Abstract
Arsenic in drinking water threatens public health worldwide. Phytoremediation has brought new vitality to solve this problem. The aim of this work was to study the role of emergent macrophyte sweet flag (Acorus calamus L.) in phytoremediation of arsenate [As(V)] and arsenite [As(III)] from polluted water. For that, the methods of analytic chemistry and physiology were used. The results showed that As(III) could be removed by A. calamus more efficiently than As(V). The removal efficiencies of As(V) and As(III) both reached more than 95%. In As(V)- and As(III)-exposed A. calamus, the arsenic contents were much higher in root than in stem and leaf. The translocation factors of As(V) and As(III) were no more than 0.152. Both As(V) and As(III) were found in the whole plant, whereas dimethylarsinic acid (DMA, 0.06‒0.13 mg kg‒1) was only present in the aboveground part. As(V) was the main species in the As(V)-exposed plants (45.86–70.21%). As(III) was the main species in stem and leaf of As(III)-exposed plants (55.76–85.52%), while As(V) was still dominant in root. A. calamus could keep its green leaves during the 31 days of inorganic arsenic (iAs) exposure. However, iAs had a little inhibitory effect on biomass accumulation, and high-concentration iAs was beneficial to promote root growth. The concentrations of malondialdehyde (MDA) and hydrogen peroxide (H2O2), as well as the activity of catalase (CAT) were significantly higher in root than those in stem and leaf. The oxidative stress response of A. calamus to As(III) was more than that to As(V). The findings of this study indicated that A. calamus was regarded as a promising material for the phytoremediation of arsenic from water.
Highlights
-
calamus L. exhibited high tolerance to As(III) and As(V).
-
As(III) can be methylated to DMA in the aboveground part of A. calamus L.
-
Most of arsenic was accumulated in the roots.
-
High-concentration iAs was beneficial to promote root growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic is widely distributed in the Earth’s crust mostly as minerals and in inorganic forms. Arsenic may enter the air, water, and soil through various geochemical processes and anthropogenic activities such as mining, smelting, fossil fuels burning, and the application of pesticides and herbicides (Ostovar et al. 2023; Raju 2022; Chen et al. 2022). Human exposure to arsenic is primarily a result of ingestion of arsenic-contaminated drinking water or food, inhalation of arsenic dust in the air, and dermal absorption (Parviainen et al. 2015). However, ingestion through food and water is the predominant type of arsenic exposure (Kong et al. 2018). The ingestion of arsenic-contaminated drinking water can cause various health problems including skin, lung, liver, and bladder cancers (Sodhi et al. 2019). Given its toxicity, the International Agency for Research on Cancer (IARC) classified arsenic as a Group I carcinogen. The World Health Organization (WHO) has set a provisional guideline limit of 10 µg L‒1 for arsenic in drinking water. In China, more than 19 million people are exposed to arsenic-rich water (Rodriguez-Lado et al. 2013). Thus, it is urgent to remediate the arsenic-contaminated water by effective methods.
Arsenic can occur in four different oxidation states (− 3, 0, + 3, + 5) in the environment. However, the inorganic forms as pentavalent arsenate [As(V)] and trivalent arsenite [As(III)] are mostly found in natural waters, with As(V) more predominant and thermodynamically stable than As(III) (Gao et al. 2020). Various methods have been developed to remove inorganic arsenic (iAs) from water, e.g., chemical precipitation, ion exchange, membrane filtration, electrocoagulation, adsorption, and biological treatments (Mulligan et al. 2001; Rezania et al. 2016; Dadwal and Mishra 2017; Alka et al. 2021). Nevertheless, these physicochemical methods have their advantages and boundaries in field application. Chemical precipitation requires large quantities of chemicals and generates a great amount of sludge (Hashim et al. 2011). Ion exchange and adsorption methods have some limitations, for instance, high capital and operational costs, low adsorption capacity, or weak binding strength (Alka et al. 2021). Biological treatments (phytoremediation and bioremediation) are now widely used as an alternative to the equivalent physicochemical methods owing to their low-cost and eco-friendly advantages (Dangi et al. 2019; Wei et al. 2021; Ostovar et al. 2022). Phytoremediation is the use of plants to reduce the environmental contaminants in surface water and sediments/soils (Manoj et al. 2020). In nature, there are several plants existing with the ability to remove toxic metals from the surrounding environment as a survival strategy. The well-studied strategies are phytoextraction (or phytoaccumulation), rhizofiltration (or phytofiltration), phytostabilization, phytovolatilization, and phytodegradation (Dalcorso et al. 2019). Rhizofiltration uses adsorption or absorption via the plant roots and is useful to treat polluted surface water, sewage, and wastewater containing low amounts of toxic metals (Rezania et al. 2016). The other desirable characteristic permitting phytoremediation as an ideal method is arsenic biomethylation by plants. Tape grass (Vallisneria natans (Lour.) Hara) was found to be able to metabolize the iAs to organic arsenic (oAs) forms of dimethylarsinic acid (DMA) and arsenobetaine (AsB) (Li et al. 2018). In general, oAs is less toxic than iAs, and complex organoarsenicals (e.g., AsB) are considered to be nontoxic. Hence, rhizofiltration is emerging as an attractive option for the cleanup of arsenic-contaminated water.
Aquatic macrophytes (including emergent, floating-leaved, submerged, and floating macrophytes and hygrophytes) are key components of wetland ecosystems as they help in degrading and removing organic and inorganic contaminants. Some floating macrophytes have been investigated for the removal of metals. Water lettuce (Pistia stratiotes L.) had the highest total Mn removal from the water (Hua et al. 2012). Both duckweed (Lemna minor L.) and water hyacinth (Eichhornia crassipes (Mart.) Solms) were also effective in the removal of metals (i.e., Cu) (Rezania et al. 2016). Azolla (Azolla pinnata R. Brown) could remove 65‒95% of applied metals (Zn, Cu, Pb, Cr, and Cd) from wastewater (Akhtar et al. 2021). Compared to floating macrophytes, less information has been gathered to date regarding metals removal by emergent macrophytes. Emergent macrophytes are rooted in sediments and undergo photosynthesis above the water. They can absorb toxic metals from water and sediments (Lin et al. 2018). Several emergent macrophytes have been studied as adsorbents to remove toxic metals, for instance, sweet flag (Acorus calamus L.), oriental cattail (Typha orientalis Presl), reed (Phragmites australis L.), and purple loosestrife (Lythrum salicaria L.) (Sesin et al. 2021). A. calamus is a perennial herbaceous plant with rhizome that is indefinitely branched, smooth, pinkish or pale green, and widely distributed in China. Although A. calamus is a highly valued medicinal plant, it has been used for the removal of contaminants such as hexavalent chromium and divalent lead ions (Shooto 2020). Arsenic is a non-essential metalloid that is highly toxic to organisms including plants. Nevertheless, less is known about the strategy for arsenic removal from water by A. calamus. A better understanding of the arsenic removal mechanism by A. calamus will help to assess the rhizofiltration potential of emergent macrophytes.
Given the above-mentioned reasons, A. calamus was selected as a case (1) to compare the removal efficiencies of As(V) and As(III); (2) to investigate the biotransformation of iAs to oAs; and (3) to clarify the physiological responses to the iAs stress. This study aimed to provide experimental evidence for the application of A. calamus to the phytoremediation of arsenic-contaminated water.
Materials and Methods
Plant Material and Treatment Conditions
Acorus calamus was obtained from an aquatic plant nursery in Suqian City, Jiangsu Province, China. All plants were washed with running tap water to thoroughly remove attached soil particles and rinsed three times with ultrapure water (18.2 MΩ cm–1). Then, the clean plants with similar size (30.0 ± 2.0 cm in height and 6.7 ± 0.3 g in weight for As(V) treatment, 39.5 ± 2.0 cm in height and 11.0 ± 1.0 g in weight for As(III) treatment) were selected and acclimatized for 7 days in laboratory conditions [15% Hoagland’s solution (75.80 mg L‒1 KNO3, 1.77 mg L‒1 Ca(NO3)2·4H2O, 73.90 mg L‒1 MgSO4·7H2O, 2.04 mg L‒1 KH2PO4, 0.42 mg L‒1 H3BO3, 0.18 mg L‒1 MnCl2·4H2O, 0.03 mg L‒1 ZnSO4·7H2O, 0.01 mg L‒1 CuSO4·5H2O, 0.01 mg L‒1 NaMoO4·2H2O, 0.83 mg L‒1 FeSO4·7H2O, and 1.12 mg L‒1 EDTA-Na2; Chen et al. 2015), pH 6.5, 112 μmol m−2 s−1, 12L:12D, 22 ± 1 °C]. After acclimatization, every three plants were washed using ultrapure water, and then transferred to a hydroponic box (127 × 87 × 114 mm3 in size) containing 800 mL of 15% Hoagland’s solution with As(III) or As(V) at various concentrations [0 (control), 200, 500, and 1,000 μg L−1] under the above-mentioned laboratory conditions for treatment. Experiments were set up in triplicate. The treatment without arsenic was used as blank control. The depth of 15% Hoagland’s solution in the hydroponic box was about 80 mm. Ultrapure water was replenished to 80 mm in height every day due to evaporation losses. Two milliliters of the water samples were taken from the hydroponic box every 2 days and filtered through a 0.22-µm filter (Xinya Purify Device Company, Shanghai, China). For arsenic analysis, the water samples were acidified with 1% HNO3 solution (v/v) and immediately cooled to 4 °C. The plant samples were collected on day 31 of the treatment based on the pre-experimental results. The collected plants were first washed with running tap water and then with ultrapure water to remove apoplastic arsenic. After being dried with filter papers, the plant samples were used to measure height and fresh weight, and then divided into root, stem, and leaf. Some fresh samples were frozen at − 70 °C for subsequent determination of lipid peroxidation level and antioxidant enzyme activity. Some were freeze-dried and grounded in an agate mortar. The powdered dry samples were sealed in PE bags and stored in a desiccator before arsenic analysis. The removal efficiency (RE) of arsenic was calculated according to the following equation (Jiang et al. 2022):
where C0 and Ct are the initial and final arsenic concentrations in water, respectively.
To estimate the potential of A. calamus for phytoremediation purposes, the bioconcentration factor (BCF) and translocation factor (TF) were used as indicators. BCF is described as the ability of plants for arsenic accumulation from water. TF is used to evaluate the pollutant transport capacity of plants from underground part to overground part. They can be calculated by the following equations (Bello et al. 2018):
where C is the arsenic concentration in the plant (mg kg–1), Cw is the arsenic concentration in the water (mg L–1), Coverground is the arsenic concentration in the overground part of plant (mg kg–1), and Cunderground is the arsenic concentration in the underground part of plant (mg kg–1).
Relative growth rate (RGR) is an indicator of the suitability of the species for phytoremediation (Compaore et al. 2020). Root tolerance index (TIr) is used to assess the rhizofiltration potential of toxic metals (EI-Mahrouk et al. 2020). RGR and TIr were calculated as follows:
where W0 and W1 are fresh weight of plant at the beginning (t0) and the next sampling (t1), respectively (g), Lt is average root length of the arsenic-treated plant (cm), Lc is average root length of control plant (cm).
Digestion and Analysis of Total Arsenic
Each plant sample (0.2 g of leaf or 0.1 g of root) was digested using 4.0 mL of 70% HNO3 (≥ 99.999% metals basis) and 1.0 mL of 30% H2O2 (guaranteed reagent grade) in a microwave-assisted digestion system (MDS-6G, Sineo Microwave Chemistry Technology Co. Ltd, Shanghai, China). The digestion procedure was conducted in three steps: 15 min to 120 °C, 15 min to 190 °C, and followed by another 30 min at 190 °C (Li et al. 2018). After cooling down, the digested solution was diluted to 25 mL with ultrapure water, passed through a 0.22-µm filter, and kept at 4 °C before analysis. Total arsenic in both water samples and digested solutions were determined by an Agilent 7700 × ICP-MS (Agilent, Tokyo, Japan). The operating parameters for ICP-MS were as follows: RF power of 1550 W, plasma gas (argon) flow rate of 15.0 L min–1, carrier gas flow rate of 0.9 L min–1, and auxiliary gas flow rate of 0.9 L min–1. The polyatomic interference was eliminated by operating the collision/reaction cell in helium mode (4.3 L min–1 flow rate). The same method was used to analyze a certified reference material of celery (GBW-10048) from the CRM/RM Information Center of China (Beijing, China). A method blank was conducted during each batch of samples to confirm the blank value had no statistical significance. The recoveries for total arsenic in spiked samples ranged from 90 to 110%.
Extraction and Speciation of Arsenic
Arsenic species were extracted from the plant samples using a microwave-assisted extraction method. 0.2 g of leaf or root powder was weighed into a Teflon digestion vessel and 10 mL of 1% HNO3 solution (v/v) was added. The digestion vessel was placed in a microwave-assisted digestion system at 90 °C for 90 min. After cooling, the extracted solution was centrifuged at 9,000 rpm for 30 min. The supernatant was then filtered through a 0.22-µm filter and stored at 4 °C before analysis. Arsenic speciation analysis in plant samples was conducted using Agilent 1260 HPLC (Agilent, Tokyo, Japan) with Agilent 7700 × ICP–MS. The separation of arsenic species was performed using a Hamilton PRP-X100 anion-exchange column (4.1 mm × 250 mm, 10 μm) equipped with a Hamilton PRP-X100 guard column (2.0 mm × 20 mm, 10 μm), in which the 10 μL sample was eluted by the mobile phase (A: 4 mmol L–1 NH4HCO3, pH 8.6; B: 4 mmol L–1 NH4HCO3/40 mmol L–1 NH4NO3, pH 8.6) at a flow rate of 1.0 mL min–1. The extraction efficiency of arsenic was validated by analyzing the certified reference material GBW-10048. The extraction efficiency was 69–98%.
Determination of Lipid Peroxidation and Antioxidant Enzyme Activities
About 0.3 g of leaf or root sample was homogenized in 2.7 mL of precooled (4 °C) 0.9% saline solution in an ice-bath. The homogenate was then centrifuged at 4,000 rpm for 10 min at 4 °C. The sample solution for the measurement of H2O2 concentration was centrifuged again at 10,000 rpm for 10 min. The obtained supernatant was used for further analysis. Malondialdehyde (MDA) content was determined using the thiobarbituric acid (TBA) reaction. H2O2 was measured in the reaction mixture by the molybdic acid (MA) method. Catalase (CAT) activity was assessed in the reaction with ammonium molybdate. All the kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Statistical Analysis
All values are presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) with Duncan's multiple range test (DMRT) was run to test the differences among groups. Values of P < 0.05 were considered statistically significant. All statistical analysis was performed with SPSS 25.0 (SPSS Inc., Chicago, IL, USA). Graphics were generated using Origin 2018C (OriginLab, Inc., Northampton, MA, USA).
Results and Discussion
Comparison of As(V) and As(III) Removal by A. calamus
The removal efficiencies of As(V) and As(III) by A. calamus were compared during a 31-day exposure. As shown in Fig. 1, the remaining concentrations of As(V) and As(III) in water showed a trend of continuous decrease. Differently, the remaining concentrations of As(III) dropped more quickly than those of As(V). For As(V) treatment, the arsenic removal efficiencies increased first slightly for 5 or 7 days and then sharply from 28.39% ± 0.72% to 96.50% ± 0.33% (200 μg L‒1), from 6.13% ± 1.60% to 95.03% ± 0.13% (500 μg L‒1), and from 10.66% ± 2.14% to 97.09% ± 0.36% (1,000 μg L‒1), respectively. For As(III) treatment, the removal efficiencies of arsenic presented a linear increase since the beginning of the experiment and then kept nearly invariant between the 13th and 31st day. The removal efficiencies of arsenic reached 97.10% ± 0.67% (200 μg L‒1), 97.41% ± 0.08% (500 μg L‒1), and 95.45% ± 0.40% (1,000 μg L‒1), respectively. In general, A. calamus could remove more than 95% of As(V) or As(III) from water. Compared with As(V), As(III) could be removed by A. calamus more efficiently.
Arsenic removal from water by A. calamus treated with different concentrations of As(V) (a 200 μg L‒1, b 500 μg L‒1, and c 1,000 μg L‒1) and As(III) (d 200 μg L‒1, e 500 μg L‒1, and f 1,000 μg L‒1). Different English letters (a–d) indicate significant differences (P < 0.05) among the means of residual As(III) or As(V) concentrations with time. Data are expressed as the mean ± SD (n = 3)
In this study, more than 95% of the total arsenic was removed from As(V)-/As(III)-contaminated water. Nevertheless, de Souza et al. (2019) reported that 1 g fresh weight of L. valdiviana was able to remove 82% of its initial As(III) concentration (500 μg L‒1) under optimal conditions. The other three aquatic macrophytes E. crassipes, L. minor, and Spirodela polyrhiza could remove no more than 80% of arsenic (initial concentration 50 μg L‒1) during 21 days (Mishra et al. 2008). E. crassipes had an arsenic removal efficiency of only 18% when exposed to 150 μg L‒1 or less arsenic concentrations (Alvarado et al. 2008). It was suggested that A. calamus could be an effective plant for phytoremediation. On the other hand, the pre-oxidation of As(III) to As(V) is a desirable process for the effective removal of iAs from water using physicochemical methods. This results showed that A. calamus could efficiently remove As(III) as well as As(V) without a pre-oxidation process. Accordingly, A. calamus could be suitable for iAs phytoremediation.
Accumulation and Transformation of Arsenic Species by A. calamus
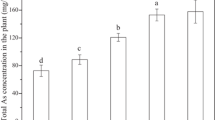
Accumulation of arsenic in A. calamus is presented in Fig. 2. After 31-day exposure, the total arsenic in different vegetative organs of A. calamus increased with the increase of initial As(V) concentration. About 34.24 ± 0.49 mg kg‒1 (200 μg L‒1), 46.76 ± 5.39 mg kg‒1 (500 μg L‒1), and 292.77 ± 36.18 mg kg‒1 (1,000 μg L‒1) of arsenic have been found to be accumulated in the underground part (root) of A. calamus. Only 1.31 ± 0.06 mg kg‒1 (200 μg L‒1), 6.12 ± 0.10 mg kg‒1 (500 μg L‒1), and 3.71 ± 0.15 mg kg‒1 (1,000 μg L‒1) of arsenic were accumulated in the aboveground part (stem and leaf). Thus, it can be seen that the arsenic contents were much higher in the root than in the stem and leaf. After As(III) treatment, A. calamus accumulated 82.14 ± 11.51 mg kg‒1 (200 μg L‒1), 26.08 ± 6.36 mg kg‒1 (500 μg L‒1), and 62.38 ± 7.97 mg kg‒1 (1,000 μg L‒1) of arsenic in the roots, respectively. High arsenic accumulation by roots under low As(III) treatment might be related to the relatively low oxidative damage suffered by plant cells. Similarly, a small amount of arsenic was accumulated in the stem and leaf under As(III) exposure. It was indicated that iAs was mainly accumulated in the root of A. calamus. This conclusion was supported by the observation that the translocation factors (TFs) of As(V) and As(III) were no more than 0.044 (see Table 1).
Accumulation of arsenic in A. calamus treated with different concentrations of As(V) (a) and As(III) (b). Different English letters (a–d) indicate significant differences (P < 0.05) among each treatment group, and Latin letters (α, β, and γ) represent the significant differences between vegetative organs. Data are expressed as the mean ± SD (n = 3)
Various arsenic species were identified and quantified in the vegetative organs of A. calamus (Fig. 3). For As(V) treatment, As(V) and As(III) were found in the whole plant, whereas DMA (0.09‒0.13 mg kg‒1) was only present in the aboveground part. As(V) was the main species in the As(V)-exposed plants (45.86–70.21%). Both the content and percentage of As(III) significantly increased with the increase in the concentration of As(V) treatment. But the percentage of As(III) was no more than 51.23%. It was suggested that some of the As(V) absorbed into the root system was reduced to As(III) and further methylated to DMA in the stem and/or leaf. For As(III) treatment, As(V) and As(III) were also identified in the whole plant, but DMA (0.06 and 0.07 mg kg‒1) was detected in the leaf under the treatment of 500 and 1,000 μg As(III) L‒1. Correspondingly, As(III) was the main species in the stem and leaf of As(III)-exposed plants (55.76–85.52%), while As(V) was still dominated in the root under 200 and 500 μg As(III) L‒1 treatment (64.8% and 58.42%). Although the As(V) content increased with increasing the concentration of As(III) treatment, the percentage of As(V) decreased. It was indicated that a large amount of As(III) absorbed could be oxidized to As(V) in the root system, and its oxidation efficiency was limited by the As(III) content in cells. The methylation of As(III) to DMA seems to occur mainly in the leaf of emergent macrophyte.
Arsenic species in A. calamus under different concentrations of As(V) (a, c) and As(III) (b, d). Different English letters (a–d) indicate significant differences (P < 0.05) among each treatment group, and Latin letters (α, β, and γ) represent the significant differences between vegetative organs. Data are expressed as the mean ± SD (n = 3)
In this study, iAs was more accumulated in the root than in the stem and leaf of A. calamus at each treated concentration. The result of this study was similar to the results of several submerged macrophytes. V. natans (Lour.) Hara accumulated more arsenic in the roots (187.11‒248.65 mg kg–1) than in the leaves (19.10‒39.52 mg kg–1) after 14 days of treatment (Li et al. 2018). Arsenic was also more accumulated in the roots of alternate watermilfoil (Myriophyllum alterniflorum DC.) (156 ± 22 mg kg–1) than in its shoots (104 ± 12 mg kg–1) (Krayem et al. 2016). Hydrilla verticillata (L.f.) Royle has been shown to accumulate > 73% of arsenic in the root (Xue and Yan 2011). Additionally, the highest concentration of arsenic was found in the roots of two free-floating macrophytes [E. crassipes (Mart.) solms and water spinach (Ipomoea aquatica Forsk)] and one emergent macrophyte [narrow-leaved cattail (Typha angustifolia L.)] growing in the wastewater treatment ponds (Nateewattana et al. 2010). It was proved that the root of aquatic macrophytes acted as a barrier to limit arsenic translocation to the stem and leaf. The root is the main organ of plants for water and inorganic salts absorption. Plants also have developed many mechanisms to restrict the translocation of metal(loid) ions. For instance, the Casparian strip in plant roots could act as a diffusion barrier to block the transport of metal(loid) ions from the cortex to the vascular bundle, thus preventing their translocation to the aboveground part (Baxter et al. 2009). Free metal(loid) ions in plant cells could be sequestered into root vacuoles to reducing their translocation to the aboveground parts (Korenkov et al. 2009). This idea was further evident from the low TF values (0.007‒0.044) of arsenic in A. calamus. Furthermore, in the case of terrestrial environment phytoremediation, plants capable of translocating arsenic to the aboveground parts are prioritized (de Campos et al. 2019). Pteris vittata L. is the first known arsenic-hyperaccumulating plant that has been found to be highly efficient in translocating arsenic from roots to fronds (Zhao et al. 2023). Harvesting the aboveground parts of P. vittata can achieve the goal of removing arsenic from the terrestrial environment. However, the entire aquatic macrophytes can be collected and removed from the medium easily. Therefore, the arsenic that has accumulated in the root of aquatic macrophytes may also be removed from the aquatic environment. For A. calamus, harvesting the entire plant with consequent reseeding/reallocation of new plant is expected.
Some organisms, such as bacteria, archaea, fungi, algae, and animals (including humans), are able to methylate As(III) to various oAs species (e.g., monomethylarsonic acid (MMA) and DMA) (Zhao and Wang 2020). In organisms, As(V) can be first reduced to As(III), followed by methylation to MMA, and then methylation to DMA (Wu et al. 2015). However, few higher plants appear to have the ability to methylate As(III). In this study, DMA could be detected in the aboveground part (i.e., leaf) of A. calamus at each concentration of As(V)/As(III) treatment. This appears to be new supporting evidence that DMA can be formed in higher plants. DMA is a major metabolite of iAs in many organisms including humans (Cullen et al. 2016). In general, the biomethylation plays an important role in the metabolism of iAs. The pathway of arsenic biomethylation involves alternate steps of two-electron reduction of As(V) to As(III) and then oxidative addition of methyl groups known as oxidative methylation (Roy et al. 2015). The methylation of iAs has been regarded as a detoxification mechanism because oAs is supposed to be less toxic than iAs (Zhen et al. 2020). The presence of DMA in the aboveground part (i.e., leaf) of A. calamus was considered to be a means of detoxification.
iAs-Induced Changes in Growth Status of A. calamus
The effect of iAs concentration on the growth status of A. calamus was studied to evaluate its stress resistance. As illustrated in Table 2, the RGR of the plants subjected to 200 and 1,000 μg As(V) L‒1 were similar to that of the control group. Differently, the RGR of the plant treated with 500 μg As(V) L‒1 was lower than that of the control group. Under As(III) treatment, the RGR of A. calamus were all slightly decreased compared with the control group. The results indicated that iAs could cause a slight decrease in plant RGR. In contrast, the TIr of A. calamus with As(V) treatment (127.63–142.76%) were all much higher than that of the control group. The TIr of the plants with As(III) treatment increased linearly with increased concentration of As(III), although only the TIr of the plant subjected to 1,000 μg As(III) L‒1 was higher than 100%. It was implied that A. calamus could regulate its root growth in response to iAs exposure. In addition, A. calamus could keep its green leaves during the 31 days of iAs exposure (Fig. 4), suggesting that low content of iAs in leaves had no obvious inhibitory effect on the chlorophyll synthesis of emergent macrophyte. A. calamus has a certain resistance to arsenic pollution in water.
In nature, few plant species are capable of accumulating or detoxifying extraordinarily high levels of arsenic (Souri and Karimi 2017). Arsenic can affect the morphological, physiological, biochemical, and metabolic attributes of plants like root-shoot length and biomass, chlorophyll content, and photosynthetic rate (Bali and Sidhu 2021). Singh et al. (2019) reported As(V) treatments (100 and 200 µM) led to significant reduction in root and leaf biomass. Arsenic treatment also reduced the fresh weight by 25.8% and dry weight by 31.0% in Artemisia annua L. as compared to the respective control (Naeem et al. 2020). In this study, As(V) treatment at 500 μg L‒1 was found to decrease the biomass gain of A. calamus. Conversely, As(V) treatment could promote the root elongation growth. This indicated that As(V) reduced the accumulation of biomass in aboveground plant parts, but promoted an increase in root biomass. In aqueous phase, As(III) is more toxic, more soluble, and more mobile than As(V) (Wu et al. 2019). The biomass gain was decreased more obviously in A. calamus with As(III) treatment. This phenomenon may be caused by the reduction in the photosynthesis rate under As(III) treatment, which leads to reduced organic matter content (Gupta et al. 2021). Unlike As(V), As(III) treatment at 200 and 500 μg L‒1 also inhibited the root elongation growth. But the root elongation growth was promoted under high As(III) concentration. These results suggested that A. calamus responded to iAs stress by altering its root morphology.
iAs-Induced Changes in Lipid Peroxidation and Oxidative Stress of A. calamus
MDA, a marker of lipid peroxidation, was determined in the As(V)/As(III)-exposed plants. As demonstrated in Fig. 5a, d, the MDA concentrations in the root were significantly higher than those in the stem and leaf. For As(III) treatment, the MDA concentrations showed an upward trend with the increase of treatment concentration. For As(V) treatment, the MDA concentrations in A. calamus with 500 μg As(V) L‒1 treatment were the highest. H2O2 is a normal by-product of cellular metabolism that in higher concentrations can induce oxidative stress. Similarly, the concentrations of H2O2 were the highest in the root of As(III)-exposed plants in each treated concentration (Fig. 5f). But the amount of H2O2 generated in response to As(V) was lower than that responding to As(III). CAT is involved in H2O2 catabolism, which is important in defense against oxidative stress. The activities of CAT in the As(III)-treated groups were the lowest in the leaf, and increased with the increase of the As(III) concentration (Fig. 5e). The highest CAT activity in As(V)-exposed plants was found in the root at 500 μg As(V) L‒1 (Fig. 5b).
Antioxidant physiological indices (MDA, CAT, and H2O2) of roots, stems, and leaves of A. calamus exposed to different concentrations of As(V) (a–c) and As(III) (d–f) treatment groups. All values are expressed as mean ± SD (n = 6). Different English letters (a–d) indicate significant differences among each treatment group, and Latin letters (α, β, and γ) indicate significant differences between vegetative organs
Arsenic is a redox-active element that causes oxidative stress by generating reactive oxygen species (ROS) in the plant tissues from its conversion of As(V) to As(III), leading to non-specific oxidation of biomolecules, enzyme inactivation, and membrane damage (Adhikary et al. 2022). ROS are reactive derivatives of O2 metabolism, including superoxide radical (O2·‒), singlet oxygen (1O2), hydroxyl radical (·OH), and H2O2. Out of all the ROS molecules, H2O2 has the longest stability (half-life of 10‒3 s) within plant cells (Huang et al. 2019). H2O2 is considered to be an important signaling molecule that responds to various stimuli. In A. calamus, the accumulation of H2O2 was greater in the root than in the stem and leave. The concentration of H2O2 was obviously related to the concentration of arsenic absorbed. On the other hand, plants have evolved a suite of antioxidant system to protect against oxidative stress by scavenging ROS (Singh et al. 2017). CAT is one of the crucial antioxidant enzymes that mitigates oxidative stress to a considerable extent by metabolizing H2O2 to H2O and O2. Corresponding to H2O2, CAT activity increased significantly upon As(III) treatment. As described by Liu et al. (2017), a higher activity of antioxidant enzyme generally exhibited better resistance to toxic metal stress. It was suggested that A. calamus could well resist As(III) stress. In addition to the enzymatic antioxidant defense systems, plants synthesize the non-enzymatic antioxidants such as MDA and glutathione (GSH) which are also involved in scavenging ROS (Ahire et al. 2021). MDA is generated by the peroxidation of membrane polyunsaturated fatty acids, which is the most commonly used marker for the determination of the antioxidant status. In this study, both As(V) and As(III) stress caused a significant increase in MDA levels in the root of A. calamus. At the same time, the level of MDA increased with increasing arsenic concentration. These results were in agreement with the findings of Banerjee and Roychoudhury (2022) and Chu et al. (2022), suggesting that A. calamus was equipped with an efficient antioxidant mechanism against iAs-induced oxidative stress.
Conclusions
This study quantified the contribution of emergent macrophyte A. calamus in iAs removal from water. A. calamus exhibited the excellent removal capability for As(V) and As(III). iAs could be absorbed into A. calamus and mainly accumulated in its root. Meanwhile, A. calamus favored the methylation of As(III) to DMA in the leaf. Plant growth was nearly not affected by As(V) and As(III). iAs treatment could cause an increasing generation of ROS (i.e., H2O2) in A. calamus, which was effectively scavenged by its enzymatic and non-enzymatic antioxidants (CAT and MDA). The present work concluded that A. calamus had a high level of iAs resistance in the tested conditions. In summary, A. calamus can be considered a promising plant for the treatment of iAs-contaminated water in field application.
Data Availability
The data used in this manuscript are included in the text.
References
Adhikary A, Saini R, Kumar R, Singh I, Ramakrishna W, Kumar S (2022) Pseudomonas citronellolis alleviates arsenic toxicity and maintains cellular homeostasis in chickpea (Cicer arietinum L.). Plant Physiol Biochem 184:26–39
Ahire ML, Mundada PS, Nikam TD, Bapat VA, Penna S (2021) Multifaceted roles of silicon in mitigating environmental stresses in plants. Plant Physiol Biochem 169:291–310
Akhtar M, Sarwar N, Ashraf A, Ejaz A, Ali S, Rizwan M (2021) Beneficial role of Azolla sp. in paddy soils and their use as bioremediators in polluted aqueous environments: implications and future perspectives. Arch Agron Soil Sci 67(9):1242–1255
Alka S, Shahir S, Ibrahim N, Ndejiko MJ, Vo D-V, Manan FA (2021) Arsenic removal technologies and future trends: a mini review. J Clean Prod 278:123805
Alvarado S, Guédez M, Lué-Merú MP, Nelson G, Alvaro A, Jesús AC, Gyula Z (2008) Arsenic removal from waters by bioremediation with the aquatic plants Water Hyacinth (Eichhornia crassipes) and Lesser Duckweed (Lemna minor). Bioresour Technol 99:8436–8440
Bali A, Sidhu GPS (2021) Arsenic acquisition, toxicity and tolerance in plants-From physiology to remediation: a review. Chemosphere 283:131050
Banerjee A, Roychoudhury A (2022) Rhizofiltration of combined arsenic-fluoride or lead-fluoride polluted water using common aquatic plants and use of the ‘clean’ water for alleviating combined xenobiotic toxicity in a sensitive rice variety. Environ Pollut 304:119128
Baxter I, Hosmani PS, Rus A, Lahner B, Borevitx JO, Muthukumar B, Mickelbart MV, Schreiber L, Franke RB, Salt DE (2009) Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet 5:e1000492
Bello AO, Tawabini BS, Khalil AB, Boland CR, Saleh TA (2018) Phytoremediation of cadmium-, lead- and nickel-contaminated water by Phragmites australis in hydroponic systems. Ecol Eng 120:126–133
Chen G, Liu X, Brookes PC, Xu J (2015) Opportunities for phytoremediation and bioindication of arsenic contaminated water using a submerged aquatic plant: Vallisneria natans (lour.) Hara. Int J Phytoremediat 17:249–255
Chen C, Yang B, Gao A, Yu Y, Zhao F (2022) Transformation of arsenic species by diverse endophytic bacteria of rice roots. Environ Pollut 309:119825
Chu L, Hou X, Song X, Zhao X (2022) Toxicological effects of different ionic liquids on growth, photosynthetic pigments, oxidative stress, and ultrastructure of Nostoc punctiforme and the combined toxicity with heavy metals. Chemosphere 298:134273
Compaore WF, Dumoulin A, Rousseau DPL (2020) Metal uptake by spontaneously grown Typha domingensis and introduced Chrysopogon zizanioides in a constructed wetland treating gold mine tailing storage facility seepage. Ecol Eng 158:106037
Cullen WR, Liu Q, Lu X, McKnight-Whitford A, Peng H, Popowich A, Yan X, Zhang Q, Fricke M, Sun H, Le Chris X (2016) Methylated and thiolated arsenic species for environmental and health research—a review on synthesis and characterization. J Environ Sci 49:7–27
Dadwal A, Mishra V (2017) Review on biosorption of arsenic from contaminated water. Clean: Soil, Air, Water 45(7):1600364
Dalcorso G, Fasani E, Manara A, Visioli G, Furini A (2019) Heavy metal pollutions: state of the art and innovation in phytoremediation. Int J Mol Sci 20:3412
Dangi AK, Sharma B, Hill RT, Shukla P (2019) Bioremediation through microbes: systems biology and metabolic engineering approach. Crit Rev Biotechnol 39:79–98
de Campos FV, de Oliveira JA, da Silva AA, Ribeiro C, dos Santos FF (2019) Phytoremediation of arsenite-contaminated environments: is Pistia stratiotes L. a useful tool? Ecol Indic 104:794–801
de Souza TD, Borges AC, Braga AF, Veloso RW, de Matos AT (2019) Phytoremediation of arsenic-contaminated water by Lemna Valdiviana: an optimization study. Chemosphere 234:402–408
El-Mahrouk EM, Eisa EAE, Ali HM, Hegazy MAE, Abd-EI-Gayed MES (2020) Populus nigra as a phytoremediator for Cd, Cu, and Pb in contaminated soil. BioResources 15:869–893
Gao Z, Jiang C, Lyu R, Yang Z, Zhang T (2020) Optimization of the preparation of fungal-algal pellets for use in the remediation of arsenic-contaminated water. Environ Sci Pollut R 27(29):231–239
Gupta S, Thokchom SD, Kapoor R (2021) Arbuscular mycorrhiza improves photosynthesis and restores alteration in sugar metabolism in Triticum aestivum L. grown in arsenic contaminated soil. Front Plant Sci 12:334
Hashim MA, Mukhopadhyay S, Sahu JN, Sengupta B (2011) Remediation technologies for heavy metal contaminated groundwater. J Environ Manag 92:23552388
Hua J, Zhang C, Yin Y, Chen R, Wang X (2012) Phytoremediation potential of three aquatic macrophytes in manganese-contaminated water. Water Environ J 26:335–342
Huang H, Ullah F, Zhou D, Yi M, Zhao Y (2019) Mechanism of ROS regulation of plant development and stress responses. Front Plant Sci 10:800
Jiang C, Zhang T, Li S, Yang Z (2022) A comparative study on Fe(III)-chitosan and Fe(III)-chitosan-CTAB composites for As(V) removal from water: preparation, characterization and reaction mechanism. Environ Sci Pollut R 29:77851–77863
Kong C, Yang L, Yu J, Wei B, Li H, Cui N, Guo Z (2018) Assessment of arsenic exposure and carcinogenic risk in an endemic arsenism area in inner Mongolia caused by exposure to arsenic in drinking water. J Ecol Rural Environ 34:456–462
Korenkov V, King B, Hirschi K, Wagner GJ (2009) Root-selective expression of AtCAX4 and AtCAX2 results in reduced lamina cadmium in field-grown Nicotiana tabacum L. Plant Biotechnol J 7:219–226
Krayem M, Baydoun M, Deluchat V, Lenain J, Kazpard V, Labrousse P (2016) Absorption and translocation of copper and arsenic in an aquatic macrophyte Myriophyllum alterniflorum DC. in oligotrophic and eutrophic conditions. Environ Sci Pollut Res 23:11129–11136
Li B, Gu B, Yang Z, Zhang T (2018) The role of submerged macrophytes in phytoremediation of arsenic from contaminated water: a case study on Vallisneria natans (Lour.) Hara. Ecotox Environ Saf 165:224–231
Lin H, Liu J, Dong Y, Ren K, Zhang Y (2018) Absorption characteristics of compound heavy metals vanadium, chromium, and cadmium in water by emergent macrophytes and its combinations. Environ Sci Pollut Res 25:17820–17829
Liu H, Xia Y, Cai W, Zhang Y, Zhang X, Du S (2017) Enantioselective oxidative stress and oxidative damage caused by Rac-and S-metolachlor to Scenedesmus obliquus. Chemosphere 173:22–30
Manoj SR, Karthik C, Kadirvelu K, Arulselvi PI, Shanmugasundaram T, Bruno B, Rajkumar M (2020) Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: a review. J Environ Manage 254:109779
Mishra VK, Alka RU, Vinita P, Tripathi BD (2008) Phytoremediation of mercury and arsenic from tropical opencast coalmine effluent through naturally occurring aquatic macrophytes. Water Air Soil Pollut 192:303–314
Mulligan CN, Yong RN, Gibbs BF (2001) Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng Geol 60:193–207
Naeem M, Nabi A, Aftab T, Khan MMA (2020) Oligomers of carrageenan regulate functional activities and artemisinin production in Artemisia annua L. exposed to arsenic stress. Protoplasma 257:871–887
Nateewattana J, Trichaiyaporn S, Saouy M, Thavornyutikarn P, Pengchai P, Choonluchanon S (2010) Monitoring of arsenic in aquatic plants, water, and sediment of wastewater treatment ponds at the Mae Moh Lignite power plant, Thailand. Environ Monit Assess 165:585–594
Ostovar M, Saberi N, Ghiassi R (2022) Selenium contamination in water; analytical and removal methods: a comprehensive review. Sep Sci Technol 57:2500–2520
Ostovar M, Ghasemi A, Karimi F, Saberi N, Vriens B (2023) Assessment of EDTA-enhanced electrokinetic removal of metal(loid)s from phosphate mine tailings. Sep Sci Technol 58:613–625
Parviainen A, Loukola-Ruskeeniemi K, Tarvainen T, Hatakka T, Härmä P, Backman B, Ketola T, Kuula P, Lehtinen H, Sorvari J, Pyy O, Ruskeeniemi T, Luoma S (2015) Arsenic in bedrock, soil and groundwater—the first arsenic guidelines for aggregate production established in Finland. Earth Sci Rev 150:709–723
Raju NJ (2022) Arsenic in the geo-environment: a review of source, geochemical processes, toxicity and removal technologies. Environ Res 203:111782
Rezania S, Taib SM, Md Din MF, Dahalan FA, Kamyab H (2016) Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. J Hazard Mater 318:587–599
Rodriguez-Lado L, Sun G, Berg M, Zhang Q, Xue H, Zheng Q, Johnson CA (2013) Groundwater arsenic contamination throughout China. Science 341:866–868
Roy M, Giri AK, Dutta S, Mukherjee P (2015) Integrated phytobial remediation for sustainable management of arsenic in soil and water. Environ Int 75:180–198
Sesin V, Davy CM, Freeland JR (2021) Review of Typha spp. (cattails) as toxicity test species for the risk assessment of environmental contaminants on emergent macrophytes. Environ Pollut 284:117105
Shooto ND (2020) Removal of toxic hexavalent chromium (Cr(VI)) and divalent lead (Pb(II)) ions from aqueous solution by modified rhizomes of Acorus calamus. Surf Interfaces 20:100624
Singh S, Sounderajan S, Kumar K, Fulzele DP (2017) Investigation of arsenic accumulation and biochemical response of in vitro developed Vetiveria zizanoides plants. Ecotoxicol Environ Saf 145:50–56
Singh R, Jha AB, Misra AN, Sharma P (2019) Differential responsed of growth, photosynthesis, oxidative stress, metals accumulation and NRAMP genes in contrasting Ricinus communis genotypes under arsenic stress. Environ Sci Pollut Res 26:31166–31177
Sodhi KK, Kumar M, Agrawal PK, Singh DK (2019) Perspectives on arsenic toxicity, carcinogenicity and its systemic remediation strategies. Environ Technol Innov 16:100462
Souri Z, Karimi N (2017) Enhanced phytoextraction by As hyperaccumulator Isatis cappadocica spiked with sodium nitroprusside. Soil Sedim Contam Int J 26:457–468
Wei Z, Van Le Q, Peng W, Yang Y, Yang H, Gu H, Lam SS, Sonne C (2021) A review on phytoremediation of contaminants in air, water and soil. J Hazard Mater 403:123658
Wu F, Jasmine F, Kibriya MG, Liu M, Cheng X, Parvez F, Islam T, Ahmed A, Rakibuz-Zaman M, Jiang J, Roy S, Paul-Brutus R, Slavkovich V, Islam T, Levy D, VanderWeele TJ, Pierce BL, Graziano JH, Ahsan H, Chen Y (2015) Interaction between arsenic exposure from drinking water and genetic polymorphisms on cardiovascular disease in Bangladesh: a prospective case-cohort study. Environ Health Perspect 123:451–457
Wu D, Zong Y, Tian Z, Shao B (2019) Role of reactive oxygen species in As(III) oxidation by carbonate structural Fe(II): a surface-mediated pathway. Chem Eng J 368:980–987
Xue P, Yan C (2011) Arsenic accumulation and translocation in the submerged macrophytes Hydrilla verticillata (L.f.) Royle. Chemosphere 85:1176–1181
Zhao F, Wang P (2020) Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil 446:1–21
Zhao F, Han Y, Shi H, Wang G, Zhou M, Chen Y (2023) Arsenic in the hyperaccumulators Pteris vittata: a review of benefits, toxicity, and metabolism. Sci Total Environ 896:165232
Zhen Z, Yan C, Zhao Y (2020) Influence of epiphytic bacteria on arsenic metabolism in Hydrilla verticillate. Environ Pollut 261:114232
Funding
This work was supported by National Key Research and Development Program of China (2020YFC1807803) and Hunan Provincial Key Research and Development Program (2022NK2060).
Author information
Authors and Affiliations
Contributions
SL: Experiment conducting, Formal analysis, Writing—original draft. TZ: Experiment designing, Supervision, Writing—review and editing, Funding acquisition. GL: Funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to Participate
Not applicable.
Consent to Publication
Not applicable.
Ethics Approval
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Liu, G. & Zhang, T. Phytoremediation for Removal of Inorganic Arsenic in Water by an Emergent Macrophyte: A Case Study on Sweet Flag (Acorus calamus L.). Int J Environ Res 18, 31 (2024). https://doi.org/10.1007/s41742-024-00585-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-024-00585-7