Abstract
Purpose
Guidelines for shock recommend mean arterial pressure (MAP) targets for vasopressor therapy of at least 65 mmHg and, until recently, suggested that patients with underlying chronic hypertension and atherosclerosis may benefit from higher targets. We conducted an individual patient-data meta-analysis of recent trials to determine if patient variables modify the effect of different MAP targets.
Methods
We searched the MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials for randomized controlled trials of higher versus lower blood pressure targets for vasopressor therapy in adult patients in shock (until November 2017). After obtaining individual patient data from both eligible trials, we used a modified version of the Cochrane Collaboration’s instrument to assess the risk of bias of included trials. The primary outcome was 28-day mortality.
Results
Included trials enrolled 894 patients. Controlling for trial and site, the OR for 28-day mortality for the higher versus lower MAP targets was 1.15 (95% CI 0.87–1.52). Treatment effect varied by duration of vasopressors before randomization (interaction p = 0.017), but not by chronic hypertension, congestive heart failure or age. Risk of death increased in higher MAP groups among patients on vasopressors > 6 h before randomization (OR 3.00, 95% CI 1.33–6.74).
Conclusions
Targeting higher blood pressure targets may increase mortality in patients who have been treated with vasopressors for more than 6 h. Lower blood pressure targets were not associated with patient-important adverse events in any subgroup, including chronically hypertensive patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this individual patient-data meta-analysis, higher blood-pressure targets–i.e. more aggressive use of vasopressors - were associated with an increased risk of death in patients enrolled >6 h after initiation of vasopressors. Lower blood-pressure targets were not associated with patient-important adverse events in any subgroup, including chronically hypertensive patients. |
Introduction
Until recently, guidelines of the Surviving Sepsis Campaign [1] and the European Consensus on Circulatory Shock [2] recommended an initial mean arterial pressure (MAP) target of at least 65 mmHg (grade 1C, indicating a strong recommendation with a low quality of evidence), and suggested that patients with underlying chronic hypertension and atherosclerosis may benefit from higher blood pressure targets.
Critical care clinicians administer vasopressors to compensate for excessive vasodilatation and/or, as rescue therapy when hypotension is so severe that treating teams anticipate imminent circulatory arrest [3]. However, inducing vasoconstriction may significantly compromise blood flow to some vascular beds, and vasopressors have pleiotropic effects that make it difficult to predict their overall effects on clinical outcomes [4].
The results of two recent randomized controlled trials (RCTs) do not suggest that MAP targets for vasopressors higher than 65 reduce mortality [5, 6]. Both trials targeted a heterogeneous patient population raising the possibility that effects of target pressures may differ in subgroups of patients. This analysis follows a study-level meta-analysis conducted at the request of two societies to inform guidelines [7]. We conducted the current patient-level analysis to understand the effects of the intervention in specific patient subgroups, an objective not achievable using published, study-level data.
Specifically, the research question was: ‘In adult patients who are in shock, do higher versus lower blood pressure targets reduce 28-day mortality overall, or in subgroups defined by chronic hypertension, congestive heart failure, age and duration of vasopressor therapy before enrolment?’ Our hypothesis was that patient characteristics at baseline modify the effect of different MAP targets.
Methods
We registered the study on PROSPERO on April 8, 2016 (CRD42016037482), followed a prespecified analysis plan and present the results according to PRISMA guidance.
Search and selection criteria
To identify RCTs of higher versus lower blood pressure targets for vasopressor therapy in adult patients who are in shock, we searched the electronic databases MEDLINE (from 1946 to November 2017), EMBASE (from 1980 to November 2017), and the Cochrane Central Register of Controlled Trials, as well as reference lists of identified articles, recently published editorials and reviews, and proceedings from the annual meetings (from 2005 to 2017) of the American Thoracic Society, the Society of Critical Care Medicine, the European Society of Intensive Care Medicine, and the International Symposium on Intensive Care and Emergency Medicine. This review excludes crossover designs, trials of unconventional vasopressor agents, and studies in which the duration of the intervention was less than 24 h. There was no exclusion on the basis of language of the published report. The detailed search strategy is presented in the eAppendix.
Two reviewers independently assessed trial eligibility based on a review of titles, abstracts, and, when possibly eligible, the corresponding full text reports.
Master database
We accessed the complete databases, database dictionaries and original protocols from each trial. The project biostatistician (A.G.D.) confirmed published results of each trial and resolved any queries with the corresponding principal investigator, data manager, or statistician. We reviewed the individual study protocols, template case report forms and database dictionaries to harmonize study databases. We updated each database with unified coding across trials and merged them into one master database. Harmonized variables are listed in order of appearance in the online supplement (eTable 4).
Outcomes
All outcomes were prespecified. The primary outcome was 28-day all-cause mortality. Binary secondary outcomes were: all-cause mortality at 90 days; proportion of patients with persistent organ dysfunction—defined as continued dependency on mechanical ventilation or renal replacement therapy—or death; the number of days alive and without persistent organ dysfunction; and the occurrence of supraventricular cardiac arrhythmia during the first 5 days of vasopressor therapy, myocardial injury, digit or limb ischemia, mesenteric ischemia and major bleeding. Other secondary outcomes were the fluid balance, daily average MAP and total daily norepinephrine equivalent received over the first 5 days of vasopressor therapy.
Calculations
We transformed doses of dopamine, epinephrine, phenylephrine and vasopressin to norepinephrine equivalents [8]. Individual studies used different risk scores to describe the baseline severity of illness. We calculated the predicted mortality for each patient using the risk score of the corresponding study [9]. For each patient, we ascertained persistent organ dysfunction, defined as dependency on mechanical ventilation, renal replacement, or vasopressor therapy at day 28 and at 3 months. Patients with persistent organ dysfunction at day 28 have a higher subsequent mortality rate, prolonged hospital course, and reduced quality of life at 3 months compared to survivors without persistent organ dysfunction [10].
Risk of bias assessment for individual studies
We used a modified version of the Cochrane Collaboration’s instrument to assess risk of bias in included trials [11]. The instrument addresses the following domains: allocation sequence concealment, blinding of participants and caregivers, blinding of data collectors, blinding for outcome assessment, blinding of data analysts, loss to follow up, selective outcome reporting, and termination of enrolment before planned sample size is reached for early evidence of benefit [12]. Studies with one or more domains assessed as a potential source of bias were considered overall at high risk of bias.
Statistics
All analyses were conducted according to the predefined statistical analysis plan outlined in the protocol (CRD42016037482). We analyzed all patients in the arms to which they were randomized. The analysis of most outcomes addresses potential heterogeneity by treating site as a random effect and trial as a fixed effect. The primary outcome of 28-day mortality, and all other binary outcomes, were analyzed using a generalized linear (logistic) mixed model with a random effect for site and fixed effects for treatment assignment and trial, except, due to their small numbers, adverse events were compared between treatment groups using unadjusted odds ratios and risk differences with exact 95% confidence intervals and p values calculated by Fisher’s exact test.
We conducted sensitivity analyses of the primary outcome adjusting the odds ratio of 28-day mortality after adding, simultaneously, the following prespecified baseline covariates: age, probability of dying in hospital, baseline chronic hypertension and duration of vasopressor therapy before enrolment. We depict survival over 3 months using a Kaplan–Meier curve.
A priori, we postulated four possible subgroup effects based on (1) presence or absence of hypertension diagnosed prior to the acute illness (binary variable as defined in primary studies)—hypothesizing that hypertensive patients will benefit more from high blood pressure targets; (2) congestive heart failure (binary variable as defined in primary studies)—hypothesizing that patients with congestive heart failure benefit from lower blood pressure targets; (3) age (continuous variable)—hypothesizing that older patients benefit from lower blood pressure targets; and (4) duration of vasopressor therapy before enrolment (dichotomized at ≤ 6 vs. > 6 h as pre-specified)—hypothesizing that ≤ 6 h would benefit from higher MAP targets. Subgroup analyses were performed for every outcome. An interaction term between the subgroup and treatment was used to test the statistical significance of subgroup effect modification. We represented each subgroup by dummy variables except age, which we modeled as a continuous variable. To allow the effect modification of age to vary non-linearly, we used a restricted cubic spline with equally spaced knots between 40 and 80. We limited the knots to between 40 and 80 because too few patients were outside this age range. We considered placing knots, every 5, 10 and 20 years, and selected the model which had the lowest AIC score [13]. We used the Hosmer–Lemeshow goodness of fit test to assess the adequacy of fit of the selected model. Due to the minimal amount of missing data, our primary analysis used complete cases. However, we confirmed the primary results after multiply imputing any missing data using the fully conditional specification [14].
We present the distribution of daily average MAP and norepinephrine equivalent vasopressor dose while on vasopressors using boxplots clustered by MAP target and trial.
We used the linear mixed effects model with a random site effect and fixed trial effect to estimate the persistent organ dysfunction-free days over the first 28 days as well as the patient-averaged MAP and vasopressor dose over the first 5 study days while on vasopressors by arm. This model estimates the mean difference between patients randomized to higher versus lower MAP targets and tests if these differences were consistent across subgroups or trials. Since vasopressor dose was strongly positively skewed, it was log-transformed before modeling and parameters were exponentiated to provide estimates of geometric means and the ratio of geometric means between MAP target. Since fluid balance was strongly Kurtotic but not highly skewed, we reported it by arm as quartiles with the ratio of the median reported and p value comparing arms estimated by the Wilcoxon rank-sum test.
All between group differences are presented as point estimates with 95% confidence intervals and p values testing against the null hypothesis of no difference in the proportion (or mean or ratio) between groups. For all analyses, statistical significance was inferred when the 95% confidence interval did not cross the null effect, or equivalently, where the two-sided p value was < 0.05. We did not adjust confidence intervals or p values for multiplicity of secondary outcomes or subgroups.
All analyses were undertaken using SAS v.9.4 (SAS Institute, Cary NC, USA). We modeled binary outcomes such as 28-day mortality and 28-day persistent organ dysfunction using the GLIMMIX procedure with trial as a fixed effect and site as a random effect using residual pseudo-likelihood subject specific estimation based on Taylor expansion.
Credibility of subgroup effects
We assessed the credibility of subgroup effects using previously published criteria: (1) statistical significance, (2) consistency across studies, (3) small number of prespecified subgroups and corresponding direction of effect, (4) strong underlying biological rationale, and (5) within- rather than between-study comparisons [15]. Fragility indices were calculated for statistically significant subgroup effects [16].
Results
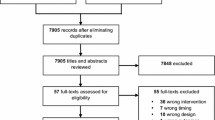
Of 8343 screened citations, we retrieved 57 full-text articles and ultimately included two randomized controlled trials [5, 6]. We identified one ongoing trial (NCT01473498). A PRISMA-IPD flowchart illustrates the selection process (Fig. 1).
The SEPSISPAM trial enrolled 776 patients with a presumptive diagnosis of septic shock within 6 h of vasopressor initiation and randomly allocated a higher (80–85 mmHg) or lower (65–70 mmHg) MAP target [5]. The Optimal Vasopressor TItration (OVATION) trial enrolled 118 patients with a presumptive diagnosis of vasodilatory shock of any etiology within 24 h of vasopressor initiation. This trial randomly allocated patients to a higher (75–80 mmHg) or lower (60–65 mmHg) MAP target [6].
Baseline
This review includes 894 patients from the SEPSISPAM trial (776 patients, 27 French sites) and the OVATION trial (118 patients, 11 North American sites). Table 1 summarizes baseline patient characteristics by treatment group. A summary of baseline characteristics in each trial is shown in the supplemental material (eTable 1). Compared to SEPSISPAM, the OVATION trial had a significantly lower proportion of males (54 vs. 67%), a higher proportion of elective surgical admissions (8 vs. 1%), and a longer duration of vasopressor therapy prior to randomization (10.6 vs. 3.6 h). The baseline illness severity, estimated using the SAPS-II score in SEPSISPAM and the APACHE II score in OVATION, yielded predicted hospital mortality rates of 59 and 52%, respectively (p = 0.003). We encountered no problems during the verification of individual patient data.
Risk of bias
In both trials, randomization was concealed but caregivers were not blinded. The analyses respected the intention-to-treat principle, follow-up was complete for the primary outcomes and enrolment was not stopped for early benefit or harm. Assessors for arrhythmia occurring during the 5-day intervention were blinded in the SEPSISPAM trial. We considered overall risk of bias to be high in both trials due to lack of blinding.
Mortality
Crude measures of 28-day mortality were 36 and 33%, respectively, in the higher and lower blood pressure target groups. In the pooled analysis controlling for trial and site, the overall odds ratio for 28-day mortality for the higher versus lower blood pressure targets was 1.15 (95% CI 0.87–1.52, p = 0.31). The odds ratio changed only trivially after adjusting for age, probability of hospital mortality, congestive heart failure, chronic hypertension and duration of vasopressor therapy before enrolment (adjusted OR = 1.19, 95% CI 0.88–1.62, p = 0.26). The 90-day mortality in the higher and lower blood pressure target groups were 43 and 41%, respectively (OR = 1.10, 95% CI 0.84–1.44, p = 0.47). A Kaplan–Meier curve (eFigure 1) of the overall survival by treatment group and a table of 28- and 90-day mortality by trial (eTable 2) appear in the Supplementary Online Content.
Figure 2 depicts 28-day mortality by trial and by subgroups defined by chronic hypertension, congestive heart failure and hours on vasopressors before enrolment. Results were similar irrespective of the presence of prior hypertension (interaction p = 0.36), congestive heart failure (p = 0.71) or age (p = 0.11; Fig. 3). However, there was a significant difference in the treatment effects by duration of vasopressors before randomization (interaction p = 0.017), with very similar mortality in high and low targets among patients on vasopressors ≤ 6 h before randomization [higher vs. lower OR = 1.01 (95% CI, 0.75–1.36)], but significantly higher mortality in the higher MAP target group among patients on vasopressors > 6 h before randomization [OR = 3.00 (95% CI, 1.33–6.74); absolute risk difference 23%]. The 90-day mortality by subgroups and corresponding fragility indices are shown in the electronic supplement (eFigure 2, eTable 3).
Higher versus lower blood pressure target odds ratio of 28-day mortality by age where age and age by target are modeled by a cubic spline with knots at 40, 60 and 80 years of age. Odds ratio > 1 favors the lower target. The solid line and shaded area depict the estimated odds ratio with 95% confidence intervals by age according to our logistic model with the age and age by treatment effect modeled by a natural cubic spline with knots at 40, 60 and 80 years of age. The test for age by MAP target effect modification was not statistically significant according to this model (Wald test for interaction p = 0.11). The Hosmer–Lemeshow test for lack of fit was p = 0.86 indicating a good fit
Secondary outcomes
By 28 days, the proportion of patients who died or had persistent organ dysfunction was 41% and 39%, in the higher and lower blood pressure groups, respectively. The estimated odds ratio of death or persistent organ dysfunction by day 28 in the higher versus lower blood pressure target was 1.06 (95% CI 0.81–1.39). The mean ± SD days alive and persistent organ dysfunction-free days during the first 28 days appears in the online supplement. The higher versus lower target treatment effect on 28-day persistent organ dysfunction or death varied by duration of vasopressor before enrolment (p = 0.013), but not by other subgroups (eFigure 3 in the Supplement). For patients on vasopressors for > 6 h prior to enrolment, the odds ratio for 28-day persistent organ dysfunction or death in the higher versus lower blood pressure target arm was 2.61 (95% CI 1.23–5.53), compared to 0.92 (95% CI 0.69–1.24) in patients enrolled within 6 h of initiating vasopressors. The expected mean difference in alive and persistent organ dysfunction-free days over the first 28 days in patients enrolled over 6 h after the initiation of vasopressor therapy was 6.8 (95% CI 2.8–10.9) days fewer in the higher versus lower blood pressure target arm, while this difference was − 0.1 (95% CI − 1.6 to 1.5) in patients enrolled within 6 h of initiating vasopressors (p for interaction = 0.002).
Supraventricular arrhythmia during the first 5 vasopressor days occurred in 40/446 (9%) of patients in the higher target arm compared to 17/448 (4%) of patients in the lower target arm [OR = 2.50 (95% CI 1.35–4.77), p = 0.002]. The association between treatment and arrhythmia did not vary significantly by any subgroup. Risks of myocardial injury, digit or limb ischemia, mesenteric ischemia and major bleeding were not statistically different (Table 2). The number of other adverse events was too small to allow adjusted and subgroup analyses.
The impact of MAP targets on fluid balance and vasopressor dose over the first 5 days while on vasopressors appears in the online supplement. The increment in vasopressor exposure associated with higher MAP targets was more pronounced among patients enrolled > 6 h after initiation of vasopressor therapy (p for interaction = 0.047).
A summary of effects appears in Table 2.
Consideration of missing data
The duration of VP prior to enrolment was unknown for 3 patients. All other variables used for the primary analysis and subgroup analyses were known for all 894 patients. We repeated the primary outcome (28-day mortality) by duration of VP prior to enrollment subgroup analyses after multiply imputing the 3 missing values. After rounding to the first decimal or second significant digit, the test for subgroup by treatment interaction and the odds ratios estimating the within subgroup estimates of treatment effects were identical to the complete case analysis reported above. A secondary analysis re-estimated the overall treatment effect after adjusting for age, probability of hospital mortality, congestive heart failure, chronic hypertension and duration of vasopressor therapy before enrolment. Other than duration of vasopressor therapy before enrolment, the only other missing data was the estimated probability of hospital mortality, which was missing for 8 patients. Again, the multiple imputed results agreed with the complete case results to the first decimal.
Discussion
In this pooled analysis of data from 894 patients enrolled in two randomized controlled trials, higher blood pressure targets for vasopressor therapy in shock were not associated with improved 28-day survival overall or in prespecified subgroups. However, we observed a higher risk of death with higher targets in patients already exposed to vasopressors for more than 6 h. In contrast, we found no effect modification with the presence of prior hypertension or heart failure, which challenges the common practice of modifying blood pressure targets for patients with these comorbidities. The results of the current analysis may warrant updating recent recommendations informed by a study-level meta-analysis that could not assess the impact of timing and age.
The reported subgroup effect is one of only four prespecified subgroups, statistically significant, consistent across trials, relies on a strong biological rationale and results from within- rather than between-study comparisons—meeting all criteria for credible subgroup effects [15]. The results suggest that, when higher MAP targets are instituted early, the adverse effects of the intervention may be balanced by beneficial effects. In contrast, when patients have already been treated with vasopressors for more than 6 h, and failed to improve rapidly, targeting higher MAP targets could be deleterious overall because, contrary to harmful effects, benefits are no longer possible. The fact that patients enrolled later also received larger cumulative doses raises the possibility of a dose effect whereby higher MAP targets become harmful beyond a dose threshold. Harm may not be apparent in the subgroup of patients enrolled very early if a significant proportion of these patients are weaned off early.
In contrast, our failure to find any effect modification, for the outcomes of interest, with prior hypertension raises questions regarding previous guidelines stating that “the optimal MAP should be individualized as it may be higher in patients with atherosclerosis and/or previous hypertension than in young patients without cardiovascular comorbidity. For example, a MAP of 65 mmHg might be too low in a patient with severe uncontrolled hypertension; in a young, previously normotensive patient, a lower MAP might be adequate.” [1, 2].
Strengths of this review include explicit and prespecified eligibility criteria, analysis plan, subgroups and direction of subgroup effects, a comprehensive literature search, duplicate adjudication of eligibility, data extraction and risk of bias assessment, relevant harmonisation metrics for catecholamine dosage and severity of illness, and strict criteria of subgroup credibility. Moreover, the definition of persistent organ dysfunction used clinically relevant, operator-independent metrics (i.e. mechanical ventilation and renal replacement therapy) over unvalidated scoring systems. All patients enrolled in published randomized controlled trials of vasopressors targeting higher versus lower blood pressure are included in this work, which allows detailed analyses related to individualization of vasopressor therapy. The statistical analysis takes into account the possible impact of study and centre, allowed exploration of four hypothesized subgroup effects, and yielded consistent results at different time points. Although the eligibility criteria and interventions were not identical, the trials shared many characteristics including the age distribution, vasopressor requirements, and consistent findings.
Limitations include the fact that, in both trials, actual MAP values while treated with vasopressors were higher than stated targets, in both arms. The effect of adhering to the targets that were protocolized in either trial remains uncertain. Exploring what constitutes the lowest safe target for patients who are most susceptible to vasopressor-induced side effects would constitute a logical next step. In both trials, norepinephrine was the vasopressor of choice. Whether higher blood pressure targets using non-catecholamine drugs would yield similar effects remains unclear. The operational definition for hypertension used in both trials relied on past medical history recorded in medical records and may not reflect actual elevated blood pressure before the acute illness. However, during the urgent initial management of shock, ascertaining the degree of control, or lack thereof, of chronic hypertension before hospitalization is seldom feasible and not easily operational. Patients treated with vasopressors for more than 6 h were not eligible for the SEPSISPAM trial. Accordingly, the SEPSISPAM participants included in this subgroup represent only 6% of the study population compared to 67% for OVATION. Finally, the analyses hinge on a small number of events and are therefore imprecise. At this stage, the lack of a statistically significant associations does not rule out potential clinical effects and, conversely, statistically significant subgroup effects could be attributable to random error.
In conclusion, the results of this study suggest that higher blood pressure targets may increase mortality in patients who have been treated with vasopressors for more than 6 h while lower blood pressure targets were not associated with patient-important adverse events in any subgroup, including chronically hypertensive patients.
References
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric S (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A (2014) Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40:1795–1815
Lamontagne F, Cohen D, Herridge M (2017) Understanding patient-centredness: contrasting expert versus patient perspectives on vasopressor therapy for shock. Intensive Care Med 43:1052–1054
Andreis DT, Singer M (2016) Catecholamines for inflammatory shock: a Jekyll-and-Hyde conundrum. Intensive Care Med 42:1387–1397
Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, Mira JP, Dequin PF, Gergaud S, Weiss N, Legay F, Le Tulzo Y, Conrad M, Robert R, Gonzalez F, Guitton C, Tamion F, Tonnelier JM, Guezennec P, Van Der Linden T, Vieillard-Baron A, Mariotte E, Pradel G, Lesieur O, Ricard JD, Herve F, du Cheyron D, Guerin C, Mercat A, Teboul JL, Radermacher P, Investigators S (2014) High versus low blood-pressure target in patients with septic shock. N Engl J Med 370:1583–1593
Lamontagne F, Meade MO, Hebert PC, Asfar P, Lauzier F, Seely AJE, Day AG, Mehta S, Muscedere J, Bagshaw SM, Ferguson ND, Cook DJ, Kanji S, Turgeon AF, Herridge MS, Subramanian S, Lacroix J, Adhikari NKJ, Scales DC, Fox-Robichaud A, Skrobik Y, Whitlock RP, Green RS, Koo KKY, Tanguay T, Magder S, Heyland DK, Canadian Critical Care Trials G (2016) Higher versus lower blood pressure targets for vasopressor therapy in shock: a multicentre pilot randomized controlled trial. Intensive Care Med 42:542–550
Hylands M, Moller MH, Asfar P, Toma A, Frenette AJ, Beaudoin N, Belley-Cote E, D’Aragon F, Laake JH, Siemieniuk RA, Charbonney E, Lauzier F, Kwong J, Rochwerg B, Vandvik PO, Guyatt G, Lamontagne F (2017) A systematic review of vasopressor blood pressure targets in critically ill adults with hypotension. Can J Anaesth 64:703–715
Brown SM, Lanspa MJ, Jones JP, Kuttler KG, Li Y, Carlson R, Miller RR 3rd, Hirshberg EL, Grissom CK, Morris AH (2013) Survival after shock requiring high-dose vasopressor therapy. Chest 143:664–671
Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G (2010) Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 303:865–873
Heyland DK, Muscedere J, Drover J, Jiang X, Day AG, Canadian Critical Care Trials G (2011) Persistent organ dysfunction plus death: a novel, composite outcome measure for critical care trials. Crit Care 15:R98
Guyatt GH, Busse JW (2016) Modification of cochrane tool to assess risk of bias in randomized trials. http://distillercer.com/resources/
Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, Heels-Ansdell D, Walter SD, Guyatt GH, Group S-S, Flynn DN, Elamin MB, Murad MH, Abu Elnour NO, Lampropulos JF, Sood A, Mullan RJ, Erwin PJ, Bankhead CR, Perera R, Ruiz Culebro C, You JJ, Mulla SM, Kaur J, Nerenberg KA, Schunemann H, Cook DJ, Lutz K, Ribic CM, Vale N, Malaga G, Akl EA, Ferreira-Gonzalez I, Alonso-Coello P, Urrutia G, Kunz R, Bucher HC, Nordmann AJ, Raatz H, da Silva SA, Tuche F, Strahm B, Djulbegovic B, Adhikari NK, Mills EJ, Gwadry-Sridhar F, Kirpalani H, Soares HP, Karanicolas PJ, Burns KE, Vandvik PO, Coto-Yglesias F, Chrispim PP, Ramsay T (2010) Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA 303:1180–1187
Harrell FE (2001) Regression modeling strategies: with application to linear models, logistic regression and survival analysis. Springer, New York
van Buuren S (2007) Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 16:219–242
Sun X, Ioannidis JP, Agoritsas T, Alba AC, Guyatt G (2014) How to use a subgroup analysis: users’ guide to the medical literature. JAMA 311:405–411
Walsh M, Srinathan SK, McAuley DF, Mrkobrada M, Levine O, Ribic C, Molnar AO, Dattani ND, Burke A, Guyatt G, Thabane L, Walter SD, Pogue J, Devereaux PJ (2014) The statistical significance of randomized controlled trial results is frequently fragile: a case for a Fragility Index. J Clin Epidemiol 67:622–628
Acknowledgements
We would like to thank Qi Zhou for additional input on the statistical analysis plan as well as nurses and physicians who contributed to both trials included in this analysis. We thank the Unité de Recherche Clinique et Épidémiologique (URCE) of the Centre de recherche du CHU de Sherbrooke for their support in coordinating the preparation and revisions of this manuscript.
Author information
Authors and Affiliations
Contributions
FL and PA had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: FL, AGD, MOM, DJC, GHG, PA. Acquisition, analysis, or interpretation of data: FL, AGD, MOM, DJC, GHG, MH, PR, J-MC, NB, PH, FD, FM, PA. Drafting the manuscript: FL, PA. Critical revision of the manuscript for important intellectual content: FL, AGD, MOM, DJC, GHG, MH, PR, J-MC, NB, PH, FD, FM, PA. Statistical analysis: FD. Study supervision: FL.
Corresponding author
Ethics declarations
Funding
This analysis was funded by a grant from the Fonds de Recherche du Québec - Santé. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of interest
The authors declare that they have no conflict of interest. Drs. Lamontagne and Asfar are the Principal Investigators of both trials included in this analysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lamontagne, F., Day, A.G., Meade, M.O. et al. Pooled analysis of higher versus lower blood pressure targets for vasopressor therapy septic and vasodilatory shock. Intensive Care Med 44, 12–21 (2018). https://doi.org/10.1007/s00134-017-5016-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-017-5016-5