Abstract
Purpose
Kinematic alignment (KA) and mechanical alignment (MA) position the prosthetic trochlea that guides patellar tracking differently. The present study determined whether KA or MA more closely restores the groove location and sulcus angle of the prosthetic trochlea to the native trochlea for three femoral component designs.
Methods
Ten 3D femur-cartilage models were created by combining computer tomographic (CT) and laser scans of native human cadaveric femurs. Three femoral component designs were positioned using KA and MA. Measurements of the prosthetic and native trochlea were made along the arc length of the native trochlear groove. The alignment technique with the smaller absolute difference between prosthetic and native for the medial–lateral and radial locations of the groove and sulcus angle of the trochlea more closely restored the native trochlea.
Results
For three femoral component designs, KA more closely restored to native the mean medial–lateral location (p = 0.0033 to < 0.0001) and mean radial location (p = 0.0150 to < 0.0001) than MA. For two femoral component designs, KA more closely restored to native the mean sulcus angle (p = 0.0326 to 0.0006) than MA. However, the differences in the mean sulcus angles between KA and MA were less than 2° for all three designs.
Conclusion
KA more closely restored the native trochlea, which explains why the reported risk of patellofemoral complications for KA is not higher than MA according to five randomized clinical trials. Small design modifications of the medial–lateral and radial locations and sulcus angle are strategies for restoring the native trochlea. Such modifications might further reduce the risk of patellofemoral complications.
Level of evidence
II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kinematic (KA) and mechanical (MA) alignment techniques are based on two different paradigms of implant positioning that use the same total knee arthroplasty (TKA) implants [22]. KA is based on a patient-specific alignment paradigm that corrects the arthritic deformity to the pre-arthritic or constitutional alignment, which varies widely from 12° varus to − 16° valgus among the world populace [27]. KA sets the femoral and tibial components coincident with the native tibial–femoral articular surfaces, thereby restoring the native joint lines, limb alignment, knee laxities, and tibial compartment forces without soft tissue release [9, 23, 24, 26]. MA is based on an average alignment paradigm that changes the constitutional alignment to a neutral hip–knee–ankle (HKA) angle. MA changes the native joint lines, limb alignment, knee laxities and tibial compartment forces by aligning the components perpendicular to lines connecting the centers of the femoral head and ankle and the center of the knee and by externally rotating the femoral component with respect to the posterior femoral joint line [10, 14, 27]. Hence, the KA and MA varus–valgus and internal–external rotations of the prosthetic trochlea are different.
The varus–valgus and internal–external rotations of the femoral component affect the orientation of the prosthetic groove that guides patella tracking during flexion–extension of the knee [1, 4, 11]. Considering the differences in the KA and MA alignment techniques [15, 16], these differences should manifest as differences in variables describing the trochlear geometry. Because the groove of the native trochlea is aligned perpendicular to the native femoral joint line [8, 18], the medial–lateral location, radial location, and the sulcus angle of the groove measured in a cylindrical coordinate system (Fig. 1) are useful variables which can serve to compute differences between the prosthetic and native trochlea for KA and MA. The alignment technique with the smaller absolute differences in these variables between the prosthetic and native groove would more closely restore the native trochlea, and the differences would provide guidance for modifying the femoral component when replicating the location of the groove and sulcus angle of the native knee which is a clinical objective.
Diagrams illustrating the three dependent variables of interest. a A 3D model of the distal femur with the cylindrical axis and an arbitrary cross section passing through the trochlea. The cylindrical axis is the axis of a cylinder best fit to the posterior articular surfaces of the femoral condyles from 10° to 110° of flexion. b An outline of the articular surface of the trochlea for an arbitrary cross section and the three dependent variables of interest. The three dependent variables are the medial–lateral location of the trochlear groove measured as the medial–lateral distance from an origin of a cylindrical coordinate system, the radial location of the trochlear groove measured as the radial distance from the cylindrical axis to the trochlear groove, and the sulcus angle. The dependent variables are determined at cross sections along the arc length of the trochlear groove. An arbitrary cross section is shown for illustrative purposes
Although several previous studies have investigated the difference between the prosthetic trochlear groove and that of the native knee [2, 22, 28], only one of these studies has investigated the difference between the prosthetic trochlear groove for a femoral component aligned in KA and MA [22]. Limitations were that an articular cartilage layer of average thickness was added, thus introducing inaccuracy into the 3D femur-articular cartilage models, only a single implant design was studied, and the sulcus angle was not determined.
The objectives of the present study were twofold. By aligning computer-aided design (CAD) models of three femoral component designs in KA and MA on highly accurate 3D femur-cartilage models of native limbs, the primary objective was to determine which alignment technique more closely restores the medial–lateral and radial locations of the groove and the sulcus angle along the arc length of the native trochlea by computing differences between the prosthetic and native trochlea. Because KA is driven by a patient-specific alignment paradigm whereas MA is driven by an average alignment paradigm, our hypothesis was that KA would more closely restore the groove location and sulcus angle to the native trochlea than MA. If this hypothesis was supported for each of the three femoral component designs, then this result might provide a biomechanical explanation for the clinical finding that the prevalence of patellofemoral complications for KA is comparable to that of MA [6, 9, 20, 29, 30]. Based on differences between the prosthetic and native trochleas, a secondary objective was to identify possible strategies for improving the prosthetic trochlea to better restore trochlear geometry to native. Implementing these strategies might reduce the risk of patellofemoral complications. If KA better restores the trochlear geometry to native than MA despite using three off-the-shelf femoral component designs presumably customized for MA, then this study would allay any concerns that patellofemoral function might be compromised in KA despite fundamental differences in alignment between KA and MA.
Materials and methods
Ten unpaired fresh-frozen human cadaveric lower limbs without evidence of prior fracture after review of computer tomographic (CT) scan and without femoral articular wear at inspection during dissection were studied (median age 75.5 years ranging from 51 to 94 years, 7 females and 3 males). Soft tissue was removed from the diaphysis of the femur. Fiducial markers consisting of a hollow sphere within a sphere 28 mm in outside diameter connected to a threaded screw were manufactured from nylon with a 3D printer (Objet Connex 260V, http://www.3dhubs.com). Nine fiducial markers were widely arrayed and rigidly fixed to the femoral diaphysis. A CT scan of the entire limb was performed using a 0.625 mm slice thickness, small scan field of view, tube potential of 140 kV, and tube current of 250 mA (General Electric Lightspeed 16, http://www3.gehealthcare.com). The femur and each fiducial marker were segmented with an automatic thresholding tool followed by manual refinement (Mimics, Materialize, Mimics, http://www.materialise.com). The “marching cubes algorithm” converted the segmented images into a 3D femur model with fiducial markers [19]. Dissection disarticulated the tibia and removed all soft tissue from the femur (Fig. 2). The bone and cartilage surfaces of the distal femur and fiducial markers were scanned with a 0.2 mm resolution laser scanner with a repeatability of < 70 microns (Metrascan 3D Scanner, http://www.creaform3d.com) [7]. A 3D distal femur-cartilage model with best-fit spherical fiducial markers was created from a point cloud. The centers of the fiducial markers were superimposed by minimizing the root mean squared distances between corresponding fiducial marker centers to register the 3D femur model and 3D distal femur-cartilage model. Once registered, the result was a 3D femur-cartilage model (Fig. 3) which was used for positioning the femoral components in KA and MA and measuring the prosthetic and native trochlea geometric variables of interest as described below (Geomagic, http://www.3dsystems.com).
Photograph shows the cartilage surface of the trochlear and femur at the time of laser scanning with nine fiducial markers (white spheres) threaded and cemented into the femoral diaphysis in the standard coronal plane with the lesser trochanter and most posterior points of the femoral condyles tangent to the standard coronal plane
The following steps describe the methods for KA and MA of the three cruciate-retaining femoral components (Persona, Nexgen, Vanguard, Zimmer-Biomet, Warsaw, IN, USA) using software (ParaView, version 4.3.1, http://www.paraview.org) and a previously described technique [5]. The 3D femur-cartilage model was projected in standard sagittal, coronal, and axial planes. For each 3D femur-cartilage model, both alignments used the same size femoral component. For KA, the varus–valgus rotation, proximal–distal location, internal–external rotation, and anterior–posterior location of the femoral components were set coincident to the distal and posterior cartilage surface of the femur at 0° and 90°, respectively. For MA the varus–valgus rotation was set perpendicular to the coronal mechanical axis defined by a line connecting the center of the femoral head and the center of the distal femur at the apex of the intercondylar notch. The proximal–distal location was set so that the thinner resection of a distal femoral condyle matched the thickness of the condyle of the femoral component. The internal–external rotation and anterior–posterior location were set by externally rotating 3° about the center of the femoral component with respect to the posterior cartilage surface of the femur such that the thinner resection was equal to the thickness of the femoral component. Matching the thinner resection to the thickness of the corresponding region of the femoral condyle insured that a minimum amount of bone would be resected from both femoral condyles, in which case the level of the joint lines would be preserved as closely as possible. For both alignment techniques, the flexion–extension rotation of the femoral component was set parallel to the sagittal projection of the mechanical axis of the femur, and the medial–lateral location was set by centering the femoral component. Femoral components were downsized when M–L overhang was 1 mm or greater. The MA femoral components averaged 4.6° ± 0.1° (mean ± standard deviation) more varus, and 2.8° ± 0.1° more externally rotated than the KA femoral components.
The following steps describe the method for computing the difference in the medial–lateral and radial locations of the groove and sulcus angle of the prosthetic minus native trochlea for KA and MA femoral components. The best fitting of a cylinder to the cartilage surface of the medial and lateral femoral condyles of the 3D femur-cartilage model established a cylindrical coordinate system (Fig. 4). Eleven cross sections of the 3D femur-cartilage model were constructed at 10% increments along the arc length of the native trochlear groove by rotating about the cylindrical axis (MATLAB, http://www.mathworks.com) (Fig. 5). These cross sections were propagated onto the KA and MA prosthetic trochlea. At each cross section, a polynomial function fit a line to all points coincident to the articular surface creating a tracing of the native and prosthetic trochlea (Fig. 6). The deepest point represented the groove, and the two highest points on the medial and lateral facets represented the boundary of the sulcus. The medial–lateral location was the distance measured along the cylindrical axis from the origin with medial as a positive value and lateral as a negative value. The radial location was the distance measured perpendicular to the cylindrical axis. The sulcus angle was the included angle between the intersection of lines connecting the deepest and two highest points on the trochlea. For KA and MA of each femoral component design, the differences in the medial–lateral and radial locations of the groove and sulcus angle of the trochlea between the prosthetic minus native were computed at each percent of arc length of the native trochlear groove, thus yielding three dependent variables for statistical analyses. Based on making five measurements of each of the dependent variables on five different specimens, the precisions in the differences in the medial–lateral and radial locations of the groove were 2.5 and 2.0 mm, respectively, while the precision in the difference in the sulcus angle of the trochlea was 6.2°.
Schematic shows the standard planes and the relationship of the cylindrical axis with respect to the standard planes on a posterior oblique view of the 3D femur-cartilage model (left), and the origin, medial–lateral (M–L) axis, radial axis, and reference plane of the cylindrical coordinate system on an anterior oblique view of the 3D femur-cartilage model (right). The cylindrical axis (black line) passes through the center of a cylinder (green) best fit to the central third of the cartilage on each femoral condyle (left). The origin of the cylindrical coordinate system (black dot) was on the M–L axis (i.e., cylindrical axis) midway between the most medial and lateral points on the femoral condyles (right). A radial axis set at the proximal edge of the groove of the native trochlea defined the plane of the 0% cross section along the arc length of the native trochlear groove. The relationship of the cylindrical axis and coordinate system to the prosthetic trochlea is not shown
Schematic shows the relationship of 11 cross sections along the arc length of the native trochlear groove with respect to the cylindrical axis on an oblique view of the 3D femur-cartilage model. The 0% cross section was set coincident to the proximal edge of the trochlear groove, and the 100% cross section was set at the most distal edge. Not shown are the projections of the cross sections on the KA and MA prosthetic trochleas
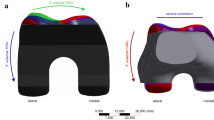
Schematic of a representative cross section of the distal femur shows the relationship between tracings of the articular surface of the native trochlea (gray), KA prosthetic trochlea (green), and MA prosthetic trochlea (blue). The landmarks of the deepest point (DP) of the groove and the highest point (HP) of the medial and lateral facets (only shown on the native trochlea) were used to determine the medial–lateral and radial locations of the groove and the sulcus angle for the native and prosthetic trochlea
Following University of California policies, this study did not require institutional review board (IRB) approval because de-identified cadaveric specimens were used.
Statistical analysis
Three observers performed KA and MA of one femoral component design (Persona) on five 3D femur models repeated five times on each model in five analysis sessions with at least 48 h between each session. All three dependent variables were determined for each cross section for each combination of observer, alignment method, and 3D femur model. The repeatability and reproducibility were quantified by computing the intraobserver and interobserver intraclass correlation coefficients (ICCs) using a three-factor ANOVA for each alignment method where the three factors were the observer at 3 levels, the specimen at 10 levels, and the cross section at 11 levels [3]. An ICC value of > 0.9 indicates excellent agreement, 0.75–0.90 indicates good agreement, 0.5 to 0.75 indicates moderate agreement, and 0.25 to 0.5 indicates fair agreement [17].
Differences between the prosthetic and native trochlea were expressed as the mean ± standard deviation. Because significant and important interactions between the factors of alignment technique and percent of arc length were evident (Fig. 7) and because our interest was to determine whether KA and MA differed at each percent of arc length, paired Student’s t tests were performed for each femoral component design at each percent of arc length for each of the three dependent variables defined above. A p value of < 0.05 indicated that the difference was significant (JMP Version 13; SAS Institute Inc, http://www.jmp.com).
Series of graphs showing the differences between the prosthetic and native for KA (green lines) and MA (blue lines) in the medial–lateral and radial locations of the groove and sulcus angle of the trochlea at intervals from 0 to 100% of normalized arc length of the native trochlear groove for three femoral component designs. The horizontal lines at 0 mm and 0° represent the baseline for no difference from the native. The values denoted by an asterisk indicate that the average difference of the medial–lateral and radial locations of the groove and sulcus angle of the trochlea between the prosthetic minus native is significant (p < 0.05) between KA and MA
A power analysis confirmed that with ten femurs, differences in groove locations between alignment methods of 2 mm, which do not cause adverse mechanical effects [12, 21], could be detected with α = 0.05 and (1 − β) ≥ 0.80 using standard deviations of the differences in groove locations between alignment methods of 1.9 mm. This value was obtained from the present study based on measurements from five specimens and subsequently checked with measurements from all ten specimens.
Results
For each femoral component design, the absolute difference between the prosthetic minus the native trochlea was smaller for KA than MA for the medial–lateral location of the groove (p = 0.0033 to < 0.0001) and the radial location of the groove (p = 0.0150 to < 0.0001) (Fig. 7). In general, the cross sections with significant differences in the medial–lateral location of the prosthetic minus native groove between the KA and MA were confined to the 0–50% of the arc length of the native groove. With respect to the radial location, all cross sections had significant differences between KA and MA. Neither the KA nor MA prosthetic trochlea overstuffed the patellofemoral joint as the radial location was always recessed with respect to the native groove (Figs. 7, 8).
Schematic of a representative knee shows tracings of the M–L and radial locations of the groove and sulcus angle of the native trochlea (black), and the KA (green) and MA (blue) positioned prosthetic trochlea of the Persona, NexGen, and Vanguard femoral components. In general, the M–L and radial locations of the KA prosthetic trochlea are closer to native than those of MA [18]. The M–L location of the MA groove was more varus, lateral, and non-anatomic because MA positions the femoral component an average of 4.6° more varus than the native distal femoral joint line. The sulcus angle for KA is somewhat closer to native than MA. The proximal limit of the prosthetic trochlea extends proximal to the native trochlea
For two of the three femoral component designs, the absolute difference between the prosthetic minus the native sulcus angle was smaller for KA than MA (p = 0.0326 to 0.0006) (Fig. 7). The Persona and Nexgen femoral components had a steeper sulcus angle than native within the cross sections ranging from 0 to 40% of the arc length and a flatter angle over the remainder of the arc length, whereas the Vanguard femoral component had a flatter sulcus angle than native over the entire arc length. Although the difference in the sulgus angle between the prosthetic and native trochlear was significant between KA and MA over the majority of the arc length for Persona and Nexgen and all of the arc length for Vanguard, the differences were small with the greatest difference limited to less than 2° when averaged over the full arc length. The proximal edge of the prosthetic trochlea extended proximal to the native trochlea for all three femoral component designs. “Appendix” contains the raw data used to make these computations.
The ICC values for repeatability (i.e., intraobserver) and reproducibility (i.e., interobserver) for both KA and MA were ≥ 0.95 for all three dependent variables except for the radial distance for MA which were 0.89 for both. Hence, the repeatability and reproducibility of both alignment methods were rated generally as excellent.
Discussion
The most important findings of the present study were that, using three femoral component designs, KA more closely restored the medial–lateral and radial locations of the groove and the sulcus angle along the majority of the arc length to the native trochlea than MA except for the sulcus angle of the Vanguard. The difference in the varus–valgus rotation and not the internal–external rotation explains the finding that significant differences in the medial–lateral location of the prosthetic groove minus the native groove were smaller for KA than MA in the 0–40% range of arc length of the native trochlea for each femoral component (Figs. 7, 8). The MA prosthetic groove was 3–4 mm more lateral than the KA prosthetic groove at the 0% cross section because MA set the femoral component in ~ 5° more varus from the native distal femoral joint line than KA. The MA prosthetic groove location 5–6 mm more lateral than the native groove at the 0% cross section is non-anatomic and oblique to the native distal joint line.
It is difficult to appreciate the rationale behind the non-anatomic and lateral location of the prosthetic groove from native for MA, because changing the limb alignment from constitutional varus to neutral would increase the Q-angle, whereas changing the limb alignment from constitutional valgus to neutral would decrease the Q-angle. Accordingly, it is impossible to compensate for the change in Q-angle for both varus and valgus limbs with a single femoral component design. Perhaps, the rationale is to avoid increasing the Q-angle, which would medialize the trochlea, for limbs that have a varus deformity which constitute the majority of limbs [25]. The non-anatomic lateral location of the prosthetic groove might be unnecessary in KA as the native limb alignment and hence Q-angle would be restored. Small adjustments of ± 2 mm in the medial–lateral location of the prosthetic groove is a strategy for enabling KA to better restore the native trochlea.
The radial location of the prosthetic groove was recessed with respect to the native groove and the KA prosthetic groove was 1–2 mm less recessed than MA, which is consistent with other studies [22, 28]. On average, the deviation of the radial location of the KA prosthetic groove from the native groove ranged from 1 to 4 mm throughout the arc length of the native trochlea with the greatest deviations confined to the 20–60% cross-sectional increments (Fig. 7). Small adjustments of 1–4 mm in the radial location of the prosthetic groove is a strategy for enabling KA to better restore the native trochlea. More closely restoring the radial location of the prosthetic groove to native would have the biomechanical advantage of increasing the moment arm of the quadriceps muscle force by radially translating the patella farther away from the center of rotation of the knee, which lowers the quadriceps muscle force needed to develop an extension moment and decreases the patellofemoral joint compression force.
Although the sulcus angle compared closely for all three femoral component designs between MA and KA, the sulcus angle differed from native and the difference depended on the femoral component design (Fig. 7). Accordingly, these differences in the sulcus angle from native are likely due to the differences in the design of the components per se rather than any effect of the alignment technique. For all three femoral component designs, the sulcus angle was steepest at the 0% cross section and gradually got flatter with increasing arc length. This feature would promote stronger engagement of the patella early in flexion, which might be important in preventing patellar subluxation/dislocation, particularly for patellar prostheses that are dome shaped and do not engage the trochlea as fully as the native patellar articular surface and for femoral prostheses such as the three studied herein where the prosthetic groove is recessed relative to the native groove.
An interesting feature of the three femoral component designs is that the proximal extension of the prosthetic trochlea extended well above the proximal extension of the native trochlea (Fig. 7). As with the steeper sulcus angle early in the arc length common to all three femoral component designs, this feature would be beneficial for engaging the patella early in flexion to compensate for the recessed location of the prosthetic groove which results in under-stuffing.
Several limitations should be discussed. First, the level of restoration of the native trochlea reported for the three femoral component designs in the present study might not apply to other femoral component designs. Second, the present study did not determine whether the relatively small differences between medial–lateral and radial locations and the sulcus angle of the prosthetic minus the native trochlea for KA and MA are large enough to be clinically important. Third, the effects of the morphology of the prosthetic trochlea on the biomechanics of tibiofemoral and patellofemoral joints depend on whether the patella is resurfaced or non-resurfaced, and the patellofemoral interaction was not studied. Finally, a single method for setting the rotation of the femoral component for MA was studied whereas multiple methods are used clinically [13, 14].
Notwithstanding the limitations noted above, our results might have clinical relevance. Although the alignment paradigms and clinical outcomes are different, the prevalence of patellofemoral complications of 0.4% is similar for the KA TKA and MA TKA treatment groups as reported by five randomized clinical trials that used three femoral component designs [6, 9, 20, 29, 30]. Our finding that KA more closely restored the native trochlea than MA might explain this low rate of patellofemoral complications for KA TKA.
Conclusion
Although neither KA nor MA restored the groove location and sulcus angle of the native trochlea using three femoral component designs, generally KA more closely restored these variables to those of the native trochlea than MA. This finding might explain the low rate of patellofemoral complications for KA. Moreover the differences observed in the groove location and sulcus angle between the prosthetic and native trochleas may provide a strategy for modifying the design of the prosthetic trochleas to achieve more natural function of the patellofemoral joint following TKA.
References
Anglin C, Ho KC, Briard JL, de Lambilly C, Plaskos C, Nodwell E, Stindel E (2008) In vivo patellar kinematics during total knee arthroplasty. Comp Aided Surg 13(6):377–391
Barink M, Van de Groes S, Verdonschot N, De Waal Malefijt M (2006) The difference in trochlear orientation between the natural knee and current prosthetic knee designs; towards a truly physiological prosthetic groove orientation. J Biomech 39(9):1708–1715
Bartlett JW, Frost C (2008) Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol 31(4):466–475
Bhattee G, Moonot P, Govindaswamy R, Pope A, Fiddian N, Harvey A (2014) Does malrotation of components correlate with patient dissatisfaction following secondary patellar resurfacing? Knee 21(1):247–251
Brar AS, Howell SM, Hull ML, Mahfouz MR (2016) Does kinematic alignment and flexion of a femoral component designed for mechanical alignment reduce the proximal and lateral reach of the trochlea? J Arthroplasty 31(8):1808–1813
Calliess T, Bauer K, Stukenborg-Colsman C, Windhagen H, Budde S, Ettinger M (2017) PSI kinematic versus non-PSI mechanical alignment in total knee arthroplasty: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc 25(6):1743–1748
Campanelli V, Howell SM, Hull ML (2016) Accuracy evaluation of a lower-cost and four higher-cost laser scanners. J Biomech 49(1):127–131
Coughlin KM, Incavo SJ, Churchill DL, Beynnon BD (2003) Tibial axis and patellar position relative to the femoral epicondylar axis during squatting. J Arthroplasty 18(8):1048–1055
Dossett HG, Estrada NA, Swartz GJ, LeFevre GW, Kwasman BG (2014) A randomised controlled trial of kinematically and mechanically aligned total knee replacements: two-year clinical results. Bone Jt J 96-B(7):907–913
Eckhoff DG, Bach JM, Spitzer VM, Reinig KD, Bagur MM, Baldini TH, Flannery NM (2005) Three-dimensional mechanics, kinematics, and morphology of the knee viewed in virtual reality. J Bone Jt Surg Am 87(Suppl):271–280
Gasparini G, Familiari F, Ranuccio F (2013) Patellar malalignment treatment in total knee arthroplasty. Joints 1(1):10–17
Ghosh KM, Merican AM, Iranpour F, Deehan DJ, Amis AA (2009) The effect of overstuffing the patellofemoral joint on the extensor retinaculum of the knee. Knee Surg Sports Traumatol Arthrosc 17(10):1211–1216
Gu Y, Howell SM, Hull ML (2017) Simulation of total knee arthroplasty in 5 degrees or 7 degrees valgus: a study of gap imbalances and changes in limb and knee alignments from native. J Orthop Res 35(9):2031–2039
Gu Y, Roth JD, Howell SM, Hull ML (2014) How frequently do four methods for mechanically aligning a total knee arthroplasty cause collateral ligament imbalance and change alignment from normal in white patients? J Bone Jt Surg Am 96(12):e101(101–109)
Hirschmann MT, Behrend H (2018) Functional knee phenotypes: a call for a more personalised and individualised approach to total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-018-4973-8
Hirschmann MT, Karlsson J, Becker R (2018) Hot topic: alignment in total knee arthroplasty-systematic versus more individualised alignment strategies. Knee Surg Sports Traumatol Arthrosc 26(6):1587–1588
Indrayan A (2013) Methods of clinical epidemiology. In: SARDaGMW (ed) Springer series on epidemiology and public health, Chap 2. Springer, Berlin, p 24. https://doi.org/10.1007/978-3-642-37131-8_2
Iranpour F, Merican AM, Dandachli W, Amis AA, Cobb JP (2010) The geometry of the trochlear groove. Clin Orthop Relat Res 468(3):782–788
Lorensen WE, Cline HE (1987) Marching cubes: a high resolution 3D surface construction algorithm. In: SIGGRAPH ‘87 proceedings of the 14th annual conference on computer graphics and interactive techniques, pp 163–169
Matsumoto T, Takayama K, Ishida K, Hayashi S, Hashimoto S, Kuroda R (2017) Radiological and clinical comparison of kinematically versus mechanically aligned total knee arthroplasty. Bone Jt J 99-B(5):640–646
Mihalko W, Fishkin Z, Krackow K (2006) Patellofemoral overstuff and its relationship to flexion after total knee arthroplasty. Clin Orthop Relat Res 449:283–287
Riviere C, Iranpour F, Harris S, Auvinet E, Aframian A, Parratte S, Cobb J (2018) Differences in trochlear parameters between native and prosthetic kinematically or mechanically aligned knees. Orthop Traumatol Surg Res 104(2):165–170
Roth JD, Howell SM, Hull ML (2015) Native knee laxities at 0 degrees, 45 degrees, and 90 degrees of flexion and their relationship to the goal of the gap-balancing alignment method of total knee arthroplasty. J Bone Jt Surg Am 97(20):1678–1684
Roth JD, Howell SM, Hull ML (2018) Kinematically aligned total knee arthroplasty limits high tibial forces, differences in tibial forces between compartments, and abnormal tibial contact kinematics during passive flexion. Knee Surg Sports Traumatol Arthrosc 26(6):1589–1601
Sharma L, Song J, Dunlop D, Felson D, Lewis CE, Segal N, Torner J, Cooke TD, Hietpas J, Lynch J, Nevitt M (2010) Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis 69(11):1940–1945
Shelton TJ, Nedopil AJ, Howell SM, Hull ML (2017) Do varus or valgus outliers have higher forces in the medial or lateral compartments than those which are in-range after a kinematically aligned total knee arthroplasty? Bone Jt J 99-B(10):1319–1328
Singh AK, Nedopil AJ, Howell SM, Hull ML (2018) Does alignment of the limb and tibial width determine relative narrowing between compartments when planning mechanically aligned TKA? Arch Orthop Trauma Surg 138(1):91–97
Varadarajan KM, Rubash HE, Li G (2011) Are current total knee arthroplasty implants designed to restore normal trochlear groove anatomy? J Arthroplasty 26(2):274–281
Waterson HB, Clement ND, Eyres KS, Mandalia VI, Toms AD (2016) The early outcome of kinematic versus mechanical alignment in total knee arthroplasty: a prospective randomised control trial. Bone Jt J 98-B(10):1360–1368
Young SW, Walker ML, Bayan A, Briant-Evans T, Pavlou P, Farrington B (2017) The Chitranjan S. Ranawat Award: no difference in 2-year functional outcomes using kinematic versus mechanical alignment in TKA: a randomized controlled clinical trial. Clin Orthop Relat Res 475(1):9–20
Acknowledgements
The authors thank the individuals who donated their remains and tissues for the advancement of education and research.
Funding
The authors are grateful to THINK Surgical for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Rocio Lozano and Valentina Campanelli are employees of THINK Surgical. Stephen Howell is a paid consultant for THINK Surgical and Medacta. Maury Hull receives research funding from THINK Surgical and Zimmer-Biomet.
Ethical approval
Following University of California policies, this study did not require institutional review board (IRB) approval because de-identified cadaveric specimens were used.
Appendix
Appendix
Plots of the medial–lateral (M–L) location and the radial location of the native trochlear groove and the sulcus angle of the native trochlea as a function of the arc length in percent. Plots show the average value (heavy line) and the data points for each of the ten specimens as a function of the normalized arc length of the native trochlea.

Rights and permissions
About this article
Cite this article
Lozano, R., Campanelli, V., Howell, S. et al. Kinematic alignment more closely restores the groove location and the sulcus angle of the native trochlea than mechanical alignment: implications for prosthetic design. Knee Surg Sports Traumatol Arthrosc 27, 1504–1513 (2019). https://doi.org/10.1007/s00167-018-5220-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-5220-z