Abstract
To test the toxic effects of tributyltin (TBT), Macrobrachium rosenbergii were exposed to three concentrations of TBT viz. 10 ng/L, 100 ng/L and 1000 ng/L for 90 days. The bioaccumulation of TBT level varied in hepatopancreas based upon dose dependent manner. Histopathological results revealed the reduction in basement membrane thickness, disruption of the hepatopancreatic tubules and abnormal lumen in hepatopancreas of TBT treated prawns. The ultrastructure of the control prawn showed normal architecture of cellular organelles with prominent nuclei in hepatocytes. On the other hand, many vacuoles, irregular arrangements of microvilli, swollen mitochondria, distorted rough endoplasmic reticulum cisternaes and abnormal nucleus were seen in the TBT treated group. Further, the biochemical and vitellogenin content were altered remarkably due to TBT exposure. It directly indicated that TBT had conspicuously inhibited the vitellogenesis. Therefore, it was inferred that the administration of TBT has considerably affected the hepatopancreatic functions in M. rosenbergii.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Tributyltin (TBT) is a common antifouling agent widely used in ship paint formulations to resist the settlement of algae, barnacles and other fouling organisms in ship hulls (Belfroid et al. 2000). Aquatic pollution resulting from its usage has been of great concern due to its bioaccumulation potential, persistence in sediment and highly toxic effects on non-target aquatic life. Despite efforts to reduce its use, considerable levels of TBT are still detected in marine ecosystems exceeding toxicity levels (Antizar-Ladislao 2008). For instance, Iyapparaj et al. (2013) reported the TBT induced damages in histocyto pathology and alterations in biochemical constituents of mussel Perna indica as it’s a well-known bioindicator of xenobiotics.
As that of the liver in mammals, hepatopancreas of crustaceans has the same function. Hepatopancreas involves in digestion and food absorption, storage of energy reserves and minerals during moulting. Hepatopancreas is the vital organ meant for detoxification of xenobiotics (Johnson et al. 1998). Assessment of the impact of environmental pollutants on aquatic animals using histopathological and ultrastructural examinations have been progressively more recognized (Paruruckumani et al. 2015). Quantification of biochemical contents in different tissues could be used as a diagnostic tool for determining the physiological status of an organism. Under stress conditions, the protein and lipid are serves as the energy source for metabolic pathways and biochemical reactions. Increasing energy demand due to toxic stress caused by pollutants will be met by the depletion of glycogen reserves (Fiona and Sherly 2016).
Our previous study, reported that the TBT significantly inhibited the organogenesis in M. rosenbergii (Revathi and Munuswamy 2010), oogenesis (Revathi et al. 2013a), spermatogenesis (Revathi et al. 2013b) and “imposex” formation (Revathi et al. 2014a). However, the effects of TBT on the hepatopancreas in M. rosenbergii have never been reported earlier. Therefore, the key objectives of the present research were to study the bioaccumulation of TBT, histopathological, ultrastructural alterations, variation in biochemical and vitellogenin content in hepatopancreas of M. rosenbergii after exposure to TBT.
Materials and Methods
Freshwater female prawns M. rosenbergii were collected from Aqua Nova Farm in Kanathur, Chennai, Tamil Nadu, India. The collected prawns were transported to the laboratory and acclimatized for 2–3 weeks by following standard methods. During acclimatization, the prawns were fed ad libitum with commercial prawn feed manufactured by Avanti Aqua Feeds, India.
Tributyltin (TBT) (95 percent purity) was purchased from Himedia (Himedia, India). TBT stock solution of 50 mM concentration was prepared in 2% of AR grade ethanol (Himedia, India). The experimental prawns weighing 16 ± 2 g were divided into five groups each containing 20 prawns exposed individually to the measured concentrations of TBT and the experimental group details are as follows. Group I served as control (without any treatment), group II control (ethanol treated), group III (10 ng/L TBT treated), group IV (100 ng/L TBT treated) and group V (1000 ng/L TBT treated). The above test concentrations of TBT is reported to be sufficient to cause adverse effects aquatic organisms especially in M. rosenbergii (Horiguchi et al., 2002; Revathi et al. 2013b, 2014a, b). The water was exchanged and the above concentrations of TBT were treated and measured in the respective experimental tanks every day as follows.
For that, 250 mL of water was taken individually from each experimental tank and to this 2 mL of 1 M hydrochloric acid and 75 mL of solvent (hexane/ethyl acetate 70:30) were added. The mixture was shaken well using magnetic stirrer for 20 min. Afterwards, the organic layer was dried with anhydrous sodium sulphate, then reduced under vacuum at 30°C to 5 mL, and stored at − 18°C. For derivatization, the concentrate was reacted with 85 mg of Sodium borohydride in 5 mL of absolute ethanol. Then the solution was washed with 10 mL of 10% sodium chloride. The resultant organic phase was dried with anhydrous sodium sulphate; 0.2 mL of durene solution (2.62 μg/L) was added as internal standard. The above mixture was reduced to 0.1 mL at room temperature and the TBT content was quantified by Gas Chromatography coupled with Mass Spectroscopy (Agilent GC–MS 5975 Inert XL MSD, United States). 2.0 µL of each derivatized sample was injected in GC–MS and operated in EMV mode. Helium was used as carrier gas with the flow rate of 1.0 mL/min. The injection port temperature was operated at 250°C. The column oven temperature was held at 80°C for 2 min then programmed at 10°C/min to 250°C which was held for 0 min, and then at 5°C/min to 280°C which was held for 9 min. Electron impact spectra in positive ionization mode were acquired between m/z 50 and 550 to ensure the TBT content (Waisbaum et al. 2010).
The first group served as control without any treatment and the second control group received an equal volume of the solvent 2% ethanol (1 µL/L). The experiment was conducted for 90 days and during the experiment the water temperature was maintained at 18 ± 2°C. At the end of the experiment, the gonado somatic index (GSI) and hepato somatic index (HSI) were calculated by following the method of Zhang et al. (2007).
At the end of experiment, the hepatopancreas tissue samples from control and treated prawns were collected and the TBT from the respective samples was extracted (Waldock and Thain 1989). Triplicate histological analyses were done by sacrificing three prawns from each group (Revathi et al. 2013a). To observe the ultrastructural changes, the hepatopancreas of control and TBT treated prawns were subjected to transmission electron microscopic study by following the method of Revathi et al. (2014a). Biochemical constituents such as protein (Bradford 1976), total lipid (Folch et al. 1957) and glycogen content (Dezwann and Zandee 1972) were estimated. As per the method of Tsukimura et al. (2000), Vitellogenin was isolated from the hepatopancreas of control and TBT treated prawns. Further, the isolated vitellogenin was purified by following the scheme of Zagalsky et al. (1967). For all the above analysis, the NHANES (National Health and Nutrition Examination Survey U.S) quality control and quality assurance protocols were followed (QA/QC) to meet the 1988 Clinical Laboratory Improvement Act mandates.
Changes in the bioaccumulation level of TBT, relative reproductive index and variation in biochemical and vitellogenin content were statistically analyzed by following one way analysis of variance (ANOVA) and Tukey–Dunnett test using SPSS 16.0 to determine the significant variations between the control and TBT treated groups.
Results and Discussion
In the present study, the impact of TBT on hepatopancreas in the freshwater female prawn M. rosenbergii was studied with the analysis of GSI, HSI, bioaccumulation of TBT, histopathological, ultrastructural changes, variations in biochemical and vitellogenin content of both control and TBT treated prawns.
In control, GSI value was 6.46% ± 0.56% and HSI was 2.01% ± 0.42%. Further, the GSI and HSI values were declined as the concentration of TBT increases. At the concentration of 10 ng/L, the GSI and HSI values were reduced to 5.24% ± 0.37% and 5.07% ± 0.26% respectively. Besides, the GSI and HSI values remarkably reduced to 4.61% ± 0.02% and 1.79% ± 0.22% respectively at 1000 ng/L (Fig. 1). Changes in the GSI and HSI values between control and experimental groups inferred that 1000 ng/L TBT treated group are statistically significant (p < 0.05); whereas the 10 and 100 ng/L TBT treated groups were not statistically significant. The present study clearly inferred that TBT had drastically reduced the reproductive activity as evidenced by the level of GSI and HSI in M. rosenbergii. The decrease in HSI in TBT exposed prawn was expected based on the common finding of hepatocellular trauma. A decrease of 1.40 fold in GSI and 1.12 fold in HSI were recorded at higher concentration of TBT (1000 ng/L). The androgenic effect of organotin compounds (Bryan et al. 1988) may support these trends in prawns exposed to TBT in this study. Overall, the present results showed a dose-dependent toxicity of TBT in M. rosenbergii. Similarly, TBT had considerably reduced the oogenesis as evident with the measurements of GSI, HSI and oocyte diameter in Macrobrachium rosenbergii (Revathi et al. 2014a).
Bioaccumulation of TBT in hepatopancreas of both control and treated prawns exhibited considerable variations. Of all tested concentrations, 10 ng/L of TBT showed the minimum accumulation of 0.036 ± 0.0001 µg/g in hepatopancreas and the maximum level of TBT accumulation was recorded in hepatopancreas (0.165 ± 0.0.31 µg/g) of 1000 ng/L TBT treated prawn (Fig. 1). The bioaccumulation of TBT in hepatopancreas varied statistically significant between control group and 100 and 1000 ng/L TBT treated groups (p < 0.05) and it was not significant (p > 0.05) for 10 ng/L TBT treated group. Bioaccumulation of TBT was reported to be high in hepatopancreas and this could suggest that the hepatopancreas is the organ responsible for the production of CYP450 to assist in metabolizing and eliminating the ingested TBT. Similarly, Revathi et al. (2013b) also registered that TBT accumulation was high in hepatopancreas than the gonad of mud crab Scylla serrata. Correspondingly, bioaccumulation level of TBT was high in hepatopancreas than gill and muscle of blue crabs (Vannuci-Silva et al. 2013). Increased level of bioaccumulation was noticed in TBT treated groups based on the dose dependent manner in minnow Gobiocypris rarus (Qun-Fang et al. 2002).
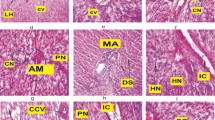
The hepatopancreas of control prawn showed the normal architecture of hepatopancreatic tubules, lumen and basement membrane thickness (Fig. 2a). At 10 ng/L TBT treatment, the hepatopancreas had shown elongation of hepatopancreatic tubule, abnormal lumen and vacuoles in the tubules (Fig. 2b). The reduction in basement membrane thickness, disruption of the hepatopancreatic tubules and vacuoles in the tubules were evident in the hepatopancreas at 100 ng/L TBT (Fig. 2c). At 1000 ng/L TBT, hepatopancreas showed remarkable variation in the cellular architecture of the hepatopancreas such as reduction in the size of hepatopancreatic tubule as well as abnormal lumen and reduced basement membrane thickness (Fig. 2d). Histopathological examination provides information on the earlier stage of pollution hazard and also useful in knowing the nature and degree of damage to cells and tissues (Shaikh et al. 2010). The effect of TBT on the hepatopancreas showed disruption in hepatopancreatic tubule, lumen and basement membrane compared to that of control. Saravana Bhavan and Geraldine (2000) reported that histopathological changes in hepatopancreas such as vacuolization of cell, winding of tubular lumen which ultimately resulted into syncytial mass containing large number of vacuolated cells and phagocytes in Macrobrachium malcolmsonii exposed to endosulfan. Histoarchitechture of hepatopancreas in TBT treated prawn Macrobrachium kistnensis also showed abnormalities and disruptions which led to improper production of digestive enzymes and impaired absorption of nutrients (Kharat et al. 2014).
a Control hepatopancreas showing the normal architecture of hepatopancreatic tubules (ht), lumen (L) and basement membrane (Bm). b TBT (10 ng/L) treated prawn hepatopancreas showing deformities in hepatopancreas such as elongated hepatopancreatic tubule (↑ Eht) and abnormal lumen (AL). c At 100 ng/L TBT treated group, hepatopancreas showing reduced hepatopancreatic tubules (↑ ht) and abnormal lumen (AL). d Hepatopancreas showing reduced hepatopancreatic tubules (↑ ht), abnormal lumen (AL) and reduced basement membrane thickness (RBm) at 1000 ng/L exposure. Bar: 50 µm
Drastic ultrastructural changes were observed in the hepatopancreas of prawn exposed to 1000 ng/L TBT for 90 days. The ultrastructure of the control prawn hepatopancreas showed the normal architecture of hepatocytes with prominent nucleus and cytoplasm (Fig. 3a). The other cellular organelles such as mitochondria, microvilli and many layers of rough endoplasmic reticulum (RER) were regularly arranged (Fig. 3b, c). Only a few vacuoles with small size were found in the control. The nucleus was seen with distinct nucleolus located in the center of hepatocytes (Fig. 3d). Electron dense heterochromatin was seen at the base of the nuclear membrane (Fig. 3e). Cellular changes were much pronounced in the hepatocytes of TBT exposed prawn. The vacuoles in the hepatocytes were increased in number and size (Fig. 3f). Moreover, irregular arrangements of microvilli, swollen mitochondria and distorted RER were noticed (Fig. 3g, h). Nuclei in the hepatocytes seemed to be abnormal in structure with condensation of more chromatin and disruption of nuclear membrane (Fig. 3i, j). TEM studies revealed the remarkable changes in the hepatopancreas of TBT treated prawn against the normal architecture of hepatocytes seen in control prawn. In concern with cellular nucleus, notable abnormalities were seen in TBT treated group than the normal control. Swollen mitochondria and deformation of cristae were found in the TBT treated group as the mitochondria is the sensitive indicator of xenobiotics. Likewise, swelling of mitochondria in hepatopancreatic cells was reported in polyaromatic hydrocarbons (PAHs) treated Palaemon serratus (Abdelmeguid et al. 2009). Swelling and shrinking of mitochondria indicated the attenuation of its functions as reported by Yamuna et al. (2009). Similarly, Atti and Saied (2018) described the deformed mitochondria, destructed rough endoplasmic reticulum, and vacuolated cytoplasm after spinosad exposure in crayfish Procambarus clarkia.
a Control prawn hepatopancreas showing normal architecture of hepatocyte with prominent nucleus (N) along with rough endoplasmic reticulum (RER), mitochondria (M) and microvilli (Mv). b Control hepatocytes showing normal architecture of RER with regularly arranged ribosomes (R) and prominent mitochondria (M) (× 20,000). c Hepatopancreas showing hepatocytes with uniformly arranged microvilli (Mv) in control prawn (× 20,000). d hepatocytes showing nucleus (N) with chromatin (Ch) evenly distributed in control prawn (× 15,000). e Higher magnification of hepatocyte showing nucleus with electron dense heterochromatin (Hc) at the base of nuclear membrane (Nm) and uniformly arranged rough endoplasmic reticulum (RER) in control prawn (× 30,000). f TBT (1000 nl/L) treated prawn hepatopancreas showing cellular abnormality in nucleus (↑) and more vacuoles (V) in the cytoplasm (× 1500). g Hepatopancreas of the treated prawn showing hepatocytes with disrupted RER (↑) (× 15,000). h Treated prawn hepatopancreas showing hepatocytes with irregular arrangement of microvilli (↑ Mv) seen along with swelling of mitochondria (↑ M) in the cytoplasm. (× 20,000). i Hepatopancreas of treated prawn showing, abnormal nucleus (↑ N) with disrupted RER (↑ RER) (× 10,000). j Hepatopancreas showing hepatocytes with abnormal nucleus (↑ N), condensation of more chromatin (↑ Ch) and disrupted of RER (↑ RER) in treated prawn (× 15,000)
Total protein, lipid and glycogen content decreased in all TBT treated prawns hepatopancreas compared to controls. Protein content (54.67 ± 2.56 mg/g) and lipid content (16.50 ± 2.04 mg/g) and glycogen content (25.42 ± 0.42 mg/g) in hepatopancreas of control prawn after 90 days. However, the protein, lipid and glycogen content were abruptly reduced in all treated groups (10 ng/L to 1000 ng/L of TBT). Statistical analysis revealed that variation in protein, lipid and glycogen content of 100 and 1000 ng/L TBT exposed prawns differed significantly (p < 0.05). However, the tested biochemical contents were found to be non significant (p > 0.05) in 10 ng/L TBT treated prawns than that of control group (Fig. 4). Present findings indicated the significant decrease in protein, lipid and glycogen content in all TBT treated groups at dose dependent manner. This could suggest that the depletion of above biochemical contents might be due to the high metabolic activity and efficiency (glycogenolysis, proteolysis and lipolysis) of the hepatopancreas under TBT stress to meet the increased energy demands for better survival. Depletion of protein content in different tissues of M. kistnensis exposed to TBT was reported by Kharat et al. (2009). Similarly monocrotophos exposure, decrease the biochemical substances like protein and lipid content in M.rosenbergii (Jayachitra et al. 2016). Correspondingly, protein, lipid and glycogen content were found to be declined with subsequent increase in concentration of pesticide nuvacron in Penaeus monodon (Fiona and Sherly 2016). Accordingly, biochemical contents were significantly decreased in fiddler crab Uca triangularis by the exposure of pesticides (Sangeetha and Deeparani 2016).
The results clearly indicated that vitellogenin content decreased significantly due to the exposure of TBT, compared to control. In control prawn, the vitellogenin content in hepatopancreas was recorded as 2.1 ± 0.15 μg/g respectively. Interestingly, vitellogenin content in the treated prawns remarkably decreased from 1.5 ± 0.12 μg/g at 10 ng/L to 0.5 ± 0.11 μg/g at 1000 ng/L TBT (Fig. 5). Statistical variation in vitellogenin content in 100 and 1000 ng/L TBT treated groups was significant when compared to control group (p < 0.05) and 10 ng/L TBT treated prawns exhibited non significance (p > 0.05). This investigation evidenced that TBT has resulted in dysfunction of hepatopancreas in M. rosenbergii. The impact of TBT on the vitellogenesis was indicated by the reduced vitellogenin content. Exposure to TBT led to decrease in vitellogenin content of M. rosenbergii (Revathi et al. 2013a). Similarly, reduction of vitellogenin and vitellin contents due to the blocking of protein synthesis or protein denaturation or interruption in the amino acid synthesis due to naphthalene toxicity was reported in Scylla serrata (Vijayavel and Balasubramanian 2006). The depletion may also be due to the rapid utilization of protein by the cells under stress condition and also indicates that the protein might undergo proteolysis which results in the production of free amino acids and is used for energy production during stress condition (Vijayavel et al. 2006). Silveyra et al. (2018) also reported that atrazine exposed crayfish Procambarus clarkia, illustrated a notable decrease in vitellogenin content of hepatopancreas. Thus, it is clear that many xenobiotic chemicals including naphthalene can disrupt vitellogenesis by direct action of the toxicants on vitellogenesis (Kime et al. 1999) as evidenced by the present results.
The present study clearly demonstrated that TBT induced the cellular alterations in hepatopancreas and changes in HSI and GSI indexes in M. rosenbergii. Histopathologic alterations in prawn exposed to TBT exhibited the vacuolization, swollen mitochondria, abnormal nuclei and decreases in RER. Besides, biochemical changes are the best indicators of stress situations caused by TBT. On the other hand, TBT had dramatically impaired the vitellogenesis and resulted in reduced vitellogenin content in M. rosenbergii. All the above findings demonstrated that the freshwater prawn M. rosenbergii was highly susceptible to reproductive toxicity of TBT in aquatic environment.
References
Abdelmeguid E, Awad HE, Ibrahim AM, Yousef NA (2009) Ultrastructural changes in hepatopancreas of Palaemon serratus, following treatment with petroleum carcinogenic compounds. Pak J Nutr 8(6):770–781
Antizar-Ladislao B (2008) Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment: a review. Environ Int 34(2):292–308
Atti MS, Saied RM (2018) Physiological and ultrastructural alterations in the crayfish Procambarus clarkia treated with spinosad (bacterial derived insecticide). Biochem Physiol 7(1):226
Belfroid AC, Purperhart M, Ariese F (2000) Organotin levels in seafood. Mar Poll Bull 40:226–232
Bradford MM (1976) A rapid and sensitive method for the qualification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bryan GW, Gibbs PE, Burt GR (1988) A comparison of the effectiveness of tri-n-butyltin chloride and five other organotin compounds in promoting the development of imposex in the dog-whelk Nucella lapillus. J Mar Biol Assoc UK 68:733–744
Dezwann D, Zandee I (1972) The utilization of glycogen and accumulation of some intermediate during anerobiosis in Mytilus edulis. Comp Biochem Physiol 43B:47–52
Fiona P, Sherly W (2016) Effect of an organophosphorus pesticide on exposure to the Indian Tiger Prawn, Penaeus Monodon. Int J Sci Res 5(1):247–252
Folch J, Lee M, Bloane-Stanley GHS (1957) A simple method for the isolation and purification to total from animal tissues. J Biochem 266:497–509
Horiguchi T, Kojima M, Kaya M, Matsuo T, Shiraishi H, Morita M, Adachi Y (2002) Tributyltin and triphenyltin induce spermatogenesis in ovary of female abalone, Haliotis gigantea. Mar Environ Res 54(3–5):679–684
Iyapparaj P, Revathi P, Ramasubburayan R (2013) Antifouling activity of the methanolic extract of Syringodium isoetifolium, and its toxicity relative to tributyltin on the ovarian development of brown mussel Perna indica. Ecotoxicol Environ Safe 89:231–238
Jayachitra J, Ilavarasi P, Subashini R (2016) Biochemical alteration in selected body tissues of freshwater prawns Macrobrachium rosenbergii exposed to monocrotophos. Worl J Pharm Pharm Sci 5(5):1469–1480
Johnson DJ, Alexander CG, Yellowlees D (1998) Epithelial cytology and function in the digestive gland of Thenus orientalis (Decapoda, Scyllaridae). J Crust Biol 18(12):271–278
Kharat PS, Laxmi B, Shejule KB, Ghoble BC (2009) Effect of TBTCL on glycogen profile in freshwater prawn, Macrobrachium kistnensis. World Appl Sci J 7(12):1534–1539
Kharat PS, Pathan TS, Shejule KB (2014) Histopanthological changes in hepatopancreas of freshwater prawn Macrobrachium kistnensis exposed to TBTCL. Mid East J Sci Res 22(9):1396–1400
Kime DE, Nash JP, Scott AP (1999) Vitellogenesis as a biomarker of reproductive disruption by xenobiotics. Aquaculture 177:345–352
Paruruckumani PS, Maharajan A, Ganapiriya V, Narayanaswamy Y, Raja Jeyasekar R (2015) Surface ultrastructural changes in the gill and liver tissue of asian sea bass Lates calcarifer (Bloch) exposed to copper. Biol Trace Elem Res. https://doi.org/10.1007/s12011-015-0370-z
Qun-Fang Z, Gui- Bin J, Ji- Yan J (2002) Effects of sublethal levels of tributyltin chloride in a new toxicity test organism: the Chinese rare minnow (Gobiocypris rarus). Arch Environ Contam Toxicol 42:332–337
Revathi P, Munuswamy N (2010) Effect of TBT on the early embryonic development in the freshwater prawn Macrobrachium rosenbergii (De Man). Chemosphere 79:922–927
Revathi P, Iyapparaj P, Arockia Vasanthi L, Munuswamy N, Krishnan M (2013a) Impact of TBT on the vitellogenesis and sex hormones in freshwater prawn Macrobrachium rosenbergii (De Man, 1879). Aquat Biosyst 9:10
Revathi P, Iyapparaj P, Munuswamy N, Vasanthi LA, Krishnan M (2013b) Bioaccumulation of tributyltin and its impact on spermatogenesis in mud crab Scylla serrata (Forskal). Turk J Biol 37(3):296–304
Revathi P, Iyapparaj P, Vasanthi L, Munuswamy N, Arun Prasanna V, Suganya T, Anantharaman P, Krishnan M (2014a) TBT effects on the development of intersex (Ovotestis) in female freshwater prawn Macrobrachium rosenbergii. BioMed Res Int. https://doi.org/10.1155/2014/412619
Revathi P, Iyapparaj P, ArockiaVasanthi L, Munuswamy N, Arun V, Pandiarajan J, Krishnan M (2014b) Influence of short term exposure of TBT on the male reproductive activity in freshwater prawn Macrobrachium rosenbergii (De Man). Bull Environ Contam Toxicol. https://doi.org/10.1007/s00128-014-1332-4
Sangeetha S, Deeparani S (2016) Histological and biochemical changes by the pesticides Endosulfan, Chlorpyrifos and Carbaryl on the gonads of Fiddler crab, Uca triangularis. World J Environ Pollut 6(1):7–14
Saravana Bhavan PS, Geraldine P (2000) Histopathology of the hepatopancreas and gills of the prawn Macrobrachium malcomsonii exposed to endosulfan. Aquat Toxicol 50:331–339
Shaikh FI, Ustad IR, Ansari NT (2010) Effect of heavy metal on the Ovary of freshwater crab, Barytelphusa cunicularis (Westwood). Bioscan 5(2):335–338
Silveyra GR, Vatnick P, Medesani I (2018) Effects of atrazine on vitellogenesis, steroid levels and lipid peroxidation, in female red swamp crayfish Procambarus clarkia. Aquat Toxicol 197:136–142
Tsukimura B, Bender JS, Linder CJ (2000) Developmental aspects of gonadal regulation in the ridgeback shrimp, Sicyonia ingentis. Comp Biochem Physiol 127A:215–224
Vannuci-Silva M, Menegario A, Franchi M, Brossi-Garcia L, Souza M, Jr Araújo, Bindes G, Govone S (2013) Bioaccumulation of tributyltin by blue crabs. J Braz Chem Soc 24(10):1642–1648
Vijayavel K, Balasubramanian MP (2006) Fluctuations of biochemical constituents and marker enzymes as a consequence of naphthalene toxicity in an estuarine edible crab Scylla serrata. Ecotoxicol Environ Saf 63(1):141–147
Vijayavel K, Anbuselvam C, Balasubramanian MP, Deepak Samuel V, Gopalakrishnan S (2006) Assessment of biochemical components and enzyme activities in the estuarine crab Scylla tranquebarica from naphthalene contaminated habitants. Ecotoxicology 15(5):469–476
Waisbaum RG, Rodriguez C, Sbarbati Nudelman N (2010) Determination of TBT in water and sediment samples along the Argentine Atlantic coast. Environ Technol 31(12):1335–1342
Waldock MJ, Thain J (1989) Shell thickening in Crassostrea gigas: organotin antifouling or sediment-induced. Mar Pollut Bull 14:411–415
Yamuna P, Bhavan Saravana, Geraldine P (2009) Ultrastructural observations in gills and hepatopancreas of prawn Macrobrachium malcolmsonii exposed to mercury. J Environ Biol 30(5):693–699
Zagalsky PF, Cheesman DF, Ceccaldi HJ (1967) Studies oncarotenoid-containing lipoproteins isolated from the eggs and ovaries of certain marine invertebrates. Comp Biochem Physiol 22:851–871
Zhang IL, Zuo ZH, Chen YX, Zhao Y, Hu S, Wang CG (2007) Effect of tributyltin on the development of ovary in female cuvier (Sebastiscus marmoratus). Aquat Toxicol 83:174–179
Acknowledgements
The author gratefully acknowledge their sincere thanks to Department of Science and Technology—National Postdoctoral Fellowship Scheme (Ref No. PDF/2017/000822) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Revathi, P., Iyapparaj, P., Vasanthi, R.A. et al. Bioaccumulation of TBT and Its Cellular Toxic Effects on the Freshwater Prawn Macrobrachium rosenbergii. Bull Environ Contam Toxicol 103, 689–696 (2019). https://doi.org/10.1007/s00128-019-02711-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02711-0