Abstract
Surface ultrastructure of the gill and liver of 3-month-old Asian sea bass, Lates calcarifer, after copper exposure, was investigated by scanning electron microscopy (SEM). Fish samples were exposed to copper concentrations of 6.83 and 13.66 ppm (sublethal) for 28 days with parallel untreated control. These structures showed structural modifications in both low and high concentrations of copper exposure. Oedema, hyperplasia, desquamation, necrosis, epithelial lifting, lamellar fusion, collapsed secondary lamellae, curling of secondary lamellae and aneurism in the secondary lamellae were observed in gill tissues exposed to copper. Hepatic lesions related to cloudy swelling of hepatocytes, congestion, vacuolar degeneration, dilation of sinusoids and nuclear hypertrophy were evident in the exposed sea bass liver tissue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper is one of the most toxic trace metals to marine biota [1] which poses considerable risk to marine ecosystems where human influences have enhanced the natural (background) copper concentrations. Potential risks occur when copper is introduced into marine ecosystems by mining activities, antifouling paints on boats, marinas, ports and jetty pylons, runoff from fungicidal uses (e.g. copper sulphate) and sewage effluent. In aquaculture industry, copper sulphate is used as an algaecide and as a therapeutic chemical for various ectoparasitic and bacterial infections [2, 3]. Excess copper can result in adverse toxicological effects and maybe poisonous to aquatic animals. Fishes are the simple and reliable biomarkers of copper pollution of aquatic bodies [4, 5].
Histopathological investigations have long been recognized as reliable biomarkers of stress in fish [6] and have been widely used as biomarkers in the evaluation of the health of fish exposed to contaminants, both in laboratory and field studies. The gills [7], liver [8] and kidney [9] are the common primary target organs for many chemicals principally because of their role within the body. The gills are multifunctional—given their large surface area, they are responsible for respiration, osmoregulation, acid–base balance and nitrogenous waste excretion. Thus, they are extremely sensitive to water contamination [10]. Due to their delicate structure, they are liable to damage by any irritant material whether dissolved or suspended in water [11] and respond to environmental changes by structural alterations. In addition, they are not irritant specific but affected by the effect of factors’ intensity and duration of exposure, especially in cases of sublethal concentration of pollutants [12–14]. Gills are efficient tools for biomonitoring potential impacts [15, 16] because of their contact with water and high permeability [17–19]. Gills also help know the environmental impact caused by pollutants [20–22].
Fish liver is an interesting model for the study of interactions between environmental factors and hepatic structures and functions. The liver of teleosts is important in the maintenance of internal homeostasis and the metabolism of xenobiotics [23]. It has also been shown to accumulate foreign compounds [24] and be susceptible to damage by toxic agent [25, 26]; the functional integrity of the liver in fish can be affected by xenobiotics [27]. According to Buck [28], the liver is the first line of defence against copper poisoning. Copper becomes toxic only when the high binding capacity of the liver exceeds and copper is released into the blood stream. In fish also, the liver is the major storage organ for copper [29, 30], and hence, research on fish liver is important especially that related to aquaculture condition and aquatic pollution-induced problems.
Asian sea bass Lates calcarifer are a commercially significant fish in tropical regions. There is a very high economic value and demand for sea bass, but their farming is threatened due to heavy metal toxicity. Only a very few studies have reported in detail on the ultrastructural effects of toxic substances in the respiratory and liver tissues [31–35]. So, through our research, we made an attempt to study surface modifications of the gill and liver tissues in response to copper in Asian sea bass.
Materials and Methods
Experimental Fish
Healthy hatchery reared 3-month-old juvenile Asian sea bass L. calcarifer with a mean total length of 7.06 ± 0.15 cm and a mean total weight of 10.18 ± 0.24 g were obtained from the Rajiv Gandhi Centre for Aquaculture, Thirumullaivasal near Sirkali, Nagapattinam Dist, Tamil Nadu, India. Fish samples were acclimatized for 2 weeks in a stock tank to the experimental glass aquaria (120 × 50 × 50 cm) filled with 250 l of water with a salinity of 27 ± 2 ppt, under a natural photoperiod 12:12 h (light:dark) cycle. The water in the tanks was passed through a 1-μm filter, UV-sterilized and refilled daily. Fish were fed twice daily with commercially prepared sea bass pellet feed which contains 2.5 mg/kg of copper. They were starved for 24 h before and during experiment.
Chemicals Used
For preparation of stock solution, 3.9 g of copper ll sulphate pentahydrate (CuSo4 5 H2O) (Merck) was dissolved in 1 l of double-distilled water and used as stock solution. It was stored in a clean standard flask at room temperature in the laboratory.
Experimental Procedures
Test Concentration
Fish were exposed to nominal 6.83 and 13.66 ppm as copper. Doses were theoretically sublethal, 10 and 20 %, respectively, of the maximum acceptable toxicant concentration (MATC), which was 68.3 ppm. The MATC was represented as no observed effect concentration (NOEC) < MATC < LOEC (lowest observed effect concentration). The test concentration was estimated using the application factor (AF) concept, by dividing the limits (NOEC and LOEC) of the MATC by the 96-h LC50 (AF = MATC / LC50 = (NOEC − LOEC) / LC50).
System Design
A recirculation closed system was set up according to Muthuwan [36]. The experiment was carried out in 360 l glass aquarium (120 × 60 × 50 cm), in which one compartment (50 × 50 × 40 cm) was partitioned by a plastic gauze (mesh size 1.5 mm) to contain a biofilter. Each aquarium was filled with 300 l of natural sea water (salinity of 27 ± 2 ppt), which was pumped continuously over a biofilter column at a rate of 4 l/min. The water was continuously aerated throughout the experiment.
Test Procedure
After 2 weeks of acclimatization in a holding tank, ten healthy fish (8.06 ± 0.19 cm in length and 11.18 ± 0.67 g in weight) were transferred to each aquarium at a loading density of 0.69 g/l. Three replicates were performed for test concentration and control. Fish were fed twice daily with chopped fresh fish at 1000 and 1400 hours. Uneaten food was quickly removed from the system. Fish were starved for 24 h before sampling. The experimental water (50 %) was changed every 2 weeks to keep the water quality within acceptable limits according to APHA [37]. Water quality (dissolved oxygen, temperature, pH and salinity) was measured daily, and water chemistry (ammonia nitrogen, nitrite nitrogen, nitrate nitrogen) was measured twice weekly using the Merck water quality analyser kit. The ammonia nitrogen and nitrite nitrogen levels were controlled and kept within 0.2 mg/l for exchanging the water in 25 %. The actual concentration of copper was measured weekly before and after its addition to maintain copper concentrations at the designed level. Mortality and behaviour were observed daily in each concentration. Two fish from each aquarium were sampled at 0, 7 and 28 days post-exposure.
Scanning Electron Microscopic Study
Fish were quickly anesthetized with 50 mg/L MS 222 (tricaine methane sulphonate) for 2–3 min. Gills and liver tissues were rapidly removed and processed routinely for scanning electron microscopic studies. Gills and liver tissues were cut into small pieces of 1 mm thickness and fixed in 2.5 % glutaraldehyde prepared in cacodylate (sodium phosphate) buffer adjusted to pH 7.4 for 4 h and afterward washed in phosphate buffer for 15 min. Then, samples were post fixed in 1 % osmium tetroxide for 80 min and washed in phosphate buffer. After dehydration in ascending series of acetone, samples were coated with gold palladium and observed through scanning electron microscope (LEO Stereoscan, 440).
Results
Visual Observations
Sublethal Exposure
Control fish were good, without any morphological deviation. Some of the fish exposed to low-concentration copper exhibited slight reductions in feeding activity during the second and third weeks. In higher concentrations of copper, the fish did not swim actively and had reduced feeding activity throughout the exposure period. These signs were dosage dependent. Mortality did not occur in control or in copper-exposed groups.
Surface Ultrastructural Observations
Gills
Control
In scanning electron microscopic studies, the architectural pattern of gills of L. calcarifer is essentially similar to that of the other teleost fish. SEM images provide good three-dimensional views of different regions of respiratory lamellae. There are four gill arches on each side of the buccal cavity. Each arch is composed of numerous gill filaments which have two rows of secondary lamellae that run perpendicular to each filament. The secondary lamellae were sequentially lined up along the two sides of the primary lamella. In control fish, the secondary lamellae constituted evenly spaced parallel plates. The epithelial cells covered both the primary and secondary lamellae. Secondary lamellae are plate-like projections at right angles to the gill filaments. They lie parallel to the adjacent lamellae and covered by a thick and coarse epithelium. Chloride and mucous cells were distributed primarily at the bases of the secondary lamellae. Numerous water pores and mucous cell openings with well-developed microridges are discernible at the lamellae (Plate 1a). Chloride cells, seen as pit openings, are found among the protuberances and cavities of the surface. In high resolution of SEM, the surface epithelium of lamellae shows clear demarcation between cells and microridges.
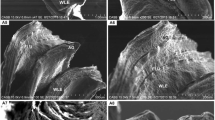
Scanning electron micrograph of gill in L. calcarifer. a Low magnification of SEM of respiratory lamellae of control fish. b Higher magnification SEM of gill tissue exposed to 6.83 ppm concentration of copper and sacrificed after 7 days displayed swelling in the secondary lamellae (SSL) and wrinkled epithelium. c, d Low magnification of SEM after 28 days of exposure to 6.83 ppm concentration of copper. Note thick blanket of mucus (TBM) and fused secondary lamellae (FSL). e SEM of gill tissue after 7 days of exposure to 13.66 ppm concentration of copper showing lifting of epithelium (LE) and note the complete fusion and distortion of secondary lamellae. f Higher magnification of SEM of gill tissue after 28 days of exposure to 13.66 ppm concentration of copper showing thinning of secondary lamellae (TSL) and sloughing of epithelial surface. Abbreviations used: PL primary lamellae, SL secondary lamellae, MRE microridged epithelial cell, IAES irregular arrangement of epithelial surface, SSL swelling of secondary lamellae, E oedema, TBM thick blanket of mucus, FSL fusion of secondary lamellae, CSL curling of secondary lamellae, LE lifting of epithelium, DSGL damaged secondary gill lamellae, SGLE sloughing of gill lamellar epithelium, TSL thinning of secondary lamellae
Treated Sublethal Exposure
The gills in the treated fish exhibited noteworthy damages than those in the control. Following the treatment at a 6.83 ppm concentration of copper after 7 days of exposure, diffuse oedema (E) and detachment of the lamellar epithelium (epithelial lifting) with the formation of large subepithelial spaces within the secondary lamellae were observed. SEM examination showed swelling and curling of secondary lamellae (SSL) (Plate 1b). After 28 days of low concentrations of copper, the gills showed extensive aneurism with some ruptures in many secondary lamellae and the breakdown of pillar cell system was seen. Moreover, partial or complete secondary lamellar fusion (FSL) (Plate 1c) and thickening of primary lamellae were encountered in the exposed sea bass as a result of inter-lamellar epithelium and chloride cell hyperplasia. SEM examination also showed complete fusion of secondary lamellae and surface wrinkling in numerous areas as a result of epithelial hyperplasia and/or hypertrophy. The surface wrinkling was severe in some regions. Mucous cell ruptures and a thick blanket of mucus (TBM) secretion (Plate 1d) were seen above the damaged epithelium. At the lowest concentration, also necrosis and leukocyte infiltration (granulocytes and macrophages) in secondary lamellae were observed. Curling of secondary lamellae (CSL) was severe in higher concentrations of copper-treated sea bass (Plate 1d). Lifting of the epithelium was common and particularly severe and extensive after 7 days of high concentrations of copper exposure (Plate 1e). In addition to this, sloughing of the primary and secondary gill epithelium (SGLE) was clearly evident in treated sea bass. The epithelial surface with microridges exhibit damaged and irregular appearance (IAES). Fusion of the secondary gill lamellar epithelium was clearly displayed in high concentrations of copper after 28 days of exposure, and this finally ended with a complete degeneration of secondary lamellae (Plate 1f). Exfoliated epithelium was a common feature in all treated fish, and the secondary lamellae were highly deformed as a consequence of the lifting of the epithelium and severe hyperplasia which led to the conjunction of adjacent filaments. In higher magnification, the SEM picture clearly depicts the denuding of the boundaries of surface epithelial cells of both primary and secondary lamellae. In some regions, the lamellae of treated fish were thinner than those of the control (Plate 1f).
Liver
Control
In all control fish, the ultrastructural morphology of hepatocytes was normal. The surface of the liver was covered with serous membrane and some connective tissue extending inward into parenchyma. It was composed of parenchymal cells (hepatocytes) (HC) and lattice fibres, which support the former. Hepatic cells were roundish polygonal, containing clear spherical nucleus (SN). They were located among sinusoids forming cord-like structures known as hepatic cell cords. Hepatic cells have many vital functions. Other than the secretion of bile, they play an important role in protein, lipid and carbohydrate metabolism. They serve as storage sites for some nutrients, and detoxification is another function attributed to them (Plate 2a).
Scanning electron micrograph of liver in L. calcarifer. a Lower magnification of scanning electron micrographs of the liver of control fish showing normal hepatocytes. b SEM of liver tissue after 7 days of exposure to 6.83 ppm concentration of copper showing highly distorted hepatocytes. c SEM of liver tissue after 28 days of exposure to 6.83 ppm concentration of copper showing cloudy swelling of hepatocytes (CSH). d, e SEM photograph of liver tissue after 7 days of exposure to 13.66 ppm concentration of copper depicting necrosis of hepatocyte (NHN) and accumulation of lipid droplets (ALD). f Higher magnification of SEM of liver tissue after 28 days of exposure to 13.66 ppm concentration of copper showing vacuolar degeneration (VD) and swelling of hepatocytes. Abbreviations used: HC hepatocyte, HN hepatocyte nucleus, VD vacuolar degeneration, CSH cloudy swelling of hepatocytes, NHN necrosis of hepatocyte nucleus, VDC vacuolar degeneration of cytoplasm, N necrosis, HS hydrophic swelling, ALD accumulation of lipid droplets, SN spherical nucleus
Treated Sublethal Exposure
Copper caused severe ultrastructural changes in the liver; the damage severity and its extension increased with the concentration of metal and duration of exposure. In exposed sea bass, the regular compartmentalization was totally lost. Other changes in lower concentrations of copper exposed to 7 and 28 days include cloudy swelling of hepatocytes, congestion, vacuolar degeneration, dilation of sinusoids and nuclear hypertrophy (Plate 2b, c). After 7 and 28 days of higher concentrations of copper exposure, the hepatocytes showed hydropic swelling (Plate 2d). Large lipid droplets and abundant glycogen occupied most of the area of hepatocytes (Plate 2e, f).
Discussion
The scanning electron micrographs of the untreated sea bass gill epithelium revealed normal architecture (Plate 1a). In contrast, the gills of L. calcarifer exposed to copper during 28 days presented a higher occurrence of histopathological lesions such as hypertrophy, fusion of secondary lamellae, oedema and mucus openings. Numerous types of gill damage have been documented in fish experimentally exposed to toxicants or in populations sampled from polluted environments [7, 38, 39]. Most of the gill histopathological changes are largely non-specific as confirmed by the occurrence of similar alterations under a wide range of toxicant-exposure conditions [40]. Hyperplasia with lamellar fusion, telangiectasia, oedema with epithelial lifting and desquamation, as documented in the present survey, are typical to organochlorines, petroleum compounds, organophosphates, carbamates, herbicides and heavy metals in other animals [41–44] and suggest an impairment to the respiratory and osmoregulatory functioning of the gills [10]. Exposure to heavy metals produces morphological and functional modifications in the branchial epithelium [7, 45]; mercury inhibits gill respiration at sub-acute and acute levels [46].
The first sign of pathology included oedema of epithelial cell in gills. This is due to the epithelium covering the secondary lamellae lifting away in a continuous sheet from the pillar cell system, thus, increasing the diffusion distance from water to blood (Plate 1e). The secondary lamellae showed capillary congestion or aneurism, similar to those reported in Gnathonemus petersii exposed to 10 mg/l of cadmium for 6 h [7]. This lamellar aneurism resulted from the collapse of the pillar cell system and the breakdown of vascular integrity with a release of large quantities of blood that push the lamellar epithelium outward [7]. The hypertrophy and hyperplasia of epithelial and chloride cells with partial or complete fusion of lamellae also occurred in this study (Plate 1c). The pathology related to the chloride cell hyperplasia was to eject the Cd2+ absorbed by the gills [47–49].
The immediate morpho-pathological response of the gills of fish exposed to ambient copper is often manifested by a significant increase in the density of its mucous cells (Plate 1c) [50–53]. The large quantity of mucous secretion acts as a defence mechanism with response to toxic substances [54, 55]. The sloughing of mucus from the surface of gills helps to remove the bound pathogens, toxicants and foreign matters [56].
Changes to the ultrastructure of fish livers have proven to be suitable and sensitive signs of toxicant-induced injury and have been used as biomarkers of chemicals in environmental risk assessments [57, 58]. There have been numerous reports on histo-cytopathological modifications in livers of fish exposed to a wide range of organic compounds and heavy metals [10, 41, 59]. The loss of the regular cytoplasmic compartmentation is a typical unspecific ultrastructural reaction of fish hepatocytes which indicates disturbance of hepatocellular homeostasis [57]. Some of the alterations observed in the hepatic cells in the present study, such as vacuolar degeneration, dilation of ER and lipid droplet accumulation, are consistent with those documented in specimens of Dicentrarcus labrax, L. calcarifer and Carassius carassius acutely treated with lead and cadmium [39, 44, 60–62]. A massive enhancement in the number of lipid droplets in the present study resulted from the decline of protein synthesis, and this accompanies with cellular injury. Glycogen deposition resulted in the disintegration of cellular organelles, and this may disturb the metabolic pathways of hepatocytes [63].
Copper reaches water bodies during treatment of the disease of fish and the control of algal blooms in sea bass hatchery. These copper will accumulate in their tissues of juvenile sea bass. Lethal concentration of copper can kill the fish. When fishes suffer from sublethal effects as a result of cumulative accumulation of copper, they may survive for longer times. In man, a daily consumption of these fishes will cause the ill effects that are specific to the toxicant. This process will damage the organism silently, without causing any immediate abrupt changes. The changes may be at genetic levels inducing genotoxicity, but concerted effort in reducing the use of copper and implementing natural remedies for disease control in sea bass hatchery can help resolve the problem of heavy metal pollution.
References
Batley GE, Apte S (1995) Trace metal speciation of labile chemical species in natural waters and sediments: non-electrochemical approaches. In: Tessier A, Turner D (eds) Metal speciation and bioavailability in aquatic systems. Wiley, New York
Straus DL, Tucker CS (1993) Acute toxicity of copper sulfate and chelated copper to channel cat fish Ictalurus punctatus. J World Aqu Soc 24:390–395
Heo GJ (1997) Antibacterial efficacy and safety of copper sulfate pentahydrate to cultured fish. Korean J Vet Res 37:203–212
Taylor LN, Mc Geer JC, Wood CM, Mc Donald DG (2000) Physiological effects of chronic copper exposure to rainbow trout (Oncorhychus mykiss) in hand and soft water, evaluation of chronic indicators. Environ Toxicol Chem 19:2298–2308
Lodhi HS, Khan MA, Verma RS, Sharma UD (2006) Acute toxicity of copper sulphate to fresh water prawns. J Environ Biol 27:585–588
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Alazemi BM, Lewis JW, Andrews EB (1996) Gill damage in the fresh water fish Gnathonemus petersii (family, Mormyridae) exposed to selected pollutants: an ultrastructural study. Environ Technol 17:225–238
Braunbeck T (1998) Cytological alterations in fish hepatocytes following in vivo and in vitro sublethal exposure to xenobiotics structural biomarkers of environmental contamination. In: Braunbeck T, Streit B, Hinton DE (eds) Fish ecotoxicology. Birkhauser Verlag, Switzerland, pp 61–140
Bernet D, Schmidt H, Meier W, Brkhardt-Holm P, Wahli T (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22:25–34
Au DWT (2004) The application of histocytopathological biomarkers in marine pollution monitoring: a review. Mar Pollut Bull 48:817–834
Roberts JR (1978) The pathophysiology and systematic pathology of teleosts. In fish pathology. 1st ed. Bailliere Tindall, London 67–70
Evans DH (1987) The fish gill: site of action and model for toxic effects of environmental pollutant. Environ Heal Perspect 71:47–58
Lindesjoo E, Thulin J (1994) Histopathology of skin and gills of fish in pulp mill effluents. Dis Aqua Org 18:81–93
Karan V, Vitorovic S, Tutundzic V, Poleksic V (1998) Functional enzyme activity and gill histology of carp after copper sulfate exposure and recovery. Ecotoxicol Environ Saf 40:49–55
Oliveira Ribeiro CA, Vollaire Y, Sanchez-Chardi A, Roche H (2005) Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the eel (Anguilla anguilla) at the Camargue Nature Reserve, France. Aquat Toxicol 74:53–69
Maharajan A, Rajalakshmi S, Vijayakumaran M, Kumarasamy P (2012) Sublethal effect of copper toxicity against histopathological changes in the spiny lobster, Panulirus homarus (Linnaeus, 1758). Biol Tra Ele Rese 145:201–210
Arellano JM, Storch V, Sarasquete C (2004) Ultrastructural and histochemical study on gills and skin of the Senegal sole, Solea senegalensis. J Appl Ichthyol 20:452–460
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid–base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Vigliano FA, Aleman N, Quiroga MI, Nieto JM (2006) Ultrastructural characterization of gills in juveniles of the Argentinian Silverside, Odontesthes bonariensis (Valenciennes, 1835) (Teleostei: Atheriniformes). Anat Histol Embryol 35:76–83
Zeeman MG, Brindley WA (1981) Effects of toxic agents upon fish immune systems: a review. In: Sharma RP (ed) Immunologic considerations in toxicology. CRC Press/Lewis Publisher, Boca Raton, pp 1–60
Schwaiger J, Wanke R, Adam S, Pawert M, Honnen W, Triebskorn R (1997) The use of histopathological indicators to evaluated contaminant-related stress in fish. J Aqua Ecosyst Str Recov 6:75–86
Teh SJ, Adams SM, Hinton DE (1997) Histopathological biomarkers in feral fresh water fish populations exposed to different types of contaminant stress. Aquat Toxicol 37:51–70
Chambers JE, Yarbrough JD (1976) Xenobiotic transformation systems in fishes. Comp Biochem Physiol 55C:77–84
Statham CN, Croft WA, Lech JJ (1978) Uptake, distribution, and effects of carbon tetrachloride in rainbow trout (Salmo gairdneri). Toxicol Appl Pharmacol 45:131–140
Racicot CG, Gaudet M, Leray C (1975) Blood and liver enzymes in rainbow trout (Salmo gairdneri) with emphasis on their diagnostic use study of CCl4 toxicity and a case of Aeromonas infection. J Fish Biol 7:825–835
Gingerich WH, Weber LJ, Larson RE (1978) Carbontetrachloride-induced retention of sulfobromophthalein in the plasma of rainbow trout. Toxicol Appl Pharmacol 43:147–158
Gingerich WH (1982) Hepatic toxicology of fishes. In: Weber LJ (ed) Aquatic toxicology. Raven, New York, pp 55–105
Buck WB (1978) Copper/molybdenum toxicity in animals. In: Oehme FW (ed) Toxicity of heavy metals in the environment. Marcel Dekker, Inc, New York, pp 491–515, Part I
Buckley LA, Jiang XZ, James RA, Morgan KT, Barrow CS (1982) Respiratory tract lesions induced by sensory irritants at the RD50 concentration. Toxicol Appl Pharmacol 74:417–429
Shearer KD (1984) Changes in elemental composition of hatchery reared rainbow trout, Salmo gairdneri, associated with growth and reproduction. Can J Fish Aqua Sci 41:1592–1600
Li J, Quabius SE, Wendelaar Bong SE, Flick G, Lock RAC (1998) Effects of waterborne copper on branchial chloride cells and Na+/K+-ATPase activities in Mozambique tilapia (Oreochromis mossambicus). Aquat Toxicol 43:1–11
Dang Z, Lock R, Flik G, Wendelaar Bonga SE (2000) Na+/ K+- ATPase immuno reactivity in branchial chloride cells of Oreochromis mossambicus exposed to copper. J Exp Bio 203:379–387
Monteiro SM, Rocha E, Fontaínhas-Fernandes AA, Sousa M (2008) Quantitative histopathology of Oreochromis niloticus gills after copper exposure. J Fish Biol 73:1376–1392
Monteiro SM, Rocha E, Mancera JM, Fontaínhas- Fernandes AA, Sousa M (2009) A stereological study of copper toxicity in gills of Oreochromis niloticus. Ecotoxicol Environ Saf 72:213–223
Monteiro SM, Rocha E, Mancera JM, Fontaínhas- Fernandes AA, Sousa M (2009) Copper toxicity in gills of the teleost fish, Oreochromis niloticus: effects in apoptosis induction and cell proliferation. Aquat Toxicol 94:219–228
Muthuwan V (1998) Green water recirculation system for intensive marine shrimp culture. PhD thesis, School of environmental, resource and development, Asian Institute of Technology 91–120
APHA (1995) Standard methods for the examination of water and waste water, 19th edn. American Public Health Association, American Water Works Association, and Water Pollution Control Federation, Washington, D.C
Pawert M, Mu¨ller E, Triebskorn R (1998) Ultrastructural changes in fish gills as biomarker to assess small stream pollution. Tissue Cell 30(6):617–626
Thophon S, Kruatrache M, Upatham ES, Pokethitiyook S, Sahaphong P, Jaritkhuan S (2003) Histopathological alterations of white sea bass Lates calcarifer, in acute and subchronic cadmium exposure. Environ Pollut 121:307–320
Mallatt J (1985) Fish gill structural changes induced by toxicants and other irritants, a statistical review. Can J Fish Aqua Sci 42:630–648
Global Tox A (1997) Technical evaluation of histopathology as an environmental monitoring tool for the mining industry in Canada. Report prepared for Aquatic Effects Technology Evaluation (AETE) Program, Ottawa. 1997, Natural Resources Canada by Global Tox International Consultants Inc. 153
Jiraung Koorskul W, Upatham ES, Kruatrachue M, Vichasrigrams S, Pokelhitiyook P (2003) Biochemical and histopathological effects of glyphosate herbicide on Nile tilapia (Oreochromis niloticus). Environ Toxicol 18(4):260–267
Dezfuli BS, Simoni E, Giari L, Manera M (2006) Effects of experimental terbuthylazine exposure on the cells of Dicentrarchus labrax (L.). Chemistry 64:1684–1694
Giari L, Manera M, Simoni E, Dezfuli BS (2007) Cellular alterations in different organs of European sea bass Dicentrarchus labrax (L.) exposed to cadmium. Chemistry 67(6):1171–1181
Mauceri A, Fossi MC, Leonzio C, Ancora S, Minniti F, Malsano M, Lo Cascio P, Ferrando S, Fasulo S (2005) Stress factors in the gills of (Mugilidae, Teleosts) living in polluted environments. Ital J Zool 72:285–293
Jagoe CH, Faivre A, Newman MC (1996) Morphological and morphometric changes in the gills of mosquitofish (Gambusia holbrooki) after exposure to mercury. Aquat Toxicol 34:163–183
Oransaye JAO, Brafield AE (1984) The effect of dissolved cadmium on the chloride cells of the gills of the stickleback, Gasterosteus aculeatus L. J Fish Biol 25:253–258
Gill TS, Pant JC, Pant J (1988) Gill, liver and kidney lesions associated with experimental exposures to carbaryl and dimethoate in the fish (Puntius conchonius Ham). Bull Environ Contam Toxicol 41:71–78
Fu H, Steinbach OM, Van den Hamer CJA, Balm PHM, Lock RAC (1990) Involvement of cortisol and metallothionein like proteins in the physiological responses to tilapia (Oreochromis mossambicus) to sublethal cadmium stress. Aquat Toxicol 16:257–270
Bradbury L (1987) British Columbia: metropolis and hinterland in microcosm. In: McCann LD (ed) Heartland and hinterland: a geography of Canada. Prentice-Hall Canada Inc, Scarborough, pp 400–441
Wise ML, Stiebel CL, Grizzp JM (1987) Acute toxicity of nitrofurazone to channel catfish, Ictalurus punctatus and gold fish Carassius auratus. Bull Environ Contam Toxicol 38:42–46
Dutta HM (1997) A composite approach for evaluation of the effect of pesticides on fish. In: Munshi JSD, Dutta HM (eds) Fish morphology: horizon of new research. Sci Pub Inc, USA, pp 249–277
Hemalatha S, Banerjee TK (1997) Histopathological analysis of sublethal toxicity of zinc chloride to the respiratory organs of the air-breathing cat fish Heteropneustes fossilis (Bloch). Biol Res 30:11–21
Handy RD, Eddy FB (1991) The absence of mucus on the secondary lamellae of unstressed rainbow trout. Oncorhynchus mykiss (Walbaum). J Fish Biol 38(1):153–155
Mazon AF, Cerqueira CCC, Monteiro EAS, Fernandes MN (1999) Acute copper exposure in freshwater fish: morphological and physiological effects. In: Val AL, Almeida-Val VMF (eds) Biology of tropical fishes. INPA, Manaus, pp 263–275
Powell MD, Speare DJ, Burka JF (1992) Fixation of mucous on rainbow trout (Oncorhynchus mykiss Walbaum) gills for light and electron microscopy. J Fish Biol 41:813–824
Braunbeck T, Volkl A (1991) Induction of biotransformation in the liver of eel (Anguilla anguilla L.) by sublethal exposure to dinitro-o-cresol: ultrastructural and biochemical study. Ecotoxi Environ Saf 21:109–127
Al-Bairuty GA, Shaw BJ, Handy RD, Henry TB (2013) Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aqua Toxicol 126:104–115
Hinton DE, Lauren DJ (1990) Liver structural alterations accompanying chronic toxicity in fishes potential biomarkers of exposure. In: McCarthy JF, Shugart LR (eds) Biomarkers of environmental contamination. Lewis Publishers, Boca Raton, pp 17–57
Franchini A, Barbanti E, Bolognani Fantin AM (1991) Effects of lead on hepatocyte ultrastructure in Carassius carassius (L.) var.auratus. Tiss Cell 23(6):893–901
Thophon S, Pokethitiyook P, Chalermwat K, Upatham ES, Sahaphong S (2004) Ultrastructural alterations in the liver and kidney of white sea bass, Lates calcarifer, in acute and subchronic cadmium exposure. Environ Toxicol 19(1):11–19
Ezhilmathy R, Rajalakshmi K, Chezhian A (2014) Histological alterations in sea bass. Lates calcarifer exposed to combined stressors of pesticide and metal (Profenofos and lead nitrate). Inter J Rese Mar Sci 3(2):44–47
Cheville NF (1994) Ultrastructural pathology: an introduction to interpretation, 1st edn. Iowa State University Press, Ames, pp 67–68
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paruruckumani, P.S., Maharajan, A., Ganapiriya, V. et al. Surface Ultrastructural Changes in the Gill and Liver Tissue of Asian Sea Bass Lates calcarifer (Bloch) Exposed to Copper. Biol Trace Elem Res 168, 500–507 (2015). https://doi.org/10.1007/s12011-015-0370-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0370-z