Abstract
The toxicity of high-chlorinated polychlorinated biphenyls (PCBs) can be efficiently reduced through anaerobic dechlorination. However, this approach suffers a lot in face of in situ microbial remediations, like a shortage of biomass. In this study, we showed that the amendment of organic matters could help microbiota in paddy soil with anaerobic dechlorination and greatly shortened the lag period. The presence of organic matters offered a better environment for dechlorinating bacteria. They provided not only a more strictly anaerobic milieu but also copious carbon sources. By using high-throughput 16S rRNA gene sequencing, genera Dehalobacter, Dehalobacterium, and Desulfitobacterium capable of dechlorination were identified in enriched cultures. Taken together, this study proved that extra organic matters can promote anaerobic dechlorination in paddy soil slurry microcosm systems, which provides new insights into the bioremediation of PCB-contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polychlorinated biphenyls (PCBs) are a family of 209 congeners, with six hydrogen atoms alternatively replaced by chlorine atoms in different positions. Since their excellent property in heat resistance, chemical inertness, and high dielectric constant, PCBs had been widely used in industrial production as dielectrics, hydraulic fluids, lubricants and adhesives (Jahnke and Hornbuckle 2019). However, it has been extensively reported that PCBs can negatively cause neurological, reproductive, endocrine, and cutaneous diseases, indicating that the presence of PCBs may pose long-term public health and ecosystem risks (Cogliano 1998; Beyer and Biziuk 2009). Although PCB production was banned in the late 1970s, PCBs still persist in soil, water, and air, becoming an environmental issue worldwide (Zhu et al. 2022).
PCBs in contaminated soils and sediments usually show a low level. In this case, bioremediation is a cost-effective and environmentally friendly approach. With the help of microorganisms, while PCBs with fewer chlorine atoms can be aerobically metabolized, those with more could only be degraded by reductive dechlorination, followed by aerobic mineralization of lower-chlorinated products (Field and Sierra-Alvarez 2008). To date, dozens of strains that can utilize PCBs as a carbon source or energy source have been obtained and reported (Xiang et al. 2020). They performed quite well on the lab scale, suggesting their potential for remediation of contaminated sites (Abraham et al. 2002).
Given the nutritional and environmental conditions required for dechlorinating bacteria, constructing an effective in situ dechlorinating microbial community faces many challenges. It was reported that the commercial PCB mixture Aroclor1260 could be dechlorinated in mixed cultures only when the cell density exceeds a threshold value (Bedard et al. 2007). Indeed, dechlorinating bacteria face energy limitations in most environments, where PCBs are often in low concentrations. These places are generally unable to provide sufficient biomass for dechlorination (Yan et al. 2012). In the natural environment, microbes utilizing electron acceptors like nitrate and sulfate may become dominant over dechlorinating bacteria. Therefore, in situ microbial remediations for PCB-contaminated soils still requires further investigation.
The dechlorination of PCBs requires a low redox potential. As the largest artificial wetland in Asia, paddy fields could provide favorable conditions for dechlorination. Our previous study depicted the role of paddy fields as natural sequential anaerobic-aerobic bioreactors for the transformation of PCBs, as well as its superiority for PCBs dechlorination under redox conditions (Chen et al. 2014a, b). Besides, it was reported that PCB-degrading activity had been successfully maintained for more than 3 years in a static flooded soil medium from Japanese paddy soil (Baba et al. 2007). Hence, research on the dechlorination of PCBs in paddy soil and other natural anaerobic wetlands owns practical significance.
The object of this study is to explore the effects of organic matters in the dechlorination process in a paddy soil slurry microcosm system. By adding extra organic matters, we concurrently investigated the dechlorination of PCBs and the mineralization of organic matters in paddy soil. Eh and pH were measured, and the dechlorination process was monitored by the changes in the concentration of H2, Cl- and PCBs. Moreover, variations in the structure of microbial communities under methanogenic conditions were also analyzed by using high-throughput 16S rRNA gene sequencing. Our study not only proves the important role of organic matters in PCB dechlorination but also provides a theoretical basis for the optimization of in situ microbial remediations.
Materials and methods
Anaerobic dechlorination of Aroclor1260
The paddy soil for the experiment was collected from a PCB-contaminated paddy field in Taizhou, Zhejiang, China, where a large amount of electronic waste was recycled nearby. The soil was firstly sorted as silty clay for use. To explore the natural dechlorination of paddy soil, a slurry reactor was constructed as the following: 250 g paddy soil, 70 g organic fertilizer and 600 mL ultrapure water were added into a 1000 mL brown jar, which was sealed and cultivated at 30℃. The experiment consisted of three groups: group A went without the addition of Aroclor1260 and sterilization; group B was added with Aroclor1260 (AccuStandard Inc., USA) to a final concentration of 40 mg/kg; the control group CK was added with the same amount of Aroclor1260 but sterilized.
Anaerobic dechlorination of PCB116 and PCB153
To investigate the effects of organic matters on the dechlorination process, 15 g of air-dried soil was added with a mixture of PCB116 and PCB153 (100 mg/L, dissolved in the chromatographically pure acetone) to create the PCB-contaminated soil in which the concentration of PCBs was 100 mg/kg. The paddy soil slurry reactor initially consisted of 300 g of paddy soil and 150 mL of oxygen-free water. For different treatments, 15 g of PCB-contaminated soils or 25 g of organic fertilizer (organic matter content: 25%) was added, respectively. The experiment consisted of four treatments: (i) paddy soil slurry with PCBs only (PCBs-OM), (ii) with nothing (CK), (iii) with PCBs and organic matters (PCBs + OM), and (iv) with organic matter only (OM). The microcosm systems were buffered to pH 7.0, sealed and cultivated under magnetic stirring at 30℃. Samples for further experiments were collected through the sampling tube, freeze-dried and stored at 4℃.
Extraction and determination of PCBs
For paddy soil slurry samples, PCBs in 3.0 g dried soil was ultrasonically extracted with hexane/acetone (1:1, v/v) for 0.5 h. Water, organic compounds and sulfur were removed by sequential addition of anhydrous sodium sulfate, florisil silicon, concentrated sulfuric acid and copper piece. Qualitative and quantitative analyses of PCB congeners were carried out on 7890 A-5975 C GC-MS (Agilent Technologies Inc., USA) equipped with an HP-5 capillary column. The temperature of the injector and the ion source was 270℃ and 230℃, respectively. The temperature program used was set as the following: 2 min at 50℃, increase at 20℃/min to 200℃ and hold for 2 min, increase at 5℃/min to 240℃ and hold for 2 min, and finally increase at 3℃/min to 290 ℃ and hold for 15 min. For the Aroclor1260 analysis, nine mixed standard samples (AccuStandard Inc., USA) containing 209 congeners (0.1 mg/L for each) were used to identify and quantify the PCB congeners. Each congener was scanned by GC-MS to identify retention times, fragment ions and the most abundant ions. All procedures were rigorously monitored to meet the USEPA requirements.
Quantification of H2 and chloride
The H2 concentration in the paddy soil slurry system was determined by hydrogen microelectrode (Unisense Technologies Inc., Denmark) through the reserved microelectrode channel under the manufacturer’s instruction. Besides, the chloride ion concentration was determined via Dionex ICS-1100 ion chromatography (Dionex Inc., USA) using external standards (R2 = 0.999).
Fourier transform infrared (FT-IR) test
For the detection of the functional groups of organic matters, FT-IR spectra were recorded using an IR Prestige-21 FT-IR Spectrometer (Shimadzu Inc., Japan) on KBr pellets. KBr pellets were obtained by pressing uniformly prepared mixtures of 1 mg sample and 200 mg KBr (spectrometry grade). N2 was filled during the spectra recording. The range of the scanned wavenumbers was set as 400 cm-1 to 4000 cm-1, and the resolution was set as 4 cm-1. At least two KBr pellets of each sample were prepared and determined in parallel.
Enrichment cultivation
The mineral medium for enrichment culture contained the following gradients per liter: 3 g NH4HCO3, 0.18 g NH4Cl, 0.9 g NaCl, 0.45 g K2HPO4, 0.45 g KH2PO4, 1 g yeast powder, 0.17 g MgCl2·6H2O, 0.12 g CaCl2·2H2O, 0.3 g L-Cysteine hydrochloride, 0.3 g Na2S·9H2O, 1 mL trace element solution (20 g FeCl3·6H2O, 6 g MnCl2·5H2O, 0.1 g H3BO4, 0.08 g CuCl2·2H2O, 1 g CoCl2·6H2O, 0.08 g ZnCl2, 0.1 g Na2MoO4·2H2O per liter) and 1 mL vitamin solution (10 mg p-aminobenzoic acid, 50 mg riboflavin, 50 mg biotin, 50 mg niacin, 20 mg folic acid, 100 mg pyridoxine hydrochloride, 5 mg cobalamin and 50 mg thiamine per liter). For the initial microcosms, serum bottles were supplemented with 50 mL medium, acetate (100 mM), Aroclor1260 (4 mg/L) and 5 g of paddy soil, and incubated with an active methanogenic enrichment culture at 10% (v/v). For following enrichment cultures, serum bottles were supplemented with 20 mL medium, 0.5 g paddy soil, Aroclor1260 (30 mg/L), and 2,6-dibromobiphenyl (30 mg/L). Initial microcosms were transferred to fresh enrichment cultures at 20% (v/v) after 60 days of cultivation. Subsequently, the following cultivation was carried out on a cycle of approximately 60 days. A control group (CK) without acetate was set. All cultures were buffered to pH 7.0 and incubated in dark at 30℃.
16S rRNA gene high-throughput sequencing
To explore the microbial composition of enrichment cultures, high-throughput 16S rRNA gene sequencing was conducted. Enrichment cultures transferred two or three times were centrifuged at 13,000×g for 15 min. The supernatants were discarded, and the pellets were collected. DNA in pellets was extracted using PowerSoil DNA Isolation Kit (MOBIO Laboratory, USA) under the manufacturer’s protocol. The V3 region of 16S rRNA in each sample was chosen, and corresponding genes were amplified by using primers 338F and 518R. Sequencing was then conducted on the platform Illumina.
Statistical analysis
For all microcosm systems, no biological replicates were set. Instead, three independent replicates were collected from the sample and tested. The results were expressed as the mean values of three experimental replicates with standard deviations (if applicable) and were compared by Student-Newman-Keul’s multiple comparison test. All statistical analysis and plotting were carried out by using the software GraphPad Prism v9.1.1.
Results
Anaerobic microbes in paddy soil slurry systems successfully dechlorinated Aroclor1260
The paddy soil slurry microcosm systems with or without exogenous Aroclor1260 were constructed to investigate anaerobic microbes’ ability to dechlorinate various kinds of PCBs. With time going by, in group B, Aroclor1260 was gradually dechlorinated in systems, and the amounts of Aroclor1260 dechlorinated on day 14, day 44, and day 73 were 21.8%, 33.4%, and 37.8%, respectively (Fig. 1a). The changes in high-chlorinated PCBs and low-chlorinated PCBs showed significant differences. Residuals of hexa-, hepta-, and octa-chlorobiphenyl were decreased, the amounts of which decreased on day 73 were 25.1%, 51.7%, and 24.6%, respectively. However, the residual of penta-chlorobiphenyl did not show signs of dechlorination. On the contrary, residuals of low-chlorinated PCBs containing less than five chlorines increased, and were 2.5 ~ 13.5 times higher than that in the initial state, indicating the occurrence of the anaerobic dechlorination Aroclor1260. Taken together, in the paddy soil slurry microcosm systems, high-chlorinated PCBs were gradually transformed into low-chlorinated ones.
Microbiota in paddy soil slurry systems successfully dechlorinated Aroclor1260, and the concentration of Cl− significantly raised. (a) Residues of PCB congeners in group B and (b) the concentration of Cl− in three groups at the end of the experiment. Group A went without the addition of Aroclor1260 and sterilization; group B was added with Aroclor1260 to a final concentration of 40 mg/kg; the control group CK was added with the same amount of Aroclor1260 but sterilized
In addition to the decrease of high-chlorinated PCBs, it was found that at the end of the experiment, the concentration of Cl- in group A was 834.0 mg/L (Fig. 1b), almost equivalent to that in control group CK (840.8 mg/L). On the contrary, this value reached 1244.1 mg/L in group B, significantly higher than the other two groups. This again provided evidence that Aroclor1260 was successfully dechlorinated and degraded by anaerobic microbes in paddy soil slurry systems.
Amendment of organic matters enhanced the dechlorination of PCBs
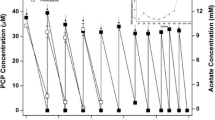
To explore the role of organic matters in the process of dechlorination, two high-chlorinated PCB congeners, PCB116 and PCB153, were chosen as electron acceptors. Chemical characteristics of the paddy soil slurry systems were manipulated by amending organic matters. It was discovered that significant dechlorination of PCBs in group PCBs + OM mainly took place during 0 ~ 24 d and 95 ~ 124 days (Fig. 2a), while group PCBs-OM had a lag period of approximately 95 days (Fig. 2b). Although the residual amounts of PCB116 and PCB153 did not show much disparity , the higher concentration of Cl- in group PCBs + OM implied that more Cl- were detached from PCBs (Fig. 2c). This suggested that extra organic matters could shorten the lag period of anaerobic dechlorination.
In group PCBs + OM, the amendment of organic matters has kept the Eh in the range of -200 mV to -300 mV (Fig. 2d), while the Eh in group PCBs-OM was between − 100 mV and − 150 mV. As shown in Fig. 2e, the pH was higher in groups PCBs + OM and OM. In groups OM and CK, the concentrations of H2 consistently increased, and the consumption of hydrogen possessed a strong connection with the dechlorination of PCBs (Fig. 2f). On day 28, The concentration of hydrogen in groups PCBs + OM and PCBs-OM was 4.69 nmol/L and 27.20 nmol/L, respectively. The concentration changed to 71.86 nmol/L and 15.17 nmol/L at the end of the cultivation. In summary, the amendment of extra organic matters created a more strictly anaerobic environment for the dechlorinating activities.
Changes in the functional groups of organic matters
FT-IR was used to investigate the changes in the functional groups of organic matters in paddy soil slurry systems (Fig. 3). The band near 3400 cm-1 was the typical characteristic of O-H stretching of hydrogen-bonded O-H groups in polysaccharides, and the presence of peaks at 2920 cm-1 and 2850 cm-1 was associated with the asymmetric stretching and stretching vibrations of -CH2- in aliphatic chains. A peak at 1730 cm-1 was generally ascribed to the C = O stretching of carbonyl functional groups, and a strong band at 1040 cm-1 was ascribed to the C-O stretching of polysaccharides. The peak at 800 cm-1 was due to the out-of-plane bending vibration of C-H of aromatics (Cocozza et al.,2003; Qin et al.,2010; Senesi et al., 1989). The FT-IR spectra dictated that two soil samples both contained substances like proteins, carboxylic acids, alkanes, aromatics and polysaccharides. One of the major differences between group PCBs + OM and group PCBs-OM was the intensity of the band at 1730 cm-1 (C = O stretching of carbonyl functional groups) relative to that at 1040 cm-1 (C-O stretching of polysaccharides), which might represent the changes in organic acids. It was clear that organic acids were consumed in group PCBs + OM but accumulated in group PCBs-OM. Another general feature of the spectra was the peaks at 2920 cm-1 to 2850 cm-1, which could be annotated as characteristic peaks of aliphatic organics. The peak at 800 cm-1 due to the out-of-plane bending vibration of aromatic C-H became sharper, inferring its involvement in the dechlorination of PCBs.
Changes in the microbial components under methanogenic conditions
Enrichment cultures under the methanogenic condition were constructed, and high-throughput 16S rRNA gene sequencing was performed on the initial microcosm (CK), G-II and G-III to see changes in the microbial components (Fig. 4). Thirteen bacterial phyla were identified from three communities, whose microbial components showed different patterns. In CK, microbes belonged to Firmicutes (58%), Bacteroidetes (18%), Euryarchaeota (10%), Proteobacteria (6%) and Chloroflexi (1%). In G-III, the relative abundance of Firmicutes increased to 84%. Besides, the main genera belonging to Firmicutes included Clostridium, Sedimentibacter, Syntrophomonas, and Desulfosporomusa (Supplementary Excel File), indicating their potential role in the dechlorination process. Notably, several reported dechlorinating bacteria including Dehalobacter, Dehalobacterium, and Desulfitobacterium were also detected. They may be in charge of the majority of clearance work.
Discussion
Anaerobic dechlorination of Aroclor1260 by the paddy soil slurry
In paddy soil slurry microcosm systems with Aroclor1260, the content of high-chlorinated PCBs decreased, while that of low-chlorinated PCBs increased, indicating the occurrence of anaerobic dechlorination. Since high-chlorinated PCBs owned a redox potential as high as 260 mV ~ 570 mV (Dolfing and Harrison 1992), they were more likely to be deoxidized as electron acceptors under an anaerobic environment. Therefore, PCB congeners containing more than four chlorines were inclined to be the target of anaerobic dechlorinating reactions (Field and Sierra-Alvarez 2008). In this experiment, hexa- and hepta-chlorobiphenyl congeners were preferentially used as electron acceptors and as a result, low-chlorinated congeners and Cl- were produced as products.
Amendment of organic matters benefits the dechlorination
Under anaerobic conditions, bacterial activities involved in the mineralization of organic matters included hydrolysis, acidification, acid decomposition and methane production, all of which led to the release of various organic acids, small molecule substances and hydrogen. Interestingly, we found that amending organic matters greatly shortened the lag period of anaerobic dechlorination, accelerating the process. PCBs have low solubility in water but a high tendency to adsorb on particles, which makes them extremely difficult to be removed from soil and sediment matrices (Passatore et al. 2014). To some extent, pH could influence the adsorption of PCBs to organic matters. If pH was appropriate, PCBs could disperse in the solid-liquid system, which improves their bioavailability (Jota and Hassett 1991). Consistent consumption of organic acid made pH stable in the range of 7.0 ~ 7.5, which was an optimal pH range for the overall removal of chlorines in PCBs (Wiegel and Wu 2000). Therefore, extra organic matters could enhance the adsorption of PCBs and increase their solubility and bioavailability. The input of exogenous organic matters not only produced more reducing substances but also enhanced the carbon cycle in which anaerobic bacteria participated. All these helped create a more anaerobic milieu (Eh≤-200 mV) suitable for the growth of dechlorinating bacteria. In addition to creating the ideal anaerobic milieu, organic matters could also offer dechlorinating bacteria with copious carbon sources. Due to the existence of other microbes, dechlorinating bacteria grew much better than in pure culture conditions (Men et al. 2014). Based on the results discovered in this study, we infer that different microbes took advantage of various carbon sources and converted lots of organic compounds into acetate and other substrates, which were subsequently accessible for dechlorinating bacteria.
The reductive dechlorination requires electron donors, which are often provided by organic substrates through microbial systems (Nies and Vogel 1990). It was reported that the addition of fatty acids (acetate, propionate, butyrate and hexanoic acid) significantly promoted dechlorination in sediment slurries of the Huston River (Alder et al. 1993). Similar results were also observed in marine sediment samples by supplying lactate (Matturro et al. 2016). Indeed, several studies have shown dechlorinating bacteria’s weak ability to synthesize specific amino acids, which might be available from the decomposition of proteins during the mineralization of organic matters (Zhuang et al. 2011, 2014). Notably, hydrogen is an effective electronic donor during dechlorination and can foster dechlorinating bacteria’s growth (Sokol et al. 1994; Rysavy et al. 2005). It was confirmed that the dehalogenators competed best against methanogens and homoacetogens when the level of hydrogen was 2 ~ 11 nM (Yang and McCarty 1998). As shown in our results, the amendment of organic matters provides more substrates for the production of hydrogen, thus providing a continuous supply for dechlorination and, at the same time, rapidly creating a low hydrogen partial pressure suitable for dechlorinating bacteria.
Microbial composition of the enrichment culture
Cocultured with Clostridium spp. and Methanosarcina barkeri, Dehalobacter restrictus was reported to dechlorinate 38% of the total chlorines of Aroclor1248 within 6 weeks (Oh et al. 2008). Desulfitobacterium dehalogenans and Desulfitobacterium PCE1 could also convert lactate to acetate, which is linked to the reduction of humic substances (Cervantes et al. 2002). Besides, the formation of OH-PCB by cytochrome P450 in Bacillus subtilis after exposure to PCBs was recently reported (Sun et al. 2018). The biotransformation increased the hydrophilicity of hydrophobic PCBs and made them more scattered in the medium.
In summary, when it comes to the anaerobic dechlorination of PCBs, the amendment of organic matters can benefit the process by not only creating a more strictly anaerobic milieu but also offering more copious carbon sources. The changes in microbial compositions during this process are worth noting. These findings provide a theoretical basis for the optimization of in situ microbial remediations.
References
Abraham W-R, Nogales B, Golyshin PN et al (2002) Polychlorinated biphenyl-degrading microbial communities in soils and sediments. Curr Opin Microbiol 5:246–253. https://doi.org/10.1016/S1369-5274(02)00323-5
Alder AC, Haggblom MM, Oppenheimer SR, Young LY (1993) Reductive dechlorination of polychlorinated biphenyls in anaerobic sediments. Environ Sci Technol 27:530–538. https://doi.org/10.1021/es00040a012
Baba D, Yasuta T, Yoshida N et al (2007) Anaerobic biodegradation of polychlorinated biphenyls by a microbial consortium originated from uncontaminated paddy soil. World J Microbiol Biotechnol 23:1627–1636. https://doi.org/10.1007/s11274-007-9409-4
Bedard DL, Ritalahti KM, Löffler FE (2007) The Dehalococcoides Population in Sediment-Free Mixed Cultures Metabolically Dechlorinates the Commercial Polychlorinated Biphenyl Mixture Aroclor 1260. Appl Environ Microbiol 73:2513–2521. https://doi.org/10.1128/AEM.02909-06
Beyer A, Biziuk M (2009) Environmental fate and global distribution of polychlorinated biphenyls. Rev Environ Contam Toxicol 201:137–158. https://doi.org/10.1007/978-1-4419-0032-6_5
Cervantes FJ, de Bok FAM, Duong-Dac T et al (2002) Reduction of humic substances by halorespiring, sulphate-reducing and methanogenic microorganisms. Environ Microbiol 4:51–57. https://doi.org/10.1046/j.1462-2920.2002.00258.x
Chen C, Yang K, Yu C et al (2014a) Influence of redox conditions on the microbial degradation of polychlorinated biphenyls in different niches of rice paddy fields. Soil Biol Biochem 78:307–315. https://doi.org/10.1016/j.soilbio.2014.08.014
Chen C, Yu C, Shen C et al (2014b) Paddy field – A natural sequential anaerobic–aerobic bioreactor for polychlorinated biphenyls transformation. Environ Pollut 190:43–50. https://doi.org/10.1016/j.envpol.2014.03.018
Cogliano VJ (1998) Assessing the cancer risk from environmental PCBs. Environ Health Perspect 106:317–323. https://doi.org/10.1289/ehp.98106317
Dolfing J, Harrison BK (1992) Gibbs free energy of formation of halogenated aromatic compounds and their potential role as electron acceptors in anaerobic environments. Environ Sci Technol 26:2213–2218. https://doi.org/10.1021/es00035a021
Field JA, Sierra-Alvarez R (2008) Microbial transformation and degradation of polychlorinated biphenyls. Environ Pollut 155:1–12. https://doi.org/10.1016/j.envpol.2007.10.016
Jahnke JC, Hornbuckle KC (2019) PCB Emissions from Paint Colorants. Environ Sci Technol 53:5187–5194. https://doi.org/10.1021/acs.est.9b01087
Jota MAT, Hassett JP (1991) Effects of environmental variables on binding of a PCB congener by dissolved humic substances. Environ Toxicol Chem 10:483–491. https://doi.org/10.1002/etc.5620100408
Matturro B, Ubaldi C, Rossetti S (2016) Microbiome Dynamics of a Polychlorobiphenyl (PCB) Historically Contaminated Marine Sediment under Conditions Promoting Reductive Dechlorination. Front Microbiol 7. https://doi.org/10.3389/fmicb.2016.01502
Men Y, Seth EC, Yi S et al (2014) Sustainable Growth of Dehalococcoides mccartyi 195 by Corrinoid Salvaging and Remodeling in Defined Lactate-Fermenting Consortia. Appl Environ Microbiol 80:2133–2141. https://doi.org/10.1128/AEM.03477-13
Nies L, Vogel TM (1990) Effects of Organic Substrates on Dechlorination of Aroclor 1242 in Anaerobic Sediments. Appl Environ Microbiol 56:2612–2617. https://doi.org/10.1128/aem.56.9.2612-2617.1990
Oh K-H, Ostrofsky EB, Cho Y-C (2008) Molecular characterization of polychlorinated biphenyl-dechlorinating populations in contaminated sediments. J Microbiol 46:165–173. https://doi.org/10.1007/s12275-007-0214-4
Passatore L, Rossetti S, Juwarkar AA, Massacci A (2014) Phytoremediation and bioremediation of polychlorinated biphenyls (PCBs): State of knowledge and research perspectives. J Hazard Mater 278:189–202. https://doi.org/10.1016/j.jhazmat.2014.05.051
Rysavy JP, Yan T, Novak PJ (2005) Enrichment of anaerobic polychlorinated biphenyl dechlorinators from sediment with iron as a hydrogen source. Water Res 39:569–578. https://doi.org/10.1016/j.watres.2004.11.009
Sokol RC, Bethoney CM, Rhee G-Y (1994) Effect of hydrogen on the pathway and products of PCB dechlorination. Chemosphere 29:1735–1742. https://doi.org/10.1016/0045-6535(94)90319-0
Sun J, Pan L, Zhu L (2018) Formation of hydroxylated and methoxylated polychlorinated biphenyls by Bacillus subtilis: New insights into microbial metabolism. Sci Total Environ 613–614:54–61. https://doi.org/10.1016/j.scitotenv.2017.09.063
Wiegel J, Wu Q (2000) Microbial reductive dehalogenation of polychlorinated biphenyls. FEMS Microbiol Ecol 32:1–15. https://doi.org/10.1111/j.1574-6941.2000.tb00693.x
Xiang Y, Xing Z, Liu J et al (2020) Recent advances in the biodegradation of polychlorinated biphenyls. World J Microbiol Biotechnol 36:145. https://doi.org/10.1007/s11274-020-02922-2
Yan J, Ritalahti KM, Wagner DD, Löffler FE (2012) Unexpected Specificity of Interspecies Cobamide Transfer from Geobacter spp. to Organohalide-Respiring Dehalococcoides mccartyi Strains. Appl Environ Microbiol 78:6630–6636. https://doi.org/10.1128/AEM.01535-12
Yang Y, McCarty PL (1998) Competition for Hydrogen within a Chlorinated Solvent Dehalogenating Anaerobic Mixed Culture. Environ Sci Technol 32:3591–3597. https://doi.org/10.1021/es980363n
Zhu M, Yuan Y, Yin H et al (2022) Environmental contamination and human exposure of polychlorinated biphenyls (PCBs) in China: A review. Sci Total Environ 805:150270. https://doi.org/10.1016/j.scitotenv.2021.150270
Zhuang W-Q, Yi S, Feng X et al (2011) Selective Utilization of Exogenous Amino Acids by Dehalococcoides ethenogenes Strain 195 and Its Effects on Growth and Dechlorination Activity. Appl Environ Microbiol 77:7797–7803. https://doi.org/10.1128/AEM.05676-11
Zhuang W-Q, Yi S, Bill M et al (2014) Incomplete Wood-Ljungdahl pathway facilitates one-carbon metabolism in organohalide-respiring Dehalococcoides mccartyi. Proceedings of the National Academy of Sciences 111:6419–6424. https://doi.org/10.1073/pnas.1321542111
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 21876149, 42077125).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jingwen Chen and Fengjun Xu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, J., Xu, F., Yang, K. et al. The amendment of Organic matters enhances the anaerobic dechlorination of Polychlorinated Biphenyls in Paddy Soil. Bull Environ Contam Toxicol 109, 393–400 (2022). https://doi.org/10.1007/s00128-022-03563-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-022-03563-x