus, establishing an objective evaluation of knee kinematics in a clinically feasible way is critical in extensive evaluation of ACL function and as valuable feedback for ACLR.
The purpose of this study was to compare 3D kinematic patterns between individuals having undergone ACLR with the healthy contralateral knee and a control group of patients who had no history of musculoskeletal injury or surgery in the lower extremities.
Material and methods
Participants
This prospective study was conducted from January 2011 to January 2014 in the facilities of the biomechanical laboratory at our clinical centre. Patients scheduled for ACLR were selected for kinematic analysis. ACL rupture was diagnosed by clinical examination and magnetic resonance imaging (MRI) and confirmed at surgery by arthroscopy. Patients with unilateral ACL rupture and a healthy contralateral knee (that had never had any kind of orthopaedic or neurological condition) were included in the study. Patients with meniscal injury where partial meniscectomy or repair was feasible or grade 1 or 2 medial collateral injury were also part of the study. Patients who had subtotal or total meniscectomy, concomitant posterior cruciate ligament (PCL) injury, restricted knee-joint movement, full-thickness cartilage defect >1 cm2 or previous history of any surgery in both knees were excluded. Of the 30 patients assessed preoperatively, 15 were available for follow-up evaluation. ACLR was done 1 day after examination. Average time to postoperative examination was 10.23 ± 1.4 months . Kinematic analyzes were compared between reconstructed and contralateral knees and knees in the control group..
Operative technique comprised double-incision isoanatomical ACLR, as previously described[8]. A patellar tendon autograft was used for all ACLRs.
Data collection
Clinical assessment was performed by two clinicians experienced in orthopaedic surgery. Static knee stability was evaluated with the manual Lachman test, drawer test and pivot-shift test. The International Knee Documentation Committee (IKDC) objective evaluation was used to assess clinical outcomes.
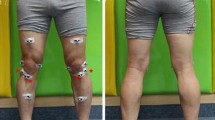
Biomechanics data for walking were collected using the Knee-KG™ System (Fig. 1), which is composed of passive motion sensors fixed on the validated knee harness [9], an infrared motion-capture system (Polaris Spectra camera, Northern Digital Inc.) and a computer equipped with the Knee-3D™ software suite (Emovi, Inc.). The system measures and analyses the position and movement of the patient’s knee [10, 11].To reduce skin-motion artifacts, the group developed a harness to fix quasi-statically on the thigh and calf [9]. This harness was shown to be accurate in obtaining 3D kinematic data that could be used to evaluate ACL and ACL graft deformation in vivo [9, 12].
Initially, calibration is performed as described by Hagemeister et al. [13] and comprises two main parts: defining joint centres, and defining the system of axes based on a predetermined posture. Calibration begins with the identification of four anatomical sites: medial and lateral malleolus, and medial and lateral condyle. Next, the operator locates the 3D position of the femoral head while the individual is asked to perform a circumduction movement of the leg. The Knee-3D™ then calculates the optimal point defining the centre of the femoral head. The next phase is defining the centre of the knee in terms of the 3D position. The study participant extends the leg completely and does repetitive leg flexion/extension motions for 10 s. Once the movement has been recorded, the Knee-3D™ calculates a mediolateral middle axis for that movement. Based on the axis, the Knee-3D™ then defines the midpoint of the knee and the 3D positions of the medial and lateral condyles measured in the previous steps. The middle of both condyles is projected on this axis, thus defining the centre of the knee. The final phase of calibration is the set of neutral transverse rotation, when the knee ia determined to be at 0° of flexion during a slight flexion–hyperextension movement.
After calibration, kinematic data were collecting during treadmill walking at a self-selected, comfortable speed; to avoid the effect of footwear on lower-limb biomechanics, all participants walked barefoot (Fig. 2). Before starting the trials, all participants walked 10 min to get used to walking on the treadmill.
Once calibration and measurements were performed, the Knee-KG™ computed kinematic knee parameters. For each participant, a database containing the four biomechanical patterns—consisting of the three knee angles (flexion–extension, abduction–adduction and internal–external tibia rotation) and anteroposterior (AP) tibial translation—was created in Microsoft Excel 2010.
Statistical analysis
Participant characteristics [age, height, weight, body mass index (BMI), gait speeds, range of motion] were tested using the Levene test to determine whether parametric assumptions were met. The Mann–Whitney U test was used for nonparametric variables, and analysis of variance (ANOVA) and the paired t test were used for parametric variables. The paired t test was used to compare kinematic parameters of the ACLD and ACLR groups and the ACLR group with the contralateral group; ANOVA was used to compare the ACLR with the control group. All statistical analyses were done using SPSS v 21 (SPSS Inc, Chicago, IL, USA). Significance was set at p 0.05.
Results
None of the participants’ characteristics were statistically different between groups (Tables 1 and 2), except walking speed after surgery, when patients walked at a higher speed compared with before surgery (Table 3).
Table 3 and Table 4 summarise the spatiotemporal and kinematic data for ACLD, ACLR and control groups during stance and swing phase.
Sagittal plane
ACLR knees showed a significantly higher extension (10.03°± 4.96°) of the knee joint during the entire stance phase compared with ACLD knees (14.22° ± 6.37°), while there was no significant difference during the swing phase (38.81° ± 3.87; 37.59° ± 6.08, respectively). No statistical difference was detected between reconstructed (10.03° ± 4.96°) and intact contralateral knees in stance (8.42° ± 5.57°) or swing (38.81° ± 3.87,) phases (38.3° ± 5.1°, respectively). However, there was a statistically significant difference between reconstructed (29.24° ± 18.06°) and healthy control (23.29° ± 18.35°) knees during terminal stance, pre-swing and initial swing phase, where the reconstructed knee showed less extension (between 46 and 74 % of the gait cycle) (Fig. 3a).
Tibiofemoral kinematics of anterior-cruciate-ligament-deficient (ACLD), -reconstructed (ACLR) and contralateral and healthy control knees. a Sagittal plane; b axial plane, c anteroposterior tibial translation, d coronal plane. *Significant difference between ACLD and ACLR knees, ^significant difference between ACLR and control knees
Axial plane
There were no statically significant differences between pre- and postoperative kinematic data in stance (-1.68° ± 2.67; -1.35° ± 1.97) or swing (0.82° ± 3.62; 1.76° ± 3.28) phases or in ACLR and intact contralateral knees in any phase of the gait cycle (stance -1.35° ± 1.97; -1.35° ± 2.97; swing: 1.76° ± 3.28; 2.16° ± 4.76). Even though the tibia of ACLR knees rotated more internally from midstance to initial swing phase compared with healthy control knees, significance was achieved only from 28 to 34 % and from 44 to 54 % of the gait cycle (-1.53° ± 0.21; -0.07° ± 0.25) (-2.78° ± 0.27; -0.86° ± 0.21, respectively) (Fig. 3b).
Anteroposterior translation and coronal plane
In ACLD knees, even though during the entire gait cycle the tibia was in an anterior position compared with ACLR knees, statistically, there were no significant differences between groups. However, there were no significant differences between ACLR knees when compared with intact contralateral knees and healthy control knees, (Fig. 3c). Although ACLR and ACLD knees remained in the adducted position in the initial and midswing phases compared with intact contralateral and healthy control knees, in the coronal plane, there were no statistically significant differences between all groups compared (Fig. 3d).
Discussion
The most important finding of this study was that ACLR knees improved significantly in extension compared with ACLD knees but not compared with the control group. There were no differences in AP translation, while ACLR knees showed more internal tibial rotation. Statistically significant differences were found in the sagittal plane, where ACLR knees showed more extension during the stance phase compared with ACLD knees, while there were no significant differences compared with intact contralateral knees. However, during the terminal stance and initial swing phase, ACLR knees showed significantly less extension than healthy control knees. Therefore, after reconstructive surgery, extension improved in comparison with ACLD knees but was not fully restored compared with the healthy control group. Findings of extension deficit were consistent with the study of Gao et al. [14], who showed that ACLR knees exhibited less extension during the stance phase and the second period of the swing phase.
As in ACLR knees passive range of motion (ROM) was fully restored, this deficit in extension could be due to quadriceps weakness. Freiwald et al. [15] found that the maximal isokinetic quadriceps ratio was 81 % of that of the normal knee 16 months after surgery, while in our study, it was 10 months. Arciero et al. [16] reported that patients regained 98.5 % thigh girth and 97 % quadriceps muscle strength at an average follow-up of 31 months. Some studies demonstrated altered muscle activity in the ACLR knee, and quadriceps weakness has been reported after harvesting the bone-patellar tendon-bone (BPTB) autograft and hamstring muscle weakness after harvesting the hamstring-tendon (HST) autograft [17]. These alterations in muscle performance could be of neural or mechanical origin. Specifically, the lack of proprioceptive activity deriving from the ruptured ligament or graft-harvest site may alter neural control of muscles around the knee [18, 19]. However, several investigations suggested that ACLR patients will return to preinjury gait status over time [16, 20].
Kinematic alterations were also identified in the axial plane, and as in the sagittal plane, there were no statistical differences in kinematic data before and after surgery between ACLR knees and intact contralateral knees; the differences were observed between ACLR knees and those in the healthy control group. Significant differences were achieved during midstance and terminal-stance phases, when the leg was under full body weight. The finding of greater internal tibial rotation in ACLR knees was consistent with other studies [14, 21], which found, respectively, that ACLR knees exhibited more internal tibial rotation during the midstance phase or throughout the entire gait cycle. This may be explained by the material properties of the graft, which were different from the native ACL [22]. Handl et al. [22] showed that the BTPB graft showed more stiffness compared with the original ACL. In addition, the native ACL has two functional bundles, and the attachment site is much larger than the insertion site of a single bundle. The decreased attachment area and posteriorly shifted insertion may affect the graft’s ability to constrain the internal tibial twisting [21]. Butler et al. [23] evaluated strain distribution along the length of the ACL and found the greatest strain at the insertion site.
While rotational instability after ACLR remains an issue, we propose that extra-articular lateral reinforcement could be a potential solution. Lording et al. [24] noted that lateral extra-articular reinforcement in conjunction with intra-articular reconstruction may be an important option for controlling rotational laxity.
While in our study there were significant differences between ACLR and healthy control knees in both planes (sagittal, axial), there were no significant difference between ACLR and intact contralateral knees. Also, there are biomechanical adaptations in the intact contralateral knee. The same phenomenon was observed also in other studies [17, 25]: Decler et al. [25] proposed that this alteration is a compensatory mechanism to help maintain some degree of symmetry between the two legs.
Even though in terms of AP translation there was improvement throughout the gait cycle in ACLR knees, our study found no significant differences between groups; also a significant difference was not achieved even when comparing ACLD and control-group knees. It is important to note, however, that while ACLD knees attained less extension during walking in order to prevent anterior translation of the tibia, ACLR knees had significantly improved extension compared with preoperatively, and there was no significant difference compared with the control group; other studies reported similar results [14, 26]. Gao and Zheng [14] found no significant statistical difference in knee-joint translations between ACLD, ACLR and ACL intact knees (ACLI). The authors explain that these results may come from a combination of two aspects: the relatively low intergroup difference and relatively high intragroup variability.
In the first part of the swing phase, ACLR and ACLD knees remain in an adducted position, even though there were no statistically significant differences. Wang et al. [21] found no significant difference between ACLR knees and controls, reporting that this does not necessarily reflect no changes in compartmental loading postoperatively; medial/lateral load sharing can be further evaluated by characterising knee kinetics, specifically abduction/adduction moments. Furthermore, Schipplein and Andriacchi [27] report co-contraction of antagonistic muscle action and/or pre-tension in passive soft tissue was necessary for dynamic joint stability during walking. Thus, further studies should assess gait-cycle kinetics to complement kinematic studies.
This study has some limitations. First, artefacts from soft-tissue movements could be considered a limitation. Nevertheless, Sati and Larouche [9] have shown that the harnesses fixed quasi-statically on the knee and tibia reduce skin-motion artefacts. Additionally, the harness is accurate in assessing 3D kinematic data, which could be used to evaluate ACL and ACL graft deformation in vivo [9, 12]. Another possible limitation is the small number of patients and controls. However, our study is consistent with other gait-analysis studies of the ACLD population. The fact that there was a prospective follow-up made it difficult to assess a greater number of patients
There is support in the literature that kinematic abnormalities in ACLR knees are associated with osteoarthritis (OA) development and progression. If the kinematic changes are sufficient to shift cyclic loading during ambulation to a region that cannot adapt to a change in the local mechanical environment, then normal homeostasis is disrupted in a manner that can initiate a degenerative pathway. The knee joint is particularly sensitive to kinematic changes, since there is a larger range of translational motion at the knee than in other joints, and the movement is dependent on stable ligaments, healthy menisci and coordinated muscular function [28]. Thus, maintaining consistent gait patterns within an envelope of healthy homeostasis between external ambulatory mechanics and cartilage metabolism is a necessary condition to sustain cartilage health [28, 29].
Conclusion
In vivo, 3D motion analysis in this study revealed that ACLR knees improve significantly in extension compared with ACLD knees, but there were still differences compared with knees in the healthy control group. In the axial plane, the tibia remains in the internal position significantly compared with a healthy control group, while there were no significant differences in anteroposterior translation in the coronal plane. These kinematic changes could lead to abnormal loading of the knee joint and may initiate the process for future chondral degeneration. However, postoperative kinematic data were collected 10 months after surgery, so a longer follow-up is needed to determine whether these kinematic changes persist overtime and their effects in joint degeneration.
References
Li RT, Lorenz S, Xu Y et al (2011) Predictors of radiographic knee osteoarthritis after anterior cruciate ligament reconstruction. Am J Sports Med 39:2595–2603. doi:10.1177/0363546511424720
Rupp S, Müller B, Seil R (2001) Knee laxity after ACL reconstruction with a BPTB graft. Knee Surg Sport Traumatol Arthrosc 9:72–76. doi:10.1007/s001670000177
Grossman MG, ElAttrache NS, Shields CL, Glousman RE (2005) Revision anterior cruciate ligament reconstruction: Three- to nine-year follow-up. Arthrosc J Arthrosc Relat Surg 21:418–423. doi:10.1016/j.arthro.2004.12.009
Asano H, Muneta T, Shinomiya K (2002) Evaluation of clinical factors affecting knee pain after anterior cruciate ligament reconstruction. J Knee Surg 15:23–28
Zhang L-Q, Shiavi RG, Limbird TJ, Minorik JM (2003) Six degrees-of-freedom kinematics of ACL deficient knees during locomotion-compensatory mechanism. Gait Posture 17:34–42
Lorbach O, Wilmes P, Theisen D et al (2009) Reliability testing of a new device to measure tibial rotation. Knee Surg Sports Traumatol Arthrosc 17:920–926. doi:10.1007/s00167-009-0772-6
Espregueira-Mendes J, Pereira H, Sevivas N et al (2012) Assessment of rotatory laxity in anterior cruciate ligament-deficient knees using magnetic resonance imaging with Porto-knee testing device. Knee Surg Sports Traumatol Arthrosc 20:671–678. doi:10.1007/s00167-012-1914-9
Arnold MP, Duthon V, Neyret P, Hirschmann MT (2013) Double incision iso-anatomical ACL reconstruction: the freedom to place the femoral tunnel within the anatomical attachment site without exception. Int Orthop 37:247–251. doi:10.1007/s00264-012-1681-8
De Guisea JA (1996) Improving in vivo knee kinematic measurements: application to prosthetic ligament analysis. The knee 3:179–190
Magnussen RA, Neyret P, Cheze L, Lustig S (2012) The KneeKG system: a review of the literature. Knee Surg Sport Traumatol Arthrosc 20:633–638. doi:10.1007/s00167-011-1867-4
Shabani B, Bytyqi D, Lustig S et al (2014) Gait changes of the ACL-deficient knee 3D kinematic assessment. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-014-3169-0
Sati M, De Guise JA, Drouin G (1997) Computer assisted knee surgery: Diagnostics and planning of knee surgery. Comput Aided Surg 2:108–123. doi:10.1002/(SICI)1097-0150(1997)2:2<108::AID-IGS4>3.0.CO;2-3
Hagemeister N, Parent G, Van de Putte M et al (2005) A reproducible method for studying three-dimensional knee kinematics. J Biomech 38:1926–1931. doi:10.1016/j.jbiomech.2005.05.013
Gao B, Zheng NN (2010) Alterations in three-dimensional joint kinematics of anterior cruciate ligament-deficient and -reconstructed knees during walking. Clin Biomech (Bristol, Avon) 25:222–229. doi:10.1016/j.clinbiomech.2009.11.006
Freiwald J, Jäger A, Starker M (1993) EMG-assisted functional analysis within the scope of follow-up of arthroscopically managed injuries of the anterior cruciate ligament. Sportverletz Sportschaden 7:122–128
Arciero RA, Scoville CR, Snyder RJ et al (1996) Single versus two-incision arthroscopic anterior cruciate ligament reconstruction. Arthroscopy 12:462–469
Ferber R, Osternig LR, Woollacott MH et al (2004) Bilateral accommodations to anterior cruciate ligament deficiency and surgery. Clin Biomech 19:136–144. doi:10.1016/j.clinbiomech.2003.10.008
Johansson H, Sjölander P, Sojka P (1990) Activity in receptor afferents from the anterior cruciate ligament evokes reflex effects on fusimotor neurones. Neurosci Res 8:54–59. doi:10.1016/0168-0102(90)90057-L
Solomonow M, Baratta R, Zhou BH et al (1987) The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sport Med 15:207–213
Bush-joseph CA, Hurwitz DE, Patel RR et al (2001) Dynamic Function After Anterior Cruciate Ligament Reconstruction with Autologous Patellar Tendon. Am J Sports Med Am J Sports Med 29:36–41
Wang H, Fleischli JE, Zheng NN (2013) Transtibial versus anteromedial portal technique in single-bundle anterior cruciate ligament reconstruction: outcomes of knee joint kinematics during walking. Am J Sports Med 41:1847–1856. doi:10.1177/0363546513490663
M H, M D, G C, et al. (2007) Reconstruction of the anterior cruciate ligament: dynamic strain evaluation of the graft. Knee Surg Sport Traumatol Arthrosc 15:233–41
Butler DL, Guan Y, Kay MD et al (1992) Location-dependent variations in the material properties of the anterior cruciate ligament. J Biomech 25:511–518. doi:10.1016/0021-9290(92)90091-E
Lording TD, Lustig S, Servien E, Neyret P (2014) ScienceDirect Lateral reinforcement in anterior cruciate ligament reconstruction. Asia-Pacific J Sport Med Arthrosc Rehabil Technol 1:3–10. doi:10.1016/j.asmart.2013.12.002
Decker LM, Moraiti C, Stergiou N, Georgoulis AD (2011) New insights into anterior cruciate ligament deficiency and reconstruction through the assessment of knee kinematic variability in terms of nonlinear dynamics. Knee Surg Sports Traumatol Arthrosc 19:1620–1633. doi:10.1007/s00167-011-1484-2
Tashman S (2004) Abnormal Rotational Knee Motion During Running After Anterior Cruciate Ligament Reconstruction. Am J Sports Med 32:975–983. doi:10.1177/0363546503261709
Schipplein OD, Andriacchi TP (1991) Interaction between active and passive knee stabilizers during level walking. J Orthop Res 9:113–119. doi:10.1002/jor.1100090114
Andriacchi TP, Favre J (2014) The nature of in vivo mechanical signals that influence cartilage health and progression to knee osteoarthritis. Curr Rheumatol Rep 16:463
Dye SF (1996) The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res 325:10–18
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shabani, B., Bytyqi, D., Lustig, S. et al. Gait knee kinematics after ACL reconstruction: 3D assessment. International Orthopaedics (SICOT) 39, 1187–1193 (2015). https://doi.org/10.1007/s00264-014-2643-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2643-0