Abstract
In this study, we examine markers of oxidative stress in the tetra Hyphessobrycon luetkenii collected from two locations in the copper contaminated João Dias creek (southern Brazil). Also, specimens were translocated from a clean reference section of the creek to a polluted stretch and vice-versa. Fish were held at in submerged cages for 96 h and then sacrificed. Nuclear abnormalities in erythrocytes and total antioxidant capacity, lipid peroxidation and protein carbonylation in gills, brain, liver and muscle displayed similar trends in both groups. Lipid peroxidation increased in all tissues of individuals translocated to the polluted site but only in liver and muscle of those translocated to the reference site. Increased protein carbonylation was also observed in gills of individuals translocated to the reference location. These results suggest similar oxidative stress among fish from the reference and polluted locations and that long-term metals exposure may require adaptations toward oxidative stress responses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well-described that the primary mechanism involved in waterborne Cu toxicity in freshwater fish is related to ionoregulatory and osmotic disturbances associated with the inhibition of branchial Na+/K+-exchanging ATPase activity (Grosell 2011; Malhotra et al. 2020; Tavares-Dias 2021). Moreover, negative effects of chronic exposure to Cu on oxidative status/profile have been described to be a key mechanism involved in Cu toxicity (Zebral et al. 2019; Malhotra et al. 2020; Tavares-Dias 2021). Other morphological, biochemical and physiological impairments associated with fish exposure to Cu are linked to negative effects on growth, reproduction and behavior (Haverroth et al. 2015; Zebral et al. 2018; Malhotra et al. 2020; Sung et al. 2020; Tavares-Dias 2021).
Transcriptome analysis in populations of the freshwater fish Hyphessobrycon luetkenii living for more than a century in a Cu mining impacted area revealed that this fish species is highly adapted to face the stressful environmental conditions imposed by this kind of economic activity. Furthermore, it indicated that several genes associated with transport, ion binding and metabolism of steroids and other lipids can be rapidly regulated in H. luetkenii (Abril et al. 2022). Therefore, aspects involved in ion regulation and oxidative status, two key mechanisms of Cu toxicity mentioned above, were shown to be detected by transcriptome analysis in this freshwater fish species exposed to waterborne Cu for several generations. Despite regulatory changes observed for genes associated with transport and ion binding (Abril et al. 2022), wild populations of H. luetkenii living in the mining impacted area of the João Dias creek showed no alterations in the blood levels of major cations (Mg2+, Na+, K+ and Ca2+), or in the activity of gill enzymes playing a key role in blood ion regulation (Borges et al. 2022). On the other hand, findings from transcriptome analysis support the idea that H. luetkenii exposure to elevated concentrations of waterborne Cu in the field affects lipid metabolism at different levels. The induction of several genes implicated in lipid and steroid transformation suggests that lipid metabolism is one of the most affected process after acute and chronic exposure of H. luetkenii to the Cu mining impacted area (Abril et al. 2022).
Considering the background described above, the possible occurrence of different oxidative status/profiles in two wild groups of H. luetkenii living in the João Dias creek was evaluated in the present study. Studies involving fish translocation into a novel environment, including those considering contaminated and uncontaminated sites, are relevant to study behavioral and fitness impacts of translocations at realistic ecological scales. It is important to note that how newly translocated individuals establish themselves in non-local, novel environments is largely unknown for aquatic species (Monk et al. 2020). The analyzed endpoints included damage to DNA in erythrocytes and levels of total antioxidant capacity, as well as lipid peroxidation and protein carbonylation in several tissues (gills, brain, liver and muscle). Therefore, we hypothesize that wild populations of H. luetkenii living in non-mining impacted (reference) site and mining impacted (polluted) site will display different oxidative status/profiles, as well as different responses to new Cu exposure scenarios inducing metal bioaccumulation (fish collected at the reference site and translocated to the polluted site) or depuration (fish collected at the polluted site and translocated to the reference site). The findings from the present study will certainly provide new insight into the key mechanisms involved in freshwater fish adaptation to chronic exposure to elevated concentrations of Cu in the field.
Materials and Methods
Fish Sample Collection and Translocation Experiment
Samples (blood, gills, brain, liver and muscle) of the fish H. luetkenii were obtained as described in a previous paper (Borges et al. 2022). One group was composed of fish living in the higher portion (non-mining impacted = reference; R site) of the João Dias creek, while the other group was represented by fish living in the lower portion (mining impacted = polluted; P site) of this creek. Additionally and differently from previous studies on Cu-induced oxidative stress, the underlining mechanisms involved in the responses of the two groups of H. luetkenii were evaluated through a 96-h translocation experiment, where fish from the reference site was translocated to the polluted site, and vice-versa. The 96-h period of translocation was selected for four main reasons: (1) acute and significant changes in several biochemical and physiological biomarkers, including oxidative stress-related parameters, are reported to occur within 96 h of freshwater fish exposure to Cu (Simonato et al. 2016; Zebral et al. 2019); (2) the 96-h exposure time is established in the “Standard Guide for Conducting Acute Toxicity Tests on Test Materials with Fishes” (ASTM 2014); (3) Cu bioaccumulation in different tissues of H. luetkenii and consequent significant effects on biomarkers of ion regulation, as well as expression of genes related to Cu transporters, metal metabolism and detoxification processes were previously reported for fish translocated for 96 h in the João Dias creek (Abril et al. 2018a, b, 2022); and (4) longer experimental periods of translocation could implicate in the intensification of possible cofounding factors related with caging the fish (Borges et al. 2022).
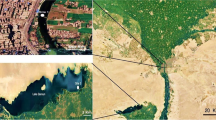
The present study was performed in the upper (non-mining impacted = reference) and lower (mining impacted = polluted) portions of the João Dias creek (Minas do Camaquã district, Caçapava do Sul municipality, Rio Grande do Sul State, southern Brazil; Fig. 1). The reference site is located 7 km upstream of the place where the mining activity occurred (30o53’47’’S − 53o25’28’’W). Furthermore, it is in a forested and pristine area, without direct influence of agricultural, industrial and urban activities (Abril et al. 2022; Borges et al. 2022). In turn, the polluted site is located within the old mining area (30o52’55’’S − 53o27’11’’W). At the beginning of mining activities, a man-made dam was built to allow the formation of a water reservoir to meet the needs of these activities. This artificial dam is located in between the two sites. Therefore, it is expected that fish from the mining-impacted site have not migrated out of the polluted area and therefore have been exposed to contamination for several generations.
Maps indicating the location of the old copper-mining area (Minas do Camaquã district, Caçapava do Sul municipality, Rio Grande do Sul State, southern Brazil). (A) General map indicating (blue marker) the location of the old copper-mining area in the Brazilian territory. (B) Map indicating (blue marker) the location of the old copper-mining area in the Rio Grande do Sul State (southern Brazil). (C) Satellite view of the Minas do Camaquã (red marker) with the course of the João Dias creek (dotted line) along the old copper-mining area.
Borges et al. (2022) described the physicochemical properties of both sites. They reported no significant differences in water pH (R site: 5.08 ± 0.06, n = 10; P site: 5.02 ± 0.02, n = 10), dissolved oxygen content (R site: 7.07 ± 0.10 mg O2/L, n = 10; P site: 6.95 ± 0.11 mg O2/L, n = 10). These authors reported a slightly higher (2.2oC) mean water temperature in the polluted site (26.8 ± 0.37 °C, n = 10) than in the reference site (24.6 ± 0.39 °C, n = 10). In addition, no significant differences were reported in the concentrations of major cations (Ca, K, Na and Mg) and several trace-metals concentrations (Cd, Fe, Mn, Pb and Zn). However, the mean concentration of waterborne Cu was reported to be two-fold higher in polluted site (8.50 ± 0.76 µg/L, n = 10) than in the reference site (4.32 ± 0.68 µg/L, n = 10). In the present study, additional water samples were collected during the translocation experiment performed and analyzed for Cu concentration, following the same technique described by Borges et al. (2022). The new mean Cu concentration observed for samples from the reference and polluted sites corresponded to 2.89 ± 0.69 µg/L (n = 10) and 10.18 ± 1.57 µg/L (n = 10), respectively. It is worth noting that Brazilian freshwater quality criteria for Cu is 9 µg/L (CONAMA, 2005), i.e. lower than the mean concentration found in the polluted site.
Thirty individuals were collected at the reference site using a fish trap. They were randomly divided into three groups. The first group of fish (R fish; n = 10) was immediately anesthetized with benzocaine, quickly rinsed in MilliQ water, euthanized by spine cord sectioning and had their blood collected and their gills, brain, liver and muscle dissected. Blood samples were immediately prepared for the analysis of nuclear abnormalities in erythrocytes. In turn, the gills, brain, liver and muscle samples were stored in liquid nitrogen for analyses of the oxidative stress-related parameters. The second group of fish was kept caged at the reference site for 96 h (RC fish; n = 10). In turn, the third group of fish was translocated and kept caged at the polluted site for 96 h (R→P fish; n = 10). Five fish from each group were caged in the same device. Therefore, two cages were used for each experimental condition to ensure a stocking density non stressing for fish (Chakraborty et al. 2010; Koh et al. 2018). After 96 h, RC and R→P fish were anesthetized, quickly rinsed in MilliQ water, euthanized by spine cord sectioning and had their tissues (blood, gills, brain, liver and muscle) collected and prepared as described for R fish.
Thirty individuals were also collected at the polluted site. They were sampled, handled and tested as described above for fish collected at the reference site. The same experimental protocol was used, but vice versa. In this case, fish were translocated from the polluted site to the reference site.
No significant difference was observed in the mean (± standard deviation) body mass and total body length of fish collected at the reference site (body mass = 0.70 ± 0.11 g; total body length = 4.21 ± 0.58 cm; n = 30) and the polluted site (body mass = 0.65 ± 0.23; total body length = 4.32 ± 0.94 cm; n = 30). The sex of the fish was not determined. Therefore, female and male fish were likely evaluated. It is worth noting that the whole-body Cu burden (mean ± standard deviation) in fish from the reference site and the polluted site were reported to be 199.6 ± 39.3 µg/kg wet body mass and 535.8 ± 139.6 µg/kg wet body mass, respectively (Borges et al. 2022).
The cages used were made with PVC pipes and were surrounded by a fine mesh nylon net allowing for adequate water flow. They measured 50 cm x 50 cm x 50 cm (total volume = 125 L). Therefore, fish were stocked at a low density (< 0.1 g fish body mass/L), considered non stressing for them (Chakraborty et al. 2010; Koh et al. 2018). Cages were anchored in the middle of the stream’s water column with the aid of local stones and using ropes. All procedures performed in the present study were approved by the Committee for Ethics in Animal Use of the Federal University of Rio Grande (CEUA; protocol # 23116.001365/2015-44) and the Brazilian Ministry of Environment (MMA; research license # 44769-1).
Oxidative Stress Parameters
Damage to DNA was measured through the evaluation of erythrocyte nuclear abnormalities (micronucleus, buds, apoptotic fragments, binuclear, and bilobed) as described by Barsiene et al. (2006). Briefly, blood samples were collected using a 1-ml disposable syringe with 21 G needle to avoid damage to erythrocytes. All fish used in each experimental condition were sampled. Samples were transferred to siliconized tubes and centrifuged (1,000 rpm) for 5 min. After centrifugation, 50 µl of pellet was pipetted from the bottom of the tube. Erythrocytes were then gently dripped onto the side of a glass slide and then spread by smear using another glass slide. Three slides were prepared for each fish. Slides were dried at room temperature, fixed with Carnoy solution (3:1; methanol: acetic acid) for approximately 20 min, and dried again at room temperature. Slides were stained for 20 min with 2% Giemsa prepared in a phosphate buffered solution (Na2HPO4 + KH2PO4; pH 8.0; PBS). Slides were washed with deonized water, dried at air temperature and had coverslips adhered with a rapid mounting medium for microscopy (Entellan®, Merck, Darmstadt, Germany). Slides were examined under a light microscope integrated with a computerized system for image analysis (Carl Zeiss, Oberkochen, Germany). Micronucleated cells were identified and counted using the open source software ImageJ 1.48v (https://imagej.nih.gov/ij/). For each fish, the three slides prepared were examined at 1,000x magnification. On each slide, 1,000 erythrocytes were examined and the number of micronucleated cells was counted. Results are expressed as percentage (%) of the total number of cells analyzed.
Total antioxidant capacity (TAC) was measured in the gills, brain, liver and muscle samples of all fish tested in each experimental condition. It was measured using the OxiSelect™ Total Antioxidant Capacity Assay Kit (Cell Biolabs, San Diego, CA, USA). According to the manufacturer, the kit measures the sample total antioxidant capacity by comparing to a known concentration of uric acid standard within a 96-well microtiter plate format. Fish tissues were sonicated (Qsonica Model CL-188, Newtown, CT, USA) in cold PBS. Sample homogenates and standards were diluted with a reaction reagent and, upon the addition of copper, the reaction proceeded for a few minutes. The reaction was stopped and read with a standard 96-well spectrophotometric microplate reader at 490 nm (ELx808IU, BioTek Instruments, Winooski, VT, USA). Antioxidant capacity was determined by comparison with the standard curve prepared with uric acid standard solutions. The protein content in sample homogenates was determined based on the Bradford method (Sigma-Aldrich, St. Louis, MO, USA). Data are expressed as µM copper reducing equivalents (CRE)/mg total protein.
Oxidative damage to lipids (lipoperoxidation; LPO) was measured in the gills, brain, liver and muscle samples of all fish tested in each experimental condition. It was measured as described by Oakes and Van Der Kraak (2003). Briefly, the method used measures the generation of malondialdehyde (MDA), a metabolite derived from polyunsaturated fatty acids oxidation. Fish samples were sonicated (Qsonica Model CL-188, Newtown, CT, USA) in a cold buffer solution (1.15% KCl and 35 µM butylated hydroxytoluene). LPO concentration in the sample homogenate was determined by fluorimetry (excitation: 520 nm; emission: 580 nm) using a spectrofluorometer (Victor, Perkin-Elmer, Waltham, MA, USA) in the presence of thiobarbituric acid (Baker Analyzed®; Baker Chemical, Phillipsburg, NJ, USA). Results were calculated based on a calibration curve prepared with MDA standard solutions at different concentrations (1.56, 0.78, 0.39, 0.19, 0.09, 0.04, 0.02 and 0.01 nmol). The protein content in sample homogenates was determined based on the Bradford method (Sigma-Aldrich, St. Louis, MO, USA). Data are expressed as nmol malondialdehyde (MDA)/mg total protein.
Oxidative damage to proteins (protein carbonylation, PCN) was measured in the gills, brain, liver and muscle samples of all fish tested in each experimental condition. It was analyzed using a commercial reagent kit (Protein Carbonyl Elisa kit, Cell Biolabs, San Diego, CA, USA), following the procedures described by the manufacturer. Fish samples were sonicated (Qsonica Model CL-188, Newtown, CT, USA) in cold PBS. Bovine serum albumin (BSA) as standard solutions or the protein of homogenate samples (10 µg/mL) were adsorbed onto a 96-well plate for 2 h at 37ºC. The protein carbonyls present in the sample homogenates or standards were derivatized to dinitrophenyl (DNP) hydrazone and probed with an anti-DNP antibody, followed by a horseradish peroxidase (HRP) conjugated secondary antibody. The sample protein carbonyl content was determined using a standard curve built with solutions containing different concentrations of reduced and oxidized BSA. Total protein content in sample homogenates was determined using a commercial reagent kit based on the Bradford method (Sigma-Aldrich, St. Louis, MO, USA). Results are expressed as nmol protein carbonyl/mg total protein.
Data Presentation and Statistical Analysis
Data for oxidative stress parameters analyzed are expressed as mean ± standard error (n = 10). The mean values for each parameter analyzed were compared using factorial analysis of variance (ANOVA). Data on erythrocyte nuclear abnormalities were mathematically transformed (arcsin-square-root) before analysis. Data on TAC, LPO and PCN were log transformed before analysis. ANOVA assumptions were verified using the normal probability plot for raw residuals (data normality) and the Cochran C test (homogeneity of variances). To compare the oxidative profile/status between R and P fish, data for each parameter were subjected to two-way (fish collection site and tissue) ANOVA. To evaluate the effect of translocation on each fish tissue, data were also subjected to two-way (fish collection site and translocation) ANOVA. When the factor was significant, therefore a post hoc test (Tukey test) was performed. In all cases, the significance level adopted was 95% (α = 0.05).
Results and Discussion
When fish tissues were compared, similar profiles of TAC, LPO and PCN were observed in fish collected in the reference and polluted sites (Fig. 2). Indeed, the ANOVA results indicated a significant effect of tissue for TAC and LPO, and a lack of significant effect of factors for PCN. For both groups of fish, brain and liver showed higher levels of TAC than gills and muscle (Fig. 2A). Regarding oxidative damage to lipids, the gills and liver showed higher levels of LPO than the brain and muscle (Fig. 2B). The higher total antioxidant capacity (TAC) observed in the brain of H. luetkenii living in the reference or the polluted site of the João Dias creek can be explained considering the need for a protection of the high lipid content present in the nervous tissue against oxidative damage. This is related to the proper state of brain functions associated with life and lifespan (Sanz et al. 2013). Indeed, the lower level of oxidative damage to lipids (LPO) observed in brain respect with gills and liver support this idea. In turn, the higher TAC observed in liver of H. luetkenii living in the reference or the polluted site of the João Dias creek can be explained considering the central role of this organ in fish metabolism and its consequently high susceptibility to oxidative damage. Indeed, the liver has been reported to be one of the organs showing the highest antioxidant activity in both herbivorous and omnivorous freshwater fishes (Radi et al. 1985, 1986). In turn, the higher level of damage to lipids (LPO) observed in liver and gills respect with other tissues of H. luetkenii living in the reference or the polluted site (P fish) can be explained considering the fact that these organs are the major targets for multiple environmental stressors (Gusso-Choueri et al. 2015).
Total antioxidant capacity (A), lipid peroxidation (B) and protein carbonylation (C) in tissues (gills, brain, liver and muscle) of the freshwater fish Hyphessobrycon luetkenii collected at the reference (R fish) and polluted (P fish) sites. Data are mean ± standard error (n = 10). Different letters indicate significant differences among fish collection sites and tissues (two-way ANOVA; Tukey test; P < 0.05)
Protein oxidation (PCN) is considered a natural consequence of aerobic life. In turn, the antioxidant defense system plays an essential role in the degradation of the non-functional (oxidized) proteins. In this case, oxidized cytosolic proteins are removed and degraded by the proteasomal system, and replaced by functional proteins newly synthesized by cells (Møller et al. 2011). Although the turnover rate of nitrogen, and consequently protein synthesis, is relatively slow and tissue-specific in freshwater fish (Fauconneau and Arnal 1985; Houlihan et al. 1993; Smith and Houlihan 1995; Busst and Britton 2018), the different tissues of fish living in the reference or the polluted site showed similar levels of protein carbonylation (Fig. 2C). This finding indicates that H. luetkenii living in the reference or the polluted site of the João Dias creek have adjusted, in the long term, to a common level of oxidative damage to proteins in all tissues.
No significant differences were observed between individuals freely living in the reference site and those kept caged in this site for all parameters analyzed. Additionally, no significant differences were observed between individuals freely living in the polluted site and those translocated to the reference site (Table 1: erythrocyte nuclear abnormalities; Fig. 3: TAC; Fig. 4: LPO; and Fig. 5: PCN). These findings indicate that all significant differences observed between individuals from the reference and polluted sites, individuals from the reference site and those translocated to the polluted site, as well as individuals from the polluted site and those translocated to the reference site can be solely attributed to environmental factors and not to possible stress-related effects associated with caging.
Total antioxidant capacity in the gills (A), brain (B), liver (C) and muscle (D) of the fish Hyphessobrycon luetkenii collected at the reference site (R fish), kept caged at this site (RC fish) or translocated to the polluted site (R→P fish) for 96 h. Also, fish were collected at the polluted site (P fish), kept caged at this site (PC fish) or translocated to the reference site (P→R fish) for 96 h. Data are mean ± standard error (n = 10). Different letters indicate significant differences among fish groups (collection site and translocation; two-way ANOVA; Tukey test; P < 0.05)
Lipid peroxidation level in the gills (A), brain (B), liver (C) and muscle (D) of the freshwater fish Hyphessobrycon luetkenii collected at the reference site (R fish), kept caged at this site (RC fish) or translocated to the polluted site (R→P fish) for 96 h. Also, fish were collected at the polluted site (P fish), kept caged at this site (PC fish) or translocated to the reference site (P→R fish) for 96 h. Data are mean ± standard error (n = 10). Different letters indicate significant differences among fish groups (collection site and translocation; two-way ANOVA; Tukey test; P < 0.05)
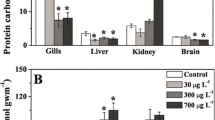
Protein carbonyl concentration in the gills (A), brain (B), liver (C) and muscle (D) of the freshwater fish Hyphessobrycon luetkenii collected at the reference site (R fish), kept caged at this site (RC fish) or translocated to the polluted site (R→P fish) for 96 h. Also, fish were collected at the polluted site (P fish), kept caged at this site (PC fish) or translocated to the reference site (P→R fish) for 96 h. Data are mean ± standard error (n = 10). Different letters indicate significant differences among fish groups (collection site and translocation; two-way ANOVA followed by the Tukey test; P < 0.05)
Similar levels of TAC were observed in individuals from the reference and the polluted site for all tissues analyzed (Fig. 3). Also, no significant difference was observed for erythrocyte nuclear abnormalities between these two groups of fish (Table 1). Furthermore, similar levels of LPO (Fig. 4) and PCN (Fig. 5) were observed in individuals from the reference and the polluted sites for all tissues analyzed. Taken altogether, these findings provide good evidence that tissue (blood cells, brain, liver and muscle) antioxidant systems of H. luetkenii living in the polluted stretch of the João Dias creek (P fish) were able to counteract, and adjust in the long term, to possibly increased oxidative stress associated with the higher level of whole-body Cu accumulation previously reported for this fish group (Borges et al. 2022).
Let us now to consider the responses observed in parameters related to oxidative stress in fish subjected to the translocation experiment. The ANOVA results indicated a significant effect of the interaction between site and translocation in muscle TAC. Indeed, no significant change was observed in the level of TAC in all tissues of H. luetkenii translocated from the reference stretch to the polluted stretch of the João Dias creek (Fig. 3). A similar result was observed for erythrocyte nuclear abnormalities (Table 1). On the other hand, the ANOVA results indicated a significant effect of site, translocation and the interaction between these two factors in gill and brain LPO. ANOVA also indicated a significant effect of translocation in liver and muscle LPO. In fact, an increased oxidative damage to lipids (LPO) was observed in all tissues analyzed in individuals translocated to the polluted site (Fig. 4). This finding can be explained considering the greater degree of oxidative stress induced by an increased level of Cu accumulated in tissues of fish translocated to the polluted site. Although data on Cu accumulation in the tissues of fish translocated to the polluted stretch of the João Dias creek are not available, an increased whole-body Cu burden was previously reported for this fish group (Borges et al. 2022). Increased whole-body Cu accumulation would imply in an increased amount of Cu accumulated in fish tissues. In fact, Cu is reported to rapidly (96 h) accumulate in freshwater fish after exposure to waterborne Cu at concentrations close to 9 µg/L (Borges et al. 2022; Zebral et al. 2019).
It is interesting to consider that carbonyl groups can be introduced in proteins by several different pathways. Metal-catalyzed oxidation, via ROS, is the predominant pathway. However, these groups can also be introduced in proteins via the adduction of oxidized lipids containing carbonyls (Møller et al. 2011). Therefore, higher levels of LPO could lead to higher levels of PCN. However, the increased LPO observed in the tissues of fish translocated to the polluted site was paralleled by a lack of change in PCN in all tissues analyzed (Fig. 5). In fact, ANOVA results indicated a lack of significant effect of factors tested and their interaction on PCN. This finding suggests that the major pathway of protein carbonylation in tissues of fish translocated to the polluted site is associated with metal-catalyzed oxidation via ROS.
The lack of full protection provided by the antioxidant defense system against ROS, indicated by the increased LPO in fish translocated to the polluted site (Fig. 4), points to the susceptibility of fish living in the reference stretch of the João Dias creek to oxidative damage associated with short-term exposure (96 h) to the higher Cu concentration found in the mining-impacted stretch of this creek (Abril et al. 2018a, b; Borges et al. 2022). This even indicate that the H. luetkenii spatial distribution in the João Dias creek may be hampered by the different levels of water Cu contamination in the different portions of this creek, at least in the short term. The results from the comparison of oxidative parameters between individuals translocated to the polluted site (acutely exposed) and those living in the polluted site (chronically exposed) indicate that H. luetkenii is capable of long-term adjustment of its antioxidant defense system to cope with a high level of Cu contamination, as observed in the mining-impacted stretch of the João Dias creek (Abril et al. 2018a, b; Borges et al. 2022). This idea is supported by the much lower levels of LPO observed in all tissues of individuals living in the polluted site respect with those of individuals translocated to the polluted site (Fig. 4).
Another response observed in H. luetkenii was associated with changes in parameters related to oxidative stress after the translocation of fish from the polluted site to the reference site. Similar levels of TAC were observed in all tissues of individuals translocated to the reference site and those living in the reference or the polluted site (Fig. 3). Therefore, as observed for individuals translocated to the polluted site, a lack of change in TAC was also observed in fish translocated to the reference site. This lack of change was paralleled by no significant change in nuclear abnormalities in erythrocytes (Table 1), PCN in all tissues (Fig. 5), as well as LPO in the gills (Fig. 4A) and brain (Fig. 4B). However, LPO levels in the liver (Fig. 4C) and muscle (Fig. 4D) were increased 96 h after fish translocation to the reference site. This finding can be explained considering the increase in oxidative stress induced by an also increased accumulation of Cu in these tissues, associated with the remobilization of this metal from other tissues during the translocation experiment, as discussed below.
Over a 72-h period of the experiment, the mean Cu concentration in the gills of H. luetkenii translocated from the polluted site to the reference site was only 55% of that observed in the gills of H. luetkenii collected and kept caged in the polluted stretch of the João Dias creek (Abril et al. 2018a). Also, Cu was shown to be reduced in the gills and brain of the freshwater fish Tilapia zilli after a 1-day or 7-day elimination period (Kalay and Canli 2000). On the other hand, over a 72-h period of the experiment with H. luetkenii, the mean Cu concentration was 1.4-fold higher in the muscle of individuals translocated to the reference site than in those translocated to the polluted site (Abril et al. 2018a). Increases in Cu concentrations were also observed in the liver of the freshwater fish Tilapia zilli after a 7-day elimination period (Kalay and Canli 2000). Altogether, these findings indicate that H. luetkenii accumulated more Cu in the liver and muscle and eliminated this metal from the gills and brain during the translocation from the polluted stretch to the reference stretch of the João Dias creek. A higher Cu accumulation in the liver and muscle would lead to increased oxidative stress, thus explaining the higher levels of LPO observed in these tissues in individuals translocated to the reference site than in those living in the polluted site.
The comparison of oxidative parameters data between fish translocated to the reference site (acutely exposed) and those living in the reference site (chronically exposed) indicate that the short-term adjustment of the antioxidant defense system to cope with the lower level of Cu contamination observed in the non-mining impacted stretch of the João Dias creek (Abril et al. 2018a, b; Borges et al. 2022) was tissue-specific. This idea is supported by the different responses in LPO showed in the gills and brain compared to those displayed in the liver and muscle. The gills (Fig. 4A) and brain (Fig. 4B) of individuals translocated to the reference site and those living in this site showed a similar LPO level. However, the LPO level was higher in the liver (Fig. 4C) and muscle (Fig. 4D) of individuals translocated to the reference site than in those living in this site. Furthermore, the PCN level was higher in the gills of individuals translocated to the reference site than in those living in this site (Fig. 5A). The tissue-specific response observed for LPO in individuals translocated to the reference site can be explained by different levels of tissue Cu accumulation, as already discussed above. In turn, the tissue-specific response of PCN can be explained considering that the turnover rate of nitrogen is also tissue-specific in freshwater fish, and is faster in more metabolically active tissues (Busst and Britton 2018). In fact, rates of protein synthesis in fish tissues are closely correlated with their synthetic capacity (Houlihan et al. 1993), which in turn is reflected by their oxygen consumption rate (Smith and Houlihan 1995). Therefore, the higher levels of PCN observed in the muscle of individuals translocated to the reference site than in those living in this site can be explained considering a combination of the greater degree of oxidative damage to proteins induced by the higher level of Cu accumulated in this tissue with a lower rate of protein synthesis in muscle with respect to the other analyzed tissues (Buchheister and Latour 2010; Xia et al. 2013; Matley et al. 2016). Therefore, it is possible that the time of exposure (96 h) of P fish to the non-mining impacted stretch of the João Dias creek was not enough for a significant reduction in the PCN level in the muscle of individuals translocated to the reference stretch of this creek. The fact that the nitrogen turnover rate, and consequently protein synthesis, is relatively slow in freshwater fish tissues, especially muscle (Fauconneau and Arnal 1985; Buchheister and Latour 2010; Xia et al. 2013; Matley et al. 2016; Busst and Britton 2018), reinforces this idea. Indeed, the half-life of isotopic nitrogen in the muscle of three different fish species was estimated to be 68 days (Xia et al. 2013), 85 days (Buchheister and Latour 2010), and 126 days (Matley et al. 2016). In fact, adjustments to behavioral and fitness impacts associated with fish translocation is shown to occur over a period of several months (Monk et al. 2020). In turn, a 96-h period of translocation was adopted in the present study to represent a short-term exposure to waterborne Cu, as indicated by the standard guide for conducting acute toxicity tests on test materials with fishes (ASTM 2014). Therefore, results gathered here can be directly compared with previous and future studies. It is important to note that longer experimental periods could intensify the possible effects of other factors related to fish captivity, such as food availability and competition-related stress.
Altogether, the findings reported in the present study indicate that wild populations of the freshwater fish H. luetkenii living in non-mining impacted and mining impacted stretches of the João Dias creek have similar profiles of tissue (blood, gills, brain, liver and muscle) oxidative status. This lack of differential response between fish groups is evidence of a long-term acclimatization process, which reinforces the ability of H. luetkenii to survive and reproduce in a metal-stressed environment, such as the old mining area of Minas do Camaquã. Interestingly, H. luetkenii living in the polluted site were able to maintain tissue levels of damage to DNA, lipid and protein without altering the total antioxidant capacity, despite their higher whole-body Cu burden. Indeed, our findings indicate that H. luetkenii living in the mining impacted stretch of the João Dias creek display a long-term adjustment in terms of tissue lipid oxidation. In turn, fish living in the non-mining impacted stretch of this creek displayed a short-term adjustment of tissue lipid and protein oxidation, except in muscle. In this case, a long-term adjustment in the level of protein oxidation was observed. The findings reported here provide key insights and a better understanding of Cu toxicology in freshwater fish exposed for several generations to elevated concentrations of Cu in the field.
References
Abril SIM, Altmüller J, Bianchini A, Nolte AW (2022) De novo transcriptome of the freshwater fish Hyphessobrycon luetkenii: Differential Gene expression in aggregates inhabiting an environment historically impacted by copper mining. Environ Toxicol Chem under review
Abril SIM, Costa PG, Bianchini A (2018a) Metal accumulation and expression of genes encoding for metallothionein and copper transporters in a chronically exposed wild population of the fish Hyphessobrycon luetkenii. Comp Biochem Physiol Part C 211:25–31. https://doi.org/10.1016/j.cbpc.2018.05.008
Abril SIM, Dalmolin C, Costa PG, Bianchini A (2018b) Expression of genes related to metal metabolism in the freshwater fish Hyphessobrycon luetkenii living in a historically contaminated area associated with copper mining. Environ Toxicol Pharmacol 60:146–156. https://doi.org/10.1016/j.etap.2018.04.019
ASTM (2014) Standard Guide for Conducting Acute Toxicity Tests on Test Materials with Fishes, Macroinvertebrates, and Amphibians. American Society for Testing and Materials. ASTM E729-96(2014), West Conshohocken, PA, USA
Barsiene J, Dedonyte V, Rybakovas A, Andreikenaite L, Andersen OK (2006) Investigation of micronuclei and other nuclear abnormalities in peripheral blood and kidney of marine fish treated with crude oil. Aquat Toxicol 78:99–104. https://doi.org/10.1016/j.aquatox.2006.02.022
Borges VD, Zebral YD, Costa PG, Fonseca JS, Klein RD, Bianchini A (2022) Metal accumulation and ion regulation in the fish Hyphessobrycon luetkenii living in a site chronically contaminated by copper: insights from translocation experiments. Arch Environ Contam Toxicol 82:62–71. https://doi.org/10.1007/s00244-021-00895-3
Buchheister A, Latour RJ (2010) Turnover and fractionation of carbon and nitrogen stable isotopes in tissues of a migratory coastal predator, summer flounder (Paralichthys dentatus). Can J Fish Aquat Sci 67:445–461. https://doi.org/10.1139/F09-196
Busst GMA, Britton JR (2018) Tissue-specific turnover rates of the nitrogen stable isotope as functions of time and growth in a cyprinid fish. Hydrobiologia 805:49–60. https://doi.org/10.1007/s10750-017-3276-2
Chakraborty SB, Mazumdar D, Banerjee S (2010) Determination of ideal stocking density for cage culture of monosex Nile tilapia (Oreochromis niloticus) in India. Proc Zool Soc 63:53–59. https://doi.org/10.1007/s12595-010-0007-3
CONAMA (2005) Conselho Nacional do Meio Ambiente, Resolução nº 357, Brasília, DF, Brazil. http://conama.mma.gov.br/?option=com_sisconama&task=arquivo.download&id=450
Fauconneau B, Arnal M (1985) In vivo protein synthesis in different tissues and the whole body of rainbow trout (Salmo gairdnerii R.). Influence of environmental temperature. Comp Biochem Physiol A Comp Physiol 82:179–187. https://doi.org/10.1016/0300-9629(85)90723-6
Grosell M (2011) Copper. In: Wood CM, Farrell T, Brauner CJ (eds) Homeostasis and toxicology of essential Metals, Fish Physiology, vol 31A. Academic Press, San Diego, pp 53–133. https://doi.org/10.1016/S1546-5098(11)31002-3
Gusso-Choueri PK, Choueri RB, Araújo GS, Cruz ACF, Stremel T, Campos S, Abessa DMS, Ribeiro CAO (2015) Assessing pollution in marine protected areas: the role of a multi-biomarker and multi-organ approach. Environ Sci Pollut Res 22:18047–18065. https://doi.org/10.1007/s11356-015-4911-y
Haverroth GM, Welang C, Mocelin RN, Postay D, Bertoncello KT, Franscescon F, Rosemberg DB, Dal Magro J, Dalla Corte CL (2015) Copper acutely impairs behavioral function and muscle acetylcholinesterase activity in zebrafish (Danio rerio). Ecotoxicol Environ Saf 122:440–447. https://doi.org/10.1016/j.ecoenv.2015.09.012
Houlihan DF, Mathers EM, Foster A (1993) Biochemical correlates of growth rate in fish. In: Rankin JC, Jensen FB (eds) Fish Ecophysiology. Chapman & Hall Fish and Fisheries Series, vol 9. Springer, Dordrecht. https://doi.org/10.1007/978-94-011-2304-4_2
Kalay M, Canli M (2000) Elimination of essential (Cu, Zn) and non-essential (Cd, pb) metals from tissues of a freshwater fish Tilapia zilli. Turk J Zool 24:429–436
Koh HL, The SY, Lee E, Tan WK, Sagathevan KA, Low AA (2018) Derivation of optimal fish stocking density via simulation of water quality model E2Algae. AIP Conference Proceedings 1974, 020042. https://doi.org/10.1063/1.5041573
Malhotra N, Ger TR, Uapipatanakul B, Huang JC, Chen KH, Hsiao CD (2020) Review of copper and copper nanoparticle toxicity in fish. Nanomaterials 10:1126. https://doi.org/10.3390/nano10061126
Matley JK, Fisk AT, Tobin AJ, Heupel MR, Simpfendorfer CA (2016) Diet-tissue discrimination factors and turnover of carbon and nitrogen stable isotopes in tissues of an adult predatory coral reef fish, Plectropomus leopardus. Rapid Commun Mass Spectrom 30:29–44. https://doi.org/10.1002/rcm.7406
Møller IM, Rogowska-Wrzesinska A, Rao RS (2011) Protein carbonylation and metal-catalyzed protein oxidation in a cellular perspective. J Proteom 74:2228–2242. https://doi.org/10.1016/j.jprot.2011.05.004
Monk CT, Chéret B, Czapla P, Hühn D, Klefoth T, Eschbach E, Hagemann R, Arlinghaus R (2020) Behavioural and fitness effects of translocation to a novel environment: whole-lake experiments in two aquatic top predators. J Anim Ecol 89:2325–2344. https://doi.org/10.1111/1365-2656.13298
Oakes KD, Van De Kraak GJ (2003) Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat Toxicol 63:447–463. https://doi.org/10.1016/s0166-445x(02)00204-7
Radi AA, Hai DQ, Matkovics B (1986) Comparative antioxidant enzyme study in freshwater fishes. II. Distribution of antioxidant enzymes and lipid peroxidation in omnivorous fish organs. Acta Biol Hung 37:135–141
Radi AA, Hay DQ, Gabrielak T, Matkovics B (1985) Comparative antioxidant enzyme study in freshwater fishes. I. distribution of superoxide dismutase, peroxide-decomposing enzymes and lipid peroxidation in herbivorous fishes. Acta Biol Hung 36:169–174
Sanz A, Trenzado CE, Botello Castro H, López-Rodríguez MJ, Tierno de Figueroa JM (2013) Relationship between brain and liver oxidative state and maximum lifespan potential of different fish species. Comp Biochem Physiol A Mol Integr Physiol 165:358–364. https://doi.org/10.1016/j.cbpa.2013.04.019
Simonato JD, Mela M, Doria HB, Guiloski IC, Randi MAF, Carvalho PSM, Meletti PC, Silva de Assis HC, Bianchini A, Martinez CBR (2016) Biomarkers of waterborne copper exposure in the neotropical fish Prochilodus lineatus. Aquat Toxicol 170:31–41
Smith RW, Houlihan DF (1995) Protein synthesis and oxygen consumption in fish cells. J Comp Physiol B 165:93–101. https://doi.org/10.1007/BF00301473
Sung J, Lee J, Lee S (2020) Exposure to copper (II) chloride induces behavioral and endocrine changes in zebrafish. J Life Sci 30:321–330. https://doi.org/10.5352/JLS.2020.30.4.321
Tavares-Dias M (2021) Toxic, physiological, histomorphological, growth performance and antiparasitic effects of copper sulphate in fish aquaculture. Aquaculture 535:736350. https://doi.org/10.1016/j.aquaculture.2021.736350
Xia B, Gao QF, Dong SL, Wang F (2013) Turnover and fractionation of nitrogen stable isotope in tissues of grass carp Ctenopharyngodon idellus. Aquac Environ Interact 3:177–186. https://doi.org/10.3354/aei00061
Zebral YD, Anni ISA, Afonso SB, Abril SIM, Klein RD, Bianchini A (2018) Effects of life-time exposure to waterborne copper on the somatotropic axis of the viviparous fish Poecilia vivipara. Chemosphere 203:410–417. https://doi.org/10.1016/j.chemosphere.2018.03.202
Zebral YD, Roza M, Fonseca JS, Costa PG, Oliveira CS, Zocke TG, Dal Pizzol JL, Robaldo RB, Bianchini A (2019) Waterborne copper is more toxic to the killifish Poecilia vivipara in elevated temperatures: linking oxidative stress in the liver with reduced organismal thermal performance. Aquat Toxicol 209:142–149. https://doi.org/10.1016/j.aquatox.2019.02.005
Acknowledgements
Thanks to Dr. Sandra I. M. Abril for her help in the field experiment. The present study was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Instituto Nacional de Ciência e Tecnologia de Toxicologia Aquática, Brasília, DF, Brazil. grant #573949/2008-5) and the International Development Research Centre (IDRC, Ottawa, ON, Canada, grant #104519-027). A. Bianchini (Proc. # 311410/2021-9) is a research fellow from the Brazilian CNPq and was supported by the International Canada Research Chair Program (IDRC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Borges, V.D., Zebral, Y.D., Costa, P.G. et al. Oxidative Stress in a Wild Population of the Freshwater Fish Hyphessobrycon Luetkenii Chronically Exposed to a Copper Mining Area: New Insights into Copper Toxicology. Bull Environ Contam Toxicol 110, 77 (2023). https://doi.org/10.1007/s00128-023-03721-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00128-023-03721-9