Abstract
Purpose
In shock, hypotension may contribute to inadequate oxygen delivery, organ failure and death. We conducted the Optimal Vasopressor Titration (OVATION) pilot trial to inform the design of a larger trial examining the effect of lower versus higher mean arterial pressure (MAP) targets for vasopressor therapy in shock.
Methods
We randomly assigned critically ill patients who were presumed to suffer from vasodilatory shock regardless of admission diagnosis to a lower (60–65 mmHg) versus a higher (75–80 mmHg) MAP target. The primary objective was to measure the separation in MAP between groups. We also recorded days with protocol deviations, enrolment rate, cardiac arrhythmias and mortality for prespecified subgroups.
Results
A total of 118 patients were enrolled from 11 centres (2.3 patients/site/month of screening). The between-group separation in MAP was 9 mmHg (95 % CI 7–11). In the lower and higher MAP groups, we observed deviations on 12 versus 8 % of all days on vasopressors (p = 0.059). Risks of cardiac arrhythmias (20 versus 36 %, p = 0.07) and hospital mortality (30 versus 33 %, p = 0.84) were not different between lower and higher MAP arms. Among patients aged 75 years or older, a lower MAP target was associated with reduced hospital mortality (13 versus 60 %, p = 0.03) but not in younger patients.
Conclusions
This pilot study supports the feasibility of a large trial comparing lower versus higher MAP targets for shock. Further research may help delineate the reasons for vasopressor dosing in excess of prescribed targets and how individual patient characteristics modify the response to vasopressor therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypotension associated with excessive vasodilation may contribute to inadequate oxygen delivery, organ failure and death [1, 2]. Medications used to increase blood pressure (vasopressors) may increase blood flow by restoring blood pressure [3] but may also hinder blood flow by inducing vasoconstriction and cause net harm [4–6]. The optimal vasopressor dosing strategy for hypotensive, critically ill patients is thus unknown. Expert opinion and practice guidelines suggest tailoring vasopressor dosing to surrogate markers of organ function [2, 7], but the association between these intermediate outcomes and survival is also unclear [8–10]. Meanwhile, clinicians must balance the risks of hypotension with the adverse effects of vasopressors.
A concurrent randomized trial comparing mean arterial pressure (MAP) targets of 65–70 versus 80–85 mmHg in critically ill adults with septic shock receiving vasopressors [11] found no difference in 28-day mortality. Higher rates of atrial fibrillation in the higher MAP group (7 versus 3 %, p = 0.02) highlighted arrhythmias as an important and previously documented complication of vasopressor therapy [12, 13]. In the subgroup of patients with chronic hypertension, the higher (versus lower) MAP approach was associated with less renal replacement therapy in the first week (32 versus 42 %, p = 0.046). This finding underscores the concept that optimal MAP targets may vary across specific patient subgroups [14]. However, this subgroup effect on a secondary outcome does not establish the superiority of higher versus lower MAP targets for chronically hypertensive patients, particularly when knowledge of group assignment may have influenced clinical decisions to initiate renal replacement therapy [15]. Further trials are needed to confirm or refute the observed effect in chronically hypertensive patients as well as other vulnerable subgroups. In this context, we conducted the Optimal Vasopressor Titration (OVATION) pilot trial to inform the design of a larger trial examining the effect of lower versus higher MAP targets in shock. To guide further investigations, we report the results of exploratory subgroups analyses based on age, chronic hypertension, congestive heart failure and prior duration of vasopressor therapy.
Methods
Study design
From May 2013 to August 2014, intensive care units (ICUs) at 11 academic hospitals in Canada and the USA participated in this open-label randomized controlled trial with approval from their local research ethics boards.
Study participants
Adults over 16 years of age receiving vasopressors in ICU for presumed vasodilatory shock regardless of admission diagnosis were eligible for enrolment if the treating physician judged that they were adequately fluid resuscitated and that ongoing vasopressor therapy was expected for at least 6 h. We did not specify a minimum amount of intravenous fluids before enrolment. We excluded patients who had received vasopressors for more than 24 h, were expected to die within 48 h, or required vasoactive agents for reasons unrelated to hypotension (e.g. intracranial hypertension, allergic angioedema). Patients were also ineligible if the treating ICU physician believed the main cause of hypotension was overt cardiogenic, haemorrhagic or neurogenic shock or if hypotension occurred in the immediate post-cardiac surgery period.
Study procedures
Dedicated research personnel in each ICU screened patients for eligibility. Upon confirming eligibility, they solicited written consent from the patients or their legal representatives. When patients were incapacitated and their representatives were unavailable, we used a deferred consent model in all but two participating sites. Randomization was stratified by centre and used permuted blocks of variable and undisclosed size. Group assignment was accessed by the local research personnel through a secure Web-based system that ensured concealment. Patients were allocated 1:1 to the two study groups.
Study interventions
We randomly assigned study participants to a lower (60–65 mmHg) versus a higher (75–80 mmHg) target MAP range. Immediately following randomization, the allocated blood pressure target was prescribed by the treating ICU physician. Study MAP targets were followed for the entire period of vasopressor infusions (including during transports and surgical interventions) and ended when patients maintained a MAP within or above the prescribed range without vasopressors. When ICU physicians judged that a patient was no longer in need of vasopressor therapy, the infusion was stopped even if the resultant MAP was no longer in range. If vasopressor therapy resumed, the assigned MAP range was reapplied. The duration of the study protocol was capped at 28 days. Administration of fluids, inotropes and corticosteroids was at the discretion of ICU physicians. Research personnel recorded MAP and vasopressor data hourly during infusion and daily thereafter until ICU discharge. They also recorded data related to life support, monitoring devices, cointerventions, laboratory tests and clinical outcomes on a daily basis.
Outcomes
The primary feasibility outcome was the between-group difference in mean MAP during vasopressor therapy. Specifically, we reasoned that a larger study would not be feasible if the separation in MAP was less than 5 mmHg. We also assessed patient accrual, number of protocol deviations, duration of vasopressor therapy and choice of vasopressor as secondary measures of feasibility. Deviations were defined as MAP out of range for four consecutive hours if vasopressor infusions were not modified to achieve target MAP (i.e. MAP below target did not constitute a deviation if MAP remained low despite increasing vasopressors). Other process of care measures include dose and duration of vasopressor therapy. We recorded clinical outcomes relevant to a larger trial, including mortality at ICU discharge, 28 days, hospital discharge and at 6 months. We also measured a composite outcome consisting of death or persistent organ dysfunction (POD) [16], which was recorded each day to day 28, and the occurrence of potential vasopressor-induced adverse events that developed after randomization (i.e. cardiac arrhythmias, myocardial injury, bowel ischemia, digit or limb necrosis, gastric intolerance, venous thromboembolic events, vasopressor extravasation, major haemorrhage). Along with intravenous fluid use and urine output over the course of the ICU stay, the change in renal Sequential Organ Failure Assessment (SOFA) score [17] during the first 2 days in the study captured the early effect of the interventions on renal function. At 6 months, we verified the occurrence of stroke and change in functional autonomy from baseline by telephone interview. We assessed functional autonomy using the alpha Functional Independence Measure (alphaFIM instrument), which measures cognitive and motor disability and helps predict functional status at discharge [18]. For FIM measures at baseline, we asked the patients’ representatives to assess their functional status before the onset of the acute illness that resulted in the ICU admission. The 6-month FIM interview was completed by the same person.
Study oversight
All investigators contributed to the study design. The Canadian Institutes for Health Research and the Fonds de Recherche du Québec–Santé funded this project but were not involved in its design, conduct or interpretation. The investigators reported to an independent data monitoring committee.
Statistical analyses
We report continuous variables using means and standard deviations (SD) or medians and quartiles, depending on the distribution. We report the between-group difference in the patient-averaged MAP during vasopressor therapy with the corresponding 95 % confidence interval (CI). The patient-averaged MAP was the average of all hours for days where some vasopressor was received. As prespecified, we used a two-sample t test with a one-sided alpha of 0.025 to test the null hypothesis that the mean difference in the patient-averaged MAP was less than 5 mmHg. Other comparisons between groups used the non-parametric Wilcoxon rank-sum test for continuous variable or Fisher’s exact test for dichotomous clinical outcomes. We transformed doses of dopamine, epinephrine, phenylephrine and vasopressin to norepinephrine equivalents as per similar trials [19].
We prespecified four subgroup analyses of the primary outcome (hospital mortality) planned for a subsequent confirmatory trial. Following the publication of the SEPSISPAM trial, we elected to report prespecified subgroup analyses following the conclusion of the pilot study to inform the design of future trials. These subgroups were defined by age (greater than or equal to 75 years versus less than 75 years), chronic hypertension (as reported in the medical record), chronic congestive heart failure (as reported in the medical record) and duration of vasopressors at enrolment (greater or less than 6 h). Although the specific age cut-off of 75 years was arbitrary, we hypothesized that older patients have a greater risk of suffering from vasopressor-associated harm due to cardiovascular senescence. We assumed that in patients with congestive heart failure, lower MAP targets would result in reduced afterload and better cardiac output but that chronically hypertensive patients would benefit from higher MAP targets. If any treatment effect was observed, we hypothesized that it would be greater in patients enrolled within the first 6 h of vasopressor therapy. We tested for subgroup interactions by applying a Wald test to the treatment by subgroup product term estimated from linear or logistic regression models for continuous and binary outcomes, respectively.
Except for the primary feasibility outcome, which is one-sided, we considered observed differences between groups to be statistically significant at a two-sided, nominal alpha of 0.05. All analyses conformed to group assignment and were performed using SAS 9.4 (Cary, NC).
Originally a sample size of 40 patients per arm was proposed to achieve 80 % power at a one-sided alpha of 0.025 to reject the null hypothesis that the average MAP in the higher arm was not at least 5 mmHg higher than the average MAP in the lower arm. This calculation assumed that the true mean MAP in the higher arm would be 10 mmHg higher than the lower arm, the SD of the MAP would be 7.5 mmHg in both arms and that at least 37 patients per arm would be evaluable. Prior to trial initiation, we increased the target sample size to 120 to assess feasibility across more centres and increase the external validity of the study results.
Role of funding sources
The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Results
Participants
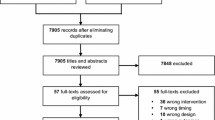
We identified 238 eligible patients, of whom 120 were enrolled (Fig. 1). The number of patients enrolled in each centre ranged from 3 to 25. We completed the pilot trial on schedule, enrolling an average of 2.3 patients per centre per month of screening. Most (n = 82, 68 %) patients participated using a deferred consent model. Patient representatives declined deferred consent for two randomized patients and no patients declined or withdrew consent after they regained capacity. Thus, the remainder of this report is based on 118 patients. Baseline characteristics appear in Table 1. Mean (SD, min–max) age was 65 (13, 25–92) years and APACHE II score was 25 (7, 10–48). Before enrolment, patients received vasopressors for a median (first–third quartiles) of 11 (4–17) h. A higher proportion of patients had chronic hypertension in the lower MAP group compared with the higher MAP group (57 versus 33 %, respectively); otherwise the two groups were balanced with respect to important prognostic variables.
Vasopressors
The separation in MAP between groups during vasopressor therapy exceeded our prespecified threshold for study feasibility (9 mmHg, 95 % CI 7–11). The mean (SD) MAP was above target in the lower MAP arm [70 (5) mmHg] and on target in the higher MAP arm [79 (5) mmHg, Fig. 2]. We observed similar separation in all the predefined subgroups except in patients with chronic congestive heart failure where the high MAP arm was only 2 (95 % CI −5 to 10) mmHg higher than the low MAP arm (test for interaction between MAP separation and chronic congestive heart failure p = 0.03). In the lower and higher MAP groups, we observed deviations on 12 versus 8 % of all days on vasopressors (p = 0.059) and 10 % of all deviations occurred on the last vasopressor day. However, 68 and 69 % of hourly MAP measurements were out of range in the higher and lower MAP arm, respectively. Half of the MAP measurements that were out of range were above target in the higher MAP arm and 12 % were below target in the lower MAP arm.
The median (Q1, Q3) number of hours from start of vasopressors to enrolment was 9 (3, 17) in the lower MAP versus 11 (3, 17) in the higher MAP arm (p = 0.63) and 30 versus 36 % (p = 0.56) of the patients in the lower and higher MAP arms were enrolled within 6 h of vasopressor initiation. The median (Q1, Q3) number of days receiving vasopressor was 3 (2, 5) days in the lower MAP arm compared to 5 (3, 8) days in the higher MAP arm (p = 0.0075). In 8 % of the cases (15 % in lower MAP arm versus 0 % in higher MAP arm, p = 0.003), vasopressors were discontinued within less than 6 h of initiation. The daily dose of norepinephrine equivalent for days on vasopressors was 10 (2, 19) mg in the lower MAP arm compared with 14 (8, 29) mg in the higher MAP arm (p = 0.017). Selection of vasopressor agents was similar in the two groups, with norepinephrine administered more frequently (92 % of patients) than other agents (vasopressin 48 %, phenylephrine 14 %, epinephrine 14 %, dopamine 4 %).
Other resuscitative measures
We observed no difference in the overall fluid balance during vasopressor therapy [lower MAP 1337 (744, 2285) mL versus higher MAP 1179 (299, 2222) mL, p = 0.40] or after discontinuation [lower MAP −286 (−975, 437) mL versus higher MAP −337 (−1169, 328) mL, p = 0.94]. Daily urine output during vasopressor therapy was not statistically different [1009 (255, 2046) mL versus 1696 (555, 2541) mL, p = 0.07). Blood products were used in 49 and 71 % of the lower and higher MAP arms, respectively (p = 0.024). Colloids (albumin solutions) were used in 49 and 64 % of the lower and higher MAP arms, respectively (p = 0.14). Use of the following were similar in the lower and higher arms: central venous catheters (97 and 100 %, p = 0.50), arterial catheters (97 and 98 %, p = 1.00), sedatives (propofol, midazolam or lorazepam 85 and 83 %, p = 0.81), systemic corticosteroids (51 and 52 %, p = 1.00), dobutamine (12 and 14 %, p = 0.79) and milrinone (3 versus 7 %, p = 0.44).
Clinical outcomes
Mortality rates were 28 % in ICU, 30 % at 28 days, 31 % in hospital and 39 % at 6 months. Differences between groups were not statistically significant. Similarly, the proportion of patients who had died or had POD at day 28 were not different between groups (lower MAP 44 % versus higher MAP 46 %, p = 0.21). The median (Q1, Q3) number of POD-free days to day 28 was 21 (1, 25) days in the lower MAP arm and 20 (4, 25) in the higher MAP arm (p = 0.61). Although fewer patients had cardiac arrhythmias in the lower MAP arm (20 versus 36 %), the difference was not significant (Table 2). The average day 1 and day 2 renal SOFA scores were 1.3 and 1.1 in both groups, respectively. The median (Q1, Q3) change in FIM among survivors from baseline to 6 months was also identical in both groups at 0 (−10, 0).
Subgroups
Among patients 75 years of age and older (n = 25), but not in those under 75 years of age, the lower MAP target was associated with reduced hospital mortality (Fig. 3). The test for interaction between age and MAP target was statistically significant (p = 0.015). The treatment effect did not differ significantly between patients in the subgroups defined by chronic hypertension, congestive heart failure and duration of vasopressor therapy before enrolment (Fig. 3).
Discussion
This multicentre, open-label pilot randomized controlled trial comparing lower (60–65 mmHg) to higher (75–80 mmHg) MAP targets for fluid resuscitated hypotensive patients treated with vasopressors confirmed the feasibility of a larger randomized controlled trial. There was a clear separation in MAP, comparable to the one achieved in the SEPSISPAM trial [11], which can reasonably be regarded as clinically relevant and this separation paralleled differential vasopressor use. Larger volumes of blood products were also used in the higher MAP arm, which suggests that raising MAP may signify more intensive use of other therapies as well. Measured clinical outcomes and exploratory subgroup analyses are potentially unreliable because they are based on a small number of clinical events. We did not measure an increase in adverse effects that would preclude a larger trial designed to delineate the risk–benefit trade-off of lower MAP targets among older patients who are also more likely to suffer from chronic underlying hypertension.
Strengths of this trial include its pragmatic eligibility criteria and interventions reflecting usual care in general adult critical care units, complete follow-up for consenting patients, rigorous concealment of the randomization sequence, strict application of the intention-to-treat principle and the highly accurate monitoring of protocol adherence.
The fact that achieved MAP was frequently above the prescribed range (even in the higher MAP arm) is a limitation. Interestingly, the SEPSISPAM investigators faced similar issues which suggests that nurses and physicians take great care to avoid underdosing vasopressors but may underappreciate or undervalue the potential risks of excessive vasopressor therapy in excess of prescribed MAP targets. Hourly MAP values represent discrete measurements every hour, not the hourly average. Moreover, the interventions being compared were MAP targets, not actual MAP measures. Accordingly, we predefined protocol deviations as a failure to adjust vasopressor infusions when MAP measurements were out of range for four consecutive hours. This explains why the number of deviations is lower than the high percentage of hourly MAP measurements that were out of range. The arbitrary definition for protocol deviations is a limitation. In this trial, vasopressors were not adjusted according to markers of organ function such as serum lactate levels or urine output. The justification for a strictly MAP-based protocol is that MAP remains the most readily available and pragmatic endpoint to guide vasopressor therapy. Moreover, there are no validated and treatment-responsive markers for tissue perfusion. Recent trials of perfusion target-directed resuscitation did not find that this approach improved survival compared to current protocols of care [8–10]. This evidence collectively suggests that while individualized vasopressor management may modify physiologic outcomes such as urine output, these do not correlate with more meaningful clinical outcomes. Although a single MAP target may not be optimal for all patients with presumed vasodilatory shock, dosing vasopressors in relation to age, a reliable baseline characteristic documented at admission, may have the greatest chance of avoiding harm while improving the chance of survival. While this trial targeted patients suffering from vasodilatory shock, we relied on the treating ICU physicians’ clinical judgement and initiated the research protocol after the clinical decision to initiate vasopressor had already been made. This pragmatic approach reflects usual care but results in a more heterogeneous study population and introduces the possibility that vasodilation may not have been the only or even the dominant cause of hypotension. Abnormal left ventricular function, which could be acute and therefore unaccounted for in the subgroup defined by chronic congestive heart failure, may be over-represented among older patients and explain excess mortality in the higher MAP arm. By design, patients could be enrolled later than in the SEPSISPAM trial. Considering that vasopressors were continued for many days, we believe that this represents a good balance between feasibility imperatives and early initiation of the study protocol. However, delayed initiation of a superior intervention may reduce the probability of observing a benefit.
In summary, this pilot study supports the feasibility of a larger trial comparing MAP targets below those applied in the SEPSISPAM trial. Further research may help delineate the reasons for vasopressor dosing in excess of prescribed targets and how individual patient characteristics modify the response to vasopressor therapy.
References
Angus DC, van der Poll T (2013) Severe sepsis and septic shock. N Engl J Med 369:2063. doi:10.1056/NEJMc1312359
Vincent JL, De Backer D (2013) Circulatory shock. N Engl J Med 369:1726–1734. doi:10.1056/NEJMra1208943
Bellomo R, Kellum JA, Wisniewski SR et al (1999) Effects of norepinephrine on the renal vasculature in normal and endotoxemic dogs. Am J Respir Crit Care Med 159:1186–1192. doi:10.1164/ajrccm.159.4.9802055
Dunser MW, Mayr AJ, Tur A et al (2003) Ischemic skin lesions as a complication of continuous vasopressin infusion in catecholamine-resistant vasodilatory shock: incidence and risk factors. Crit Care Med 31:1394–1398. doi:10.1097/01.CCM.0000059722.94182.79
Schmittinger CA, Torgersen C, Luckner G et al (2012) Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med 38:950–958. doi:10.1007/s00134-012-2531-2
Weil MH, Tang W (2009) Challenging the rationale of routine vasopressor therapy for management of hypotension. Crit Care 13:179. doi:10.1186/cc7976
Dellinger RP, Levy MM, Rhodes A et al (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. doi:10.1097/CCM.0b013e31827e83af
Mouncey PR, Osborn TM, Power GS et al (2015) Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 372:1301–1311. doi:10.1056/NEJMoa1500896
Peake SL, Delaney A, Bailey M et al (2014) Goal-directed resuscitation for patients with early septic shock. N Engl J Med 371:1496–1506. doi:10.1056/NEJMoa1404380
Yealy DM, Kellum JA, Huang DT et al (2014) A randomized trial of protocol-based care for early septic shock. N Engl J Med 370:1683–1693. doi:10.1056/NEJMoa1401602
Asfar P, Meziani F, Hamel JF et al (2014) High versus low blood-pressure target in patients with septic shock. N Engl J Med. doi:10.1056/NEJMoa1312173
De Backer D, Aldecoa C, Njimi H et al (2012) Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 40:725–730. doi:10.1097/CCM.0b013e31823778ee
De Backer D, Biston P, Devriendt J et al (2010) Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 362:779–789. doi:10.1056/NEJMoa0907118
Panwar R, Lanyon N, Davies AR et al (2013) Mean perfusion pressure deficit during the initial management of shock–an observational cohort study. J Crit Care 28:816–824. doi:10.1016/j.jcrc.2013.05.009
D’Aragon F, Belley-Cote EP, Meade MO et al (2015) Blood pressure targets for vasopressor therapy: a systematic review. Shock 43:530–539. doi:10.1097/SHK.0000000000000348
Heyland DK, Muscedere J, Drover J et al (2011) Persistent organ dysfunction plus death: a novel, composite outcome measure for critical care trials. Crit Care 15:R98. doi:10.1186/cc10110
Vincent JL, Moreno R, Takala J et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Hinkle JL, McClaran J, Davies J et al (2010) Reliability and validity of the adult alpha functional independence measure instrument in England. J Neurosci Nurs 42:12–18
Brown SM, Lanspa MJ, Jones JP et al (2013) Survival after shock requiring high-dose vasopressor therapy. Chest 143:664–671. doi:10.1378/chest.12-1106
Acknowledgments
We would like to express our gratitude for the contribution and support of Janet Overvelde, Shawna Froese, Jesse Gadon, the Institute for Safer Medication Practices Canada, the Canadian Critical Care Society, the Canadian Society of Hospital Pharmacists, the Canadian Association of Emergency Physicians, the Canadian Association of Critical Care Nurses and the intensive care unit nurses and physicians who collaborated on this work. Dr. Tom Stelfox conducted the internal review of this manuscript for the Canadian Critical Care Trials Group. We are also indebted to Dr. Andreas Laupacis (Chair), Dr. Lauren Griffiths and Dr. Scott Halpern who constituted the Data Monitoring Committee for this trial. This study would not have been feasible without the outstanding participation of (in alphabetical order) Sarah-Judith Breton, Katia Cauchon, Paulina Farais, Susan Fleury, Brittany Giacomino, Marnie Jakab, Lisa Julien, Catherine Krause, Chantal Langevin, Heather Langlois, Nicole Marinoff, Andrea Matte, Tracy McArdle, Katie O'Brien, Rebecca Porteus, , Sumesh Shah, Samantha Taylor, and Irene Watpool.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
None reported.
Additional information
Take-home message: This pilot study supports the feasibility of a larger trial comparing MAP targets below those applied in the SEPSISPAM trial. Further research may help delineate the reasons for vasopressor dosing in excess of prescribed targets and how individual patient characteristics modify the response to vasopressor therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lamontagne, F., Meade, M.O., Hébert, P.C. et al. Higher versus lower blood pressure targets for vasopressor therapy in shock: a multicentre pilot randomized controlled trial. Intensive Care Med 42, 542–550 (2016). https://doi.org/10.1007/s00134-016-4237-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4237-3