Abstract
The widespread contamination and persistence of the herbicide butachlor in the environment resulted in the exposure of non-target organisms. The present study investigated the toxicity effect of butachlor (1–15 µmol/L) and the protective effect of vitamin C (VC) against butachlor-induced toxicity in zebrafish. It was found that butachlor significantly increased the mortality and malformation rates in a dose-dependent manner, which caused elevation in reactive oxygen species (ROS) and malondialdehyde (MDA) after 72 h exposure. Compared with butachlor treatment group, the protective effect of VC against butachlor-induced toxicity were observed after adding 40, 80 mg/L VC respectively. VC significantly decreased the mortality, malformation rates, ROS, MDA, and normalized antioxidant enzymes activities of zebrafish after 72 h exposure. The result shows VC has mitigative effect on butachlor-induced toxicity and it can be used as an effective antioxidant in aquaculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Butachlor (N-butoxymethyl-2-chloro-2′,6′-diethyl acetanilide) is a chloroacetanilide herbicide which inhibits protein synthesis in developing plant tissue and is widely used for pre-emergence or early post-emergence control of a variety of undesirable weeds in crops (Mohanty et al. 2001), and its consumption is nearly 4.5 × 107 kg per year in Asia alone (Abigail et al. 2015). With the widespread use of butachlor, it has been detected in the soil (Shi et al. 2011), groundwater and surface water (Toan et al. 2013), and the residue concentrations range from 0.1 to 1.4 µg/L in surface water (Mamun et al. 2009), which has become one of the most serious environmental problems, pose a significant threat to aquatic ecosystems.
Like most other herbicides, butachlor has been identified as a possible carcinogen (Tu et al. 2013). Many studies have demonstrated the toxic effects of butachlor in aquatic animals, like earthworm (Chen et al. 2014), alga (He et al. 2012), frog (Geng et al. 2005; Liu et al. 2011), fish (Tu et al. 2013; Anbumani et al. 2015; Xu et al. 2015) and even humans. Whereas studies of probing the protect effects of external antioxidant substances in this field are scarce. Thus, a reliable toxicity test and finding a way to provide the protective effect are needed to ensure aquatic organism and human safety.
Oxidative stress plays an important role in evaluating toxic effects (Xin et al. 2016). Many researchers have suggested that toxic effects of pesticides could be associated with increased production of reactive oxygen species (Yu et al. 2008). ROS is the metabolic by products of oxygen (e.g., hydrogen peroxide and oxygen ions etc.) involving in cell signaling and homeostasis (Pandey et al. 2003). Previous studies were linked the toxic effects of butachlor with the oxidative stress that led to an imbalance between the antioxidant defense system and the occurrence of ROS (Farombi et al. 2008). A significant increase in MDA was observed in the liver, kidney, gills and heart of African Catfish after exposure to different concentrations of butachlor (1, 2, 2.5 mg/L) (Farombi et al. 2008). Although the toxicity of butachlor to aquatic organisms has been widely investigated, the toxicity mechanism for the butachlor-induce oxidative stress on organism is not well elucidated.
There is an increasing evidence that VC has vital antioxidant functions in aquatic organism. It is the most important free radical scavenger in extracellular fluids, trapping radicals in the aqueous phase and protecting biomembranes from peroxidative damage (El-Neweshy et al. 2016; Padayatty et al. 2003). Moreover, the anticarcinogenic, anticlastogenic and even antimutagenic roles of VC have been tested in a variety of in vivo and in vitro systems exposed to radiation and pesticides (Durak et al. 2009; El-Sayed et al. 2016).
Zebrafish is a commonly used aquatic model for early-life stage toxicity evaluation of different environmental contaminants because of similarities with the human genome, low cost, diverse adaptability, short breeding cycle, high fecundity, and transparent embryos (Dai et al. 2014; Hill et al. 2005). To date, however, the effects of butachlor on zebrafish and the influence on butachlor toxicity of a well-known antioxidant such as VC are yet to be understood.
Therefore, the aim of the present study is to evaluate the acute toxic effects of butachlor in zebrafish and to investigate the possible protect effects of VC on butachlor-induced toxicity in zebrafish.
Materials and Methods
Butachlor (Purity > 98%) was purchased from Hangzhou Qingfeng agricultural chemicals company Ltd. DMSO and VC (Purity > 99%) were purchased from Aladdin industrial Inc. Shanghai. Butachlor stock solution was prepared by diluting butachlor by DMSO. All butachlor working solutions were prepared in the Hank’s solution (137 mmol/L NaCl, 5.4 mmol/L KCl, 0.25 mmol/L Na2HPO4, 0.44 mmol/L KH2PO4, 1.3 mmol/L CaCl2, 1.0 mmol/L MgSO4, and 4.2 mmol/L NaHCO3). MDA test kit was purchased from Nanjing Jiancheng Engineering Research Institute. Milli-Q water was used to prepare all the solutions and suspensions. All chemicals used in the present study were at least of analytical grade.
Zebrafish embryos which used for chemical exposure were obtained from College of the Environment in Zhejiang University of Technology. According to the procedure specified by OECD (2011) Validation Report, the normal fertilized embryos, 6 h post-fertilization (hpf), were randomly distributed in 50 mL beakers and were exposed to various concentrations of butachlor (0, 1, 2, 5, 10, 15 μmol/L), butachlor + 40 mg/L VC solution, butachlor + 80 mg /L VC solution, respectively, with 20 embryos per dose group at 28 ± 1°C for a period of 72 h. The concentrations of butachlor were selected from previous experiment according to the LC50 value (LC50-butachlor = 19.2 μmol/L). The Hank’s solution and hank’s solution @ VC were used as the control groups. Each experiment was performed independently in triplicate. Malformation, hatching delay and mortality were examined via inverted microscope (Nikon, Japan) and recorded daily. During exposure, dead embryos/larvae were promptly removed. The exposure solutions were renewed daily.
The test concentrations of VC and butachlor were measured by UV–vis spectrophotometer (Agilent Cary 100) and GC–MS (GC 2000-Mars 6100) respectively. The maximum absorption wavelength was 261 nm and the regression equation was y = 0.02731x + 0.0698 with correlation coefficient of 0.9941 when the VC content was 10–100 mg/L. The extraction procedures of butachlor exposure solution were based on the method developed by Mamun et al. (2009). The working conditions of the equipment were the following: Helium (purity 99.999%) was used as carrier gas, volume of injection 1 μL (splitless), injector temperature 280°C, ionization mode-electron impact at 70 eV, ion source temperature was 180°C and transfer line temperature at 250°C, the column oven was programmed as follows: initial temperature set at 80°C for 2 min, then it was increased to 280°C at 20 C min−1 (hold 10 min). Selected ion monitoring scan spectra were conducted for butachlor quantitative analysis with ion fragments of 176 and 160 (Wang et al. 2008). The detailed parameters were showed in the Table 1.
Biochemical assays were performed for investigating the oxidative stress after 72 h exposure. ROS production was measured by conversion of non fluorescent 2′7′-dichlorofluorescin diacetate (DCFDA) to the higher fluorescent compound dichlorofluorescein (DCF) as described by Wang et al. (1999). Fluorescence intensities were measured by SpectraMax M5 Microplate Reader (Molecular Devices, USA) at 488 nm excited emission and 525 nm emission light. The concentration of MDA, a molecular indicator of lipid peroxidation, was evaluated using MDA assay kits according to the manufacturer’s protocol. Catalase (CAT) activity was measured according to the method described by Sinha (1972). Glutathione S-transferase (GST) activity was based on the reaction of GST with p-nitrobenzyl chloride as described by Keen et al. (1976), which formed a complex with maximal absorbance at 310 nm. The activity of acid phosphatase (AcP) was determined by the method proposed by Prazeres et al. (2004). Moreover, soluble protein content was determined according to the method of Marion and Bradford (1976) with bovine serum albumin as a standard. All biomarker concentrations were normalized to its protein content respectively. Each experiment was conducted twice with three replicates. The calculated specific activities were then expressed relative to the average value of the controls. All zebrafish were maintained in accordance with the Guidelines for the Care of Laboratory Animals of the Ministry of Science and Technology of the People’s Republic of China.
Experimental data were analyzed by Origin pro 8 and SPSS statistical software (IBM SPSS, USA) packages. Each of the toxicity data sets was compared with its corresponding control followed by ANOVA. The differences were considered statistically significant at p < 0.05.
Results and Discussion
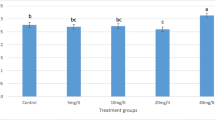
Pesticides provoked oxidative stress leading to the generation of free radicals and caused lipid peroxidation as molecular mechanism involved in pesticide-induced toxicity (Wang et al. 2013). Recent studies indicated that the toxicity effect induced by butachlor might be associated with the increasing of ROS, which might provide an explanation for the multiple types of toxic responses (Farombi et al. 2008). Butachlor were significantly (p < 0.05) lethal in a dose dependent way to early zebrafish embryos. Similar responses to butachlor exposure were observed in zebrafish (Tu et al. 2013) like pericardial edema (J, K, P, Q), yolk sac edema (G, H, K, O–Q) and crooked body (J, M, Q) in the Fig. 1. The measured test concentrations of butachlor were between 80 and 100% of the nominal test concentrations during the exposure period (Fig. 2 a). The loss might be due to sorption or volatility (Peddinghaus et al. 2012). Mortality and malformation rates of zebrafish were increased significantly with increasing concentrations of butachlor (Fig. 3). In the meantime, the hatching rate was significantly decreased. Butachlor promoted oxidative stress response in zebrafish as indicated by enhanced MDA and ROS contents and induced CAT, SOD, GST and AcP (Fig. 4). The increasing of AcP and CAT activities were induced at the low concentrations of butachlor, while their activities were inhibited at high concentration (Fig. 4). The results indicated that butachlor induced developmental toxicity in a dose dependent way.
Expression of biochemical parameters (a–e), a malondialdehyde, b reactive oxygen species, c catalase, d glutathion-S-transferase and e acid phosphatase in zebrafish after exposure to butachlor-VC mixed solutions for 72 hpf. (Means shared the same letter or asterisk are significantly different, p < 0.05)
Compared to the butachlor group, the mortality and malformation rates were significantly decreased after adding VC (Fig. 3). The decrease in mortality was greater as the concentration was higher, especially when the concentration of butachlor was 15 µmol/L (Fig. 3a). But the hatching rate showed no significant change after adding VC. Those phenomenons indicated that VC might have a beneficial role in ameliorating butachlor toxicity. To the best of our knowledge, there are no reports on the protective effect of antioxidant in butachlor toxicity. However, the protective role of antioxidant in organophosphate (OP) toxicity has been investigated in previous research (Eroglu et al. 2013), such as dichlorvos-induced oxidative stress in human erythrocytes. VC and VE could ameliorate OP-induced oxidative stress by decreasing lipid peroxidation in erythrocytes at certain doses of OP pesticides (Eroglu et al. 2013). The similar result was obtained from our research. MDA contents were obviously decreased after adding VC (Fig. 4a). The MDA content directly manifests the lipid peroxidation and membrane disruptions in response to oxidative stress. In addition, ROS contents were controlled at a low level after adding VC (Fig. 4b), which was significantly at 5 and 10 μmol/L butachlor group. But MDA and ROS contents of zebrafish were increased when 15 μmol/L butachlor co-existed with VC. CAT and AcP activities of zebrafish increased and maintained at a higher level after adding VC (Fig. 4). CAT is the enzyme which can eliminate H2O2 (Yu et al. 2008; Dale et al. 2017). VC might involve in the process of decomposition of H2O2 or O2 at the oxidative stress which was caused by butachlor. All the above demonstrated that VC could ameliorate butachlor-induced toxicity.
It was worth noting that the mortality and malformation rates were increased when exposed to pure VC solution (40, 80 mg/L VC). The measured test concentrations were significantly lower (between 0.2% and 18%) than the nominal test concentrations during the exposure (Fig. 2b). Compared to the control group, mortality and malformation rates increased by 6.67%, 5.83%, respectively, at a concentration of 40 mg/L of VC (Fig. 3). The other rose to 11.67%, 13.33% respectively (Fig. 3). Besides, the contents of MDA, ROS, GST were also increased obviously when VC existed alone (Fig. 4). It meant VC might have adverse effect when it existed alone and that should be the point for further studies.
In conclusion, VC could ameliorate butachlor-induced toxicity, as indicated by the decrease of mortality, malformation rates and the reversion in the response of the biomarkers. As an effective antioxidant, it may play an important role in chemoprotection strategies during aquaculture for pesticide pollution.
References
Abigail MEA, Samuel SM, Ramalingam C (2015) Addressing the environmental impacts of butachlor and the available remediation strategies: a systematic review. Int J Environ Sci Technol 12:4025–4036. https://doi.org/10.1007/s13762-015-0866-2
Anbumani S, Mohankumar MN (2015) Cytogenotoxicity assessment of monocrotophos and butachlor at single and combined chronic exposures in the fish Catla catla (Hamilton). Environ Sci Pollut Res 22:4964–4976. https://doi.org/10.1007/s11356-014-3782-y
Chen C, Wang YH, Zhao XP, Wang Q, Qian YZ (2014) Comparative and combined acute toxicity of butachlor, imidacloprid and chlorpyrifos on earthworm, Eisenia fetida. Chemosphere 100:111–115. https://doi.org/10.1016/j.chemosphere.2013.12.023
Dai YJ, Jia YF, Chen N, Bian WP, Li QK, Ma YB, Chen YL, Pei DS (2014) Zebrafish as a model system to study toxicology. Environ Toxicol Chem 33:11–17. https://doi.org/10.1002/etc.2406
Dale K, Rasinger JD, Thorstensen KL, Penglase S, Ellingsen S (2017) Vitamin E reduces endosulfan-induced toxic effects on morphology and behavior in early development of zebrafish (Danio rerio). Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc 101:84–93. https://doi.org/10.1016/j.fct.2017.01.004
Durak D, Uzun FG, Kalender S, Ogutcu A, Uzunhisarcikli M, Kalender Y (2009) Malathion-induced oxidative stress in human erythrocytes and the protective effect of vitamins C and E in vitro. Environ Toxicol 24:235–242. https://doi.org/10.1002/tox.20423
El-Neweshy MS, El-Sayed YS (2016) Influence of vitamin C supplementation on lead-induced histopathological alterations in male rats. Exp Toxicol Pathol 63:221–227. https://doi.org/10.1016/j.etp.2009.12.003
El-Sayed YS, El-Gazzar AM, El-Nahas AF, Ashry KM (2016) Vitamin C modulates cadmium-induced hepatic antioxidants’ gene transcripts and toxicopathic changes in Nile tilapia, Oreochromis niloticus. Environ Sci Pollut Res 23:1664–1670. https://doi.org/10.1007/s11356-015-5412-8
Eroglu S, Pandir D, Uzun FG, Bas H (2013) Protective role of vitamins C and E in dichlorvos-induced oxidative stress in human erythrocytes in vitro. Biol Res 46:33–38. https://doi.org/10.4067/S0716-97602013000100005
Farombi EO, Ajimoko YR, Adelowo OA (2008) Effect of butachlor on antioxidant enzyme status and lipid peroxidation in fresh water African catfish, (Clarias gariepinus). Int J Env Res Pub Health 5:423–427. https://doi.org/10.3390/ijerph5050423
Geng BR, Yao D, Xue QQ (2005) Acute toxicity of the pesticide dichlorvos and the herbicide butachlor to tadpoles of four anuran species. Bull Environ Contam Toxicol 75:343–349. https://doi.org/10.1007/s00128-005-0759-z
He HZ, Yu J, Chen GK, Li WY, He JB, Li HS (2012) Acute toxicity of butachlor and atrazine to freshwater green alga Scenedesmus obliquus and cladoceran Daphnia carinata. Ecotoxicol Environ Saf 80:91–96. https://doi.org/10.1016/j.ecoenv.2012.02.009
Hill AJ, Teraoka H, Heideman W, Peterson RE (2005) Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 86:6–19. https://doi.org/10.1093/toxsci/kfi110
Keen JH, Habig WH, Jakoby WB (1976) Mechanism for the several activities of the glutathione S-transferases. J Biol Chem 251:6183–6188 PMID: 977564
Liu WY, Wang CY, Wang TS, Fellers GM, Lai BC, Kam YC (2011) Impacts of the herbicide butachlor on the larvae of a paddy field breeding frog (Fejervarya limnocharis) in subtropical Taiwan. Ecotoxicology 20:377–384. https://doi.org/10.1007/s10646-010-0589-6
Mamun MIR, Park JH, Choi JH, Kim HK, Choi WJ, Han SS, Hwang K, Jang NI, Assayed ME, El-Dib MA, Shin HC, El-Aty AMA, Shim JH (2009) Development and validation of a multiresidue method for determination of 82 pesticides in water using GC. J Sep Sci 32:559–574. https://doi.org/10.1002/jssc.200800606
Marion M, Bradford (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Mohanty SR, Bharati K, Moorthy BTS, Ramakrishnan B, Rao RV, Sethunathan N, Adhya KT (2001) Effect of the herbicide butachlor on methane emission and ebullition flux from a direct-seeded flooded rice field. Biol Fertil Soils 33:175–180. https://doi.org/10.1007/s003740000301
Padayatty SJ, Katz A, Wang YH, Eck P, Kwon O, Lee JH, Chen SL, Corpe C, Dutta A, Dutta SK, Levine M (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22:18–35. https://doi.org/10.1080/07315724.2003.10719272
Pandey S, Parvez S, Sayeed I, Haque R, BinHafeez B, Raisuddin S (2003) Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci Total Environ 309:105–115. https://doi.org/10.1016/S0048-9697(03)00006-8
Peddinghaus S, Brinkmann M, Bluhm K, Sagner A, Hinger G, Braunbeck T, Eisenträger A, Tiehm A, Hollert H, Keiter SH (2012) Quantitative assessment of the embryotoxic potential of NSO-heterocyclic compounds using zebrafish (Danio rerio). Reprod Toxicol 3:224–232. https://doi.org/10.1016/j.reprotox.2011.12.005
Prazeres JN, Ferreira CV, Aoyama H (2004) Acid phosphatase activities during the germination of glycine max seeds. Plant Physiol Biochem 42:15–20. https://doi.org/10.1016/j.plaphy.2003.10.009
Shi RG, Lv JG, Feng JM (2011) Assessment of pesticide pollution in suburban soil in South Shenyang, China. Bull Environ Contam Toxicol 87:567–573. https://doi.org/10.1007/s00128-011-0401-1
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394 https://doi.org/10.1016/0003-2697(72)90132-7)
Toan PV, Sebesvari Z, Blasing M, Rosendahl I, Renaud FG (2013) Pesticide management and their residues in sediments and surface and drinking water in the Mekong Delta, Vietnam. Sci Total Environ 452:28–39. https://doi.org/10.1016/j.scitotenv.2013.02.026
Tu WQ, Niu LL, Liu WP, Xu C (2013) Embryonic exposure to butachlor in zebrafish (Danio rerio): endocrine disruption, developmental toxicity and immunotoxicity. Ecotox Environ Safe 89:189–195. https://doi.org/10.1016/j.ecoenv.2012.11.031
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616. https://doi.org/10.1016/S0891-5849(99)00107-0
Wang L, Li C, Li C, Li X, Xu C (2008) A rapid multi-residue determination method of herbicides in grain by GC–MS-SIM. J Chromatogr Sci 46:424. https://doi.org/10.1093/chromsci/46.5.424
Wang SR, Li HS, Lin CX (2013) Physiological, biochemical and growth responses of Italian ryegrass to butachlor exposure. Pestic Biochem Phys 106:21–27. https://doi.org/10.1016/j.pestbp.2013.03.007
Xin LL, Wang JS, Fan GQ, Che BZ, Cheng KM, Dong GZ (2016) Comparative oxidative stress elicited by nanosilver in stable HSPA1A promoter-driven luciferase reporter HepG2 and A549 cells. Toxicol Res 5:1298–1305. https://doi.org/10.1039/c6tx00195e
Xu HD, Wang JS, Li MH, Liu Y, Chen T, Jia AQ (2015) H-1 NMR based metabolomics approach to study the toxic effects of herbicide butachlor on goldfish (Carassius auratus). Aquat Toxicol 159:69–80. https://doi.org/10.1016/j.aquatox.2014.11.020
Yu F, Wang Z, Ju B, Wang Y, Wang J, Bai D (2008) Apoptotic effect of organophosphorus insecticide chlorpyrifos on mouse retina in vivo via oxidative stress and protection of combination of vitamin C and E. Exp Toxicol Pathol 59:415–423. https://doi.org/10.1016/j.etp.2007.11.007
Acknowledgements
This work was supported by grants from the Project of Science and Technology in Zhejiang Food and Drug Administration (SP201717) and the Key Innovation Team of Science and Technology in Zhejiang Province (2010R50018). All authors participated in writing and editing of this manuscript. There are no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiang, Q., Xu, B., Ding, Y. et al. Oxidative Stress Response Induced by Butachlor in Zebrafish Embryo/Larvae: The Protective Effect of Vitamin C. Bull Environ Contam Toxicol 100, 208–215 (2018). https://doi.org/10.1007/s00128-017-2245-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2245-9