Abstract
In the present study, the effect of tributyltin (TBT) on the histopathological and hormonal changes during spermatogenesis in freshwater prawn Macrobrachium rosenbergii was documented. Three experimental concentrations such as 10, 100 and 1,000 ng/L were selected and exposed to prawns for 45 days. After TBT exposure, the reproductive activities like sperm count and sperm length were decreased when compared with control. Further, abnormal structure of the seminiferous tubule, decrease in spermatozoa concentration, diminution of the seminiferous tubule membrane and the abundance of spermatocytes in the testis were noticed in treated prawns. Interestingly, radioimmunoassay clearly revealed the reduction of testosterone level in TBT exposed groups. Thus, TBT has considerably reduced the level of testosterone and caused the impairment of spermatogenesis in the freshwater male prawn M. rosenbergii.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Freshwater ecosystem is under increasing threat due to rapidly expanding population and subsequent modernization process, which resulted in conspicuous exploitation of nature leading to pollution. The rivers are very vulnerable, since effluents from industries, domestic and farm open directly into them. During the past few decades, rising trends of population explosion, development of modern technology, industrialization and increase in the production and consumption of large variety of new synthetic chemicals are the causatives of enhanced release of pollutants in aquatic environment (Kharat et al. 2009). Accumulation of industrial effluents and agricultural runoff into water bodies has become a major concern (FAO 1986).
Organotin compounds, particularly tributyltin (TBT), have classically been reported to be strong endocrine disrupting compound (EDC), as they have been widely used as biocides in a variety of consumer and industrial products. TBT is a ubiquitous persistent xenobiotic that can be found in freshwater, estuarine and marine ecosystem (Fent 1996). Coexistence of TBT and triphenyltin (TPT) is still reported in aquatic environments and causes ecotoxicological threats to aquatic organisms because of its industrial applications (Zuo et al. 2012; Revathi et al. 2013a).
High concentration of TBT has been detected in bivalves and fishes collected from heavy organotin contaminated sites (Morcillo and Porte 1997). TBT causes morphological abnormalities and oogenesis in molluscs and crustaceans (Weis et al. 1987; Weis and Kim 1988, Revathi et al. 2013a). Though the mechanism of toxicity is unclear, triorganotins are thought to operate by inhibiting oxidative and photosynthetic phosphorylation processes essential to most of the organisms (Duncan 1980). The wide range and fundamental toxicity have raised concern over the effects of triorganotins on non-target organisms (Chiliamovitch and Kuhn 1977).
Histological analysis appears to be a very sensitive parameter and is crucial in determining cellular changes that may occur in target organs (Dutta 1996). Several studies have assessed the ability of endocrine disruptors to alter steroid hormone metabolism in invertebrates. Some studies have additionally assessed the impact of xenobiotics on endogenous steroid levels, which may in turn be an indication of altered steroid synthesis and metabolism (Revathi 2010).
Few studies have examined the effects at gametic levels, especially on sperm production (Mansueto et al. 2011; Revathi et al. 2013b). In our previous study, TBT showed significant inhibition of organogenesis as well as embryonic development in Macrobrachium rosenbergii (Revathi and Munuswamy 2010). The effect of TBT on the gametogenesis has been mostly studied in molluscs, however, only a limited number of research has been carried out on the impact of TBT in crustaceans peculiarly no much information is available on freshwater organisms like commercially important species M. rosenbergii (Revathi and Munuswamy 2010). Our previous study addressed the variation in ultrastructural, biochemical and sex hormone level in male prawn M. rosenbergii due to the long term exposure of TBT (Revathi et al. 2013b). However, the present investigation aimed to study the impact of TBT on spermatogenesis by evaluating cellular level variations in testis and quantification of testosterone in freshwater male prawn M. rosenbergii.
Materials and Methods
TBT was obtained from Himedia, India, with a purity of greater than 98 %. All other chemicals were of analytical grade and were obtained from commercial sources. Freshwater male prawn, M. rosenbergii were collected from the Aqua Nova hatchery in Kannathur near Chennai, South India. The collected prawns were brought to the laboratory in a plastic cover with habitat water. They were introduced into plastic tanks with sufficient aeration. The water was changed daily and they were fed ad libitum with commercial pelletized feed. They were maintained in the laboratory for 2–3 weeks for acclimatization.
The 5 month old prawns weighing 16 ± 2 g were selected and twenty prawns per group were exposed to TBT. The first group served as control (without any treatment). As ethanol is a solvent used to prepare the TBT (TBT chloride) solutions, the second group served as positive control that received 2 % ethanol treatment. Remaining three groups were individually exposed to environmentally realistic concentrations of TBT i.e., 10,100 and 1,000 ng/L using water as the medium (data not shown). Each group of prawns was maintained in the individual plastic tanks containing 100 L of well aerated water and the water subjected to static renewal. Every day, the water was exchanged and the nominal concentrations of TBT were maintained in the respective experimental tanks. The experiment was performed for a period of 45 days. The water temperature was maintained at 18 ± 2°C. Three replicate tanks were maintained for each treatment group.
The water samples (250 mL) from each experimental tank were collected individually and stored at 4°C in the dark until laboratory analysis. The pH of the water samples was adjusted to 5 with 5 mL of sodium acetate buffer. To this, 100 µL of 2 % Sodium tetraethyl borate (NaBEt4) was added and the mixture was stirred for 10 s. Then the mixture was allowed to stand for 10 min in the dark for reaction. Afterwards, 500 µL of isooctane was added to the mixture with vigorous magnetic stirring for 15 min. Then, Milli-Q water was added to obtain about a 3 cm high level in the narrow neck to facilitate decantation. From the mixture, 0.5 mL supernatant and 1 mL underlying aqueous phase were transferred into 2 mL effendorf tube and centrifuged for 5 min at 10,000 rpm at 4°C. Finally, the extracts were individually transferred into uncoated graphite tubes and immediately analyzed using graphite furnace atomic absorption spectrometry (GFAAS) at 286.3 nm for the quantification of TBT in the water samples (Michel and Averty 1991).
At the end of the experiment, the prawns were weighed and the gonads were removed and the wet weight of the gonads was recorded. The GSI and HSI were calculated following the procedure described by Zhang et al. (2007).
The sperm count of extruded spermatophoric mass was done following the direct cell count method (Tomar 1970).
Spermatophores were taken from the male prawn M. rosenbergii and it was homogenized with 1 % saline. From this solution one drop of the suspension was taken onto a slide and viewed under the Leica 2500 microscope. Randomly three samples of sperm were used for measuring their length using LAS software installed in Leica 2500 microscope (Germany).
The testes of male prawn in both control and treated groups were dissected and fixed in Bouin’s fixative for histological studies. The tissues were dehydrated through a graded alcohol series and embedded in paraffin wax. Sections of 6–8 µm thickness were taken and stained with hematoxylin and eosin. The stained sections were mounted using DPX and photomicrographs were taken using Leica 2500 microscope (Germany).
The testes were dissected out from control and experimental prawns, pooled, shock frozen in liquid nitrogen and homogenized individually on ice in 100 µL deionized water using a motor driven Teflon pestle. Testosterone metabolites were extracted using 4 mL ethyl acetate (2 × 2 mL) and the organic phase was separated using centrifugation at 5,000×g for 15 min at 4°C. The ethyl acetate fractions were pooled and evaporated under a stream of nitrogen.
The level of free immunoreactive testosterone was estimated using radioimmunoassay (RIA) according to the protocol of Oreczyk et al. (1974). The testosterone extracts were reconstituted separately in 100 µL of gelatin phosphate buffer solution (GPBS) (sodium phosphate buffer 0.1 M, pH 7.2, containing 0.15 M NaCl and 0.1 % gelatin) in RIA tubes. Appropriately diluted antiserum to testosterone (New England Nuclear Corp., Boston, MA) and 0.1 mL of [3H]-steroids without antiserum (to determine non-specific binding) were included in every assay. At the end of incubation, the unbound steroids were adsorbed on dextran coated charcoal to separate it from bound steroids. For that, 0.3 mL of dextran coated charcoal (0.1 % dextran T70 and 1 % charcoal in PSB) was added to each tube. Afterwards, the tubes were centrifuged at 3,000×g for 20 min at 4°C. The supernatant was poured carefully without disturbing the charcoal pellet into the vials containing 5 mL of scintillation fluid (0.5 % PPO, 0.04 % POPOP and 25 % methanol I toluene). The vials were kept shaking at room temperature to extract steroids into aqueous phase and steroid levels were estimated using a liquid scintillation counter (Beckman, USA).
Normality and homogeneity of the relative reproductive index, sperm count, sperm length and testosterone level was statistically analyzed by following one way analysis of variance (ANOVA) and Tukey- Dunnett test using SPSS 16.0 to determine the significant variations between the control and TBT treated groups.
Results
The male reproductive system was studied by observing the cellular level changes in testis of both control and treated groups. Further, the male reproductive activity was assessed based on the GSI and HSI level, sperm count, sperm length, cellular level changes and sex steroid levels in TBT treated and control prawns.
The concentration of TBT in experimental tanks was maintained by daily dosage after water exchange. At the end of the experiment, the concentration of TBT in experimental tanks was quantified using GFAAS and the values were 8.90, 84.6 and 892.7 ng/L TBT in 10, 100 and 1,000 ng/L of TBT treated tanks respectively (Table 1). One, one, two, three, four prawns died during the 45 days exposure period in the control, control-ethanol, 10,100 and 1,000 ng/L TBT treated groups respectively.
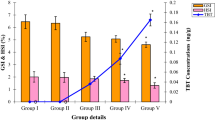
TBT had significantly reduced the GSI and HSI values in treated prawns compared to control (Fig. 1). In control, the GSI and HSI values recorded as 1.16 ± 0.09 % and 6.96 ± 0.40 % respectively. However, the GSI and HSI values were decreased to 0.49 ± 0.04 % and 3.1 ± 0.42 % at higher concentration of TBT (1,000 ng/L), respectively after 45 days of exposure. The GSI and HSI values statistically varied between control and TBT treated groups (p < 0.05 to p < 0.001).
Sperm count in the control prawn, was recorded as 2.57 ± 0.28 millions/spermatophoric mass (Table 2). In contrast, it drastically reduced to 2.43 ± 0.11 millions/spermatophoric mass, 1.32 ± 0.13 millions/spermatophoric mass and 0.33 ± 0.06 millions/spermatophoric mass, respectively in 10 mg/L, 100 mg/L and 1,000 ng/L TBT treated prawns. Statistical variations in sperm count between control and TBT treated groups was significant (p < 0.05 to p < 0.001).
Further, prawns exposed to TBT showed reduction in sperm length compared to control (Table 2). In control prawn, the sperm length was recorded as 8.11 ± 0.06 µm. However, in the treated groups, the sperm length was drastically reduced from 7.87 ± 0.54 µm (at 10 ng/L TBT) to 3.69 ± 0.09 µm (1,000 ng/L TBT). Sperm length between control and TBT treated prawns were statistically significant (p < 0.05 to p < 0.001).
In control prawn, mature spermatozoa were filled up in the lumen of the seminiferous tubules. However, in 10 ng/L TBT treatment, the testis showed decrease in seminiferous tubule diameter and decrease in the concentration of spermatozoa. However, higher concentrations of TBT such as 100 and 1,000 ng/L resulted in disruption of seminiferous tubule architecture, increase in connective tissues, abundance of immature sperm cells like spermatogonia and spermatocytes (Fig. 2a–d).
a Control prawn (without treatment) testis showing fully mature spermatozoa (Sz) tightly packed with the lumen of seminiferous tubule (St). b At 10 ng/L TBT exposure, testis showing decrease in concentration of mature spermatozoa (Sz) in the seminiferous tubule and decrease in seminiferous tubule size (up arrow). c Testis showing disruption of seminiferous tubule architecture (up arrow St), increase in connective tissue level (up arrow Ct) at 100 ng/L TBT exposure. d At 1,000 ng/L TBT exposure, testis showing minimum concentration of mature spermatozoa (Sz), disruption of seminiferous tubule architecture, abundance of spermatocytes (Sc). Note the decrease in mature spermatozoa cells level in TBT treated groups compared to control. Bar 50 µm
The testosterone level in testis of control and treated groups was depicted in Fig. 3. The testosterone level in control prawn was measured as 11.3 ± 1.06 pg/g. However, there was a remarkable reduction in the testosterone level as the concentration of TBT increased from 10 to 1,000 ng/L. At higher concentration (1,000 ng/L), testosterone level decreased to 3.7 ± 0.57 pg/g in testis. Tukey-Dunnett test revealed that, the testosterone level in testis of TBT treated and control groups were statistically significant (p < 0.05 to p < 0.001).
Discussion
The present results inferred that, TBT possesses adverse effect on male reproductive system of M. rosenbergii as evidenced by reduced GSI, HSI values, retarding spermatogenesis, sperm count and sperm length. A decrease of 1.96-fold in GSI and 1.42-fold in HSI 2.42-fold sperm count and 1.0-fold sperm length was recorded at higher concentration (1,000 ng/L) of TBT. Due to the toxic effects of TBT the size of the gonads and hepatopancreas of the treated prawns was found to be decreased. Pesticides caused partial or total arrest of spermatogenesis may responsible for the reduction in sperm count and length in TBT exposed prawns as stated by Srivastava et al. (2008). Similar observation on the decrease of GSI in male prawn M. rosenbergii ascribable to TBT toxicity in the long term study has been reported (Revathi et al. 2013b). Accordingly, Revathi et al. (2013c) also described that TBT exposure led to the reduction in GSI and HSI values in mud crab Scylla serrata. Jegou (1992) found that dysfunction may lead to a reduction in sperm quality and possible infertility. Likewise Fent and Hunn (1995) suggested that TBT can also affect sperm count and male reproductive system in aquatic organisms. Accordingly, low levels of TBT exposure for 21 days decreased sperm count in guppies (Poecilia reticulata) (Haubruge et al. 2000). Nakayama et al. (2004) reported that TBT affects sexual behavior and reproduction in medaka (Oryzias latipes). Similarly, TBT even at environmentally realistic concentrations had also adversely affected the gametogenesis in cuvier Sebastiscus marmoratus (Zhang et al. 2007).
Testicular inflammation was documented as one of the common responses in aquatic animals exposed to environmental toxicants (Sokal et al. 1985; Ruby et al. 1986, 1987). The present results clearly documented the abnormal cellular architecture in testis and impairment of spermatogenesis in M. rosenbergii due to TBT toxicity. Overall, the results claim a dose dependent toxicity of TBT on the reproductive performance of M. rosenbergii. Zutshi (2005) observed appreciable reduction in size, with spermatids and sperms in degenerating condition and necrosis of interstitial cells after fenthion treatment in fish, Glossogobius giuris. Zutshi and Murthy (2001) reported the extensive cytotoxic damage in the testis of Glossogibius giuris after fenthion exposure. Pesticides cause testicular atrophy such as disruption of seminiferous tubules and destroyed leydic cells that can arrest the spermatogenesis in fishes (Srivastava et al. 2008). Kumar et al. (2007) have also documented in vivo cytotoxic damage in testis of Heteropneustes fossilis exposed to anionic surfactant.
From the present study it is obvious that the TBT had significantly decreased testosterone level in testis of M. rosenbergii. Similar results was observed in male cover exposed to TBT (Zheng et al. 2005). Exposure of TPT led to a significant increase in fatty acid esterification of testosterone in Paracentrotus lividus (Janer et al. 2005). This is probably a mechanism to lower endogenous levels of testosterone by esterifying it with fatty acids. Increased sulfation may again be a mechanism to inhibit the biological activity of testosterone by decreasing its affinity to putative steroid receptors and facilitating its elimination (Lavado et al. 2006). The alterations of testosterone and energy metabolism are sensitive end points in mysids exposed to different xenobiotics (Verslycke et al. 2003a, b). Some other pesticides such as malathion, chlordane and lindane also caused a decrease in testosterone in aquatic organisms (Baldwin and LeBlane 1994; Parks and LeBlanc 1996). In the above context, the present work clearly manifests that TBT had deleterious effects on spermatogenesis, reduction in testosterone level and impairment in the reproductive system of freshwater male prawn, M. rosenbergii.
References
Baldwin WS, LeBlane GA (1994) Identification of multiple steroid hydroxylases in Daphnia magna and their modulation by xenobiotics. Environ Toxicol Chem 13:1013–1021
Chiliamovitch VP, Kuhn C (1977) Behavioral, hematological studies on acute toxicity of bis (tri-n-butyltin) oxide on Salmo gairdneri Richardson and Tilapia rendalli Boulenger. J Fish Biol 10:575–585
Duncan J (1980) The toxicology of molluscidies. Organotin Pharm Ther 10:407–429
Dutta GJ (1996) Uridine diphosphate glucose and the synthesis of glucosides by mollusks. Arch Biochem Biophysiol 116(1):399–405
FAO FAO/UNOP (1986) Meeting on the effect of pollution on marine ecosystem. FAO. Fish Report 352:20
Fent K (1996) Ecotoxicology of organotin compounds. Cri Rev Toxicol 26:1–117
Fent K, Hunn J (1995) Organotins in freshwater harbours and river-temporal distribution, annual trends and fate. J Environ Toxicol Chem 14:1123–1132
Haubruge E, Petit F, Gage MJG (2000) Reduced sperm counts in guppies (Poecilia reticulate) following exposure to low levels of Tributyltin and Bisphenol A. Biol Sci 267:2333–2337
Janer G, Sternberg RM, LeBlanc GA, Porte C (2005) Testosterone conjugating activities in invertebrates: are they targets for endocrine disruptors? Aquat Toxicol 71:273–282
Jegou B (1992) The sertoli cells in vivo and in vitro. Cell Biol Toxicol 8:49–54
Kharat PS, Laxmi I, Ghoble B, Shejule KB, Ghoble IBC (2009) Effect of TBTCL on glycogen profile in freshwater prawn, Macrobrachium kistnensis. World App Sci J 7(12):1534–1539
Kumar M, Trivedi SP, Misra A, Sharma S (2007) Histopathological changes in testis of the freshwater fish, Heteropneustes fossilis (Bloch) exposed to linear alkyl benzene sulphonate (LAS). J Environ Biol 28:679–684
Lavado R, Barbaglio A, Candia Carnevali MD, Porte C (2006) Steroid levels in crinoid echinoderms are altered by exposure to model endocrine disruptors. Steroids 71:489–497
Mansueto V, Vittoria CM, Faqi AS (2011) Post-embryonic development effect of bisphenol A and tributyltin effects in Ciona intestinalis. Caryologia 64(4):478–484
Michel P, Averty B (1991) Tributyltin analysis in seawater by GC-FPD after direct aqueous phase ethylation using sodium tetraethyl borate. Appl Organomet Chem 5:393–397
Morcillo Y, Porte C (1997) Interaction of tributyl and triphenyltin with the microsomal monooxygenase system of mollusks and fish from the western Mediterranean. Aquat Toxicol 38:35–46
Nakayama K, Oshima Y, Yamaguchi T, Tsuruda Y, Kang IJ, Kobayashi M, Imada N, Honjo T (2004) Fertilization success and sexual behavior in male medaka, Oryzias latipes exposed to tributyltin. Chemosphere 55:1331–1337
Oreczyk GP, Caldwell BV, Behrman HR (1974) Endocrinology. In: Jaffe BM, Behrman HR (eds) Methods of hormone radioimmunoassay. New York, Academic press, pp 256–258
Parks LG, LeBlanc GA (1996) Reductions in steroid hormone biotransformation/elimination as a biomarker of pentachlorophenol chronic toxicity. Aquat Toxicol 34:291–303
Revathi P (2010) Studies on the endocrine disruptor and its impact on the reproductive physiology of the freshwater prawn Macrobrachium rosenbergii (De Man). Ph. D., Thesis, University of Madras. Chennai, Tamil nadu, India
Revathi P, Munuswamy N (2010) Effect of TBT on the early embryonic development in the freshwater prawn Macrobrachium rosenbergii (De Man). Chemosphere 79:922–927
Revathi P, Iyapparaj P, Arockia Vasanthi L, Munuswamy N, Krishnan M (2013a) Impact of TBT on the vitellogenesis and sex hormones in freshwater prawn, Macrobrachium rosenbergii (De Man, 1879). Aquat Biosyst 9:10
Revathi P, Iyapparaj P, Arockia Vasanthi L, Munuswamy N, Krishnan M (2013b). Ultra structural changes during spermatogenesis, biochemical and hormonal evidences of testicular toxicity caused by TBT in prawn Macrobrachium rosenbergii (De Man). Environ Toxicol. doi:10.1002/tox.21848
Revathi P, Iyapparaj P, Arockia Vasanthi L, Munuswamy N, Krishnan M (2013c) Bioaccumulation of tributyltin and its impact on spermatogenesis in mud crab Scylla serrata (Forskal). Turk J Biol 37:296–304
Ruby SM, Idler DR, So YP (1986) The effect of sublethal cyanide exposure on plasma vitellogenesis levels in rainbow trout, Salmo gaidneri during early vitellogenesis. Arch Environ Contam Toxicol 15:603–607
Ruby SM, Idler DR, So YP (1987) Changes in plasma, liver and ovary vitellogenin in land-locked atlantic salmon following exposure to sublethal cyanide. Arch Environ Contam Toxicol 16:507–510
Sokal RZ, Madding CE, Swerdloff RS (1985) Lead toxicity and the hypothalamic pitutary testicular axis. Biol Reprod 33:722–728
Srivastava T, Yadav K, Sunil Trivedi P, Devi K (2008) Cyprin induced gonadal impairment in a freshwater food fish, Channa punctatus (Bloch). J Environ Biol 29(2):187–191
Tomar NS (1970) Artificial Insemination and reproduction of cattle and buffalo, 2nd edn. Saroj Prakashan, Katra, Allahabad-2
Verslycke T, Vercauteren J, DeVos C, Moens L, Sandra P, Janssen CR (2003a) Cellular energy allocation in the estuarine mysid shrimp Neomysis integer (Crustacea: Mysidacea) following tributyltin exposure. J Exp Mar Biol Ecol 288:167–179
Verslycke T, Poelmans S, De Wasch K, Vercauteren J, Devos CMoens L, Sandra P, De Brabander HF, Janssen CR (2003b) Testosterone metabolism in the estuarine mysid Neomysis integer (Crustacea: Mysidacea) following tributyltin exposure. Environ Toxicol Chem 22:2030–2036
Weis JS, Kim K (1988) Tributyltin is a teratogen in producing deformities in limbs of the fiddler crab, Uca pugilator. Arch Environ Contam Toxicol 17:583–587
Weis JS, Gottlieb J, Kwiatkowski J (1987) Tributyltin retards regeneration and produces deformities of limbs in the fiddler crab, Uca pugilator. Arch Environ Contam Toxicol 16:321–326
Zhang J, Zhenghong Z, Yixin C, Yang Z, Shuai H, Chonggang W (2007) Effect of tributyltin on the development of ovary in female cuvier (Sebasticus marmoratus). Aquat Toxicol 83:174–179
Zheng RH, Wang CG, Zhao Y, Zuo ZH, Chen YX (2005) Effect of tributyltin, benzo(a)pyrene and their mixture exposure on the sex hormone levels in gonads of cuvier (Sebastiscus marmoratus). Environ Toxicol Pharma 20:361–367
Zuo Z, Wang C, Wu M, Wang Y, Chen Y (2012) Exposure to tributyltin and triphenyltin induces DNA damage and alters nucleotide excision repair gene transcription in Sebastiscus marmoratus liver. Aquat Toxicol 122–123:106–112
Zutshi B (2005) Ultrastructure studies on the effect of fenthion on pituitary (GTH cells) and testis of Glossogobius giuris (Ham) during breeding phase. J Environ Biol 26(1):31–36
Zutshi B, Murthy PS (2001) Ultrastructural changes in testis of gold fish Glossogobius giuris (Ham.) induced by fenthion. Ind J Exp Bio 39:170–173
Acknowledgments
The authors would like to acknowledge sincere thanks to Dr. D. S. Kothari Post Doctoral Fellowship scheme of University Grants Commission (UGC), India for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Revathi, P., Iyapparaj, P., Arockia Vasanthi, L. et al. Influence of Short Term Exposure of TBT on the Male Reproductive Activity in Freshwater Prawn Macrobrachium rosenbergii (De Man). Bull Environ Contam Toxicol 93, 446–451 (2014). https://doi.org/10.1007/s00128-014-1332-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1332-4