Abstract
Male sterility induced by the cytoplasm of Triticum timopheevii Zhuk. has shown potential for hybrid seed production in common wheat (Triticum aestivum L.). As hybrids produced by this method are often partially sterile, fertility restoration is crucial for implementing this technology in breeding practice. Several restorer genes were identified, of which Rf3 is one of the most effective genes for achieving restoration. Previous studies located Rf3 on chromosome 1B in common and spelt wheat. However, the distribution of Rf3 in these taxa remained unclear. In the present study, we genetically mapped Rf3 using a BC1 population derived from CMS-Sperber and the restorer line Primepi (N = 193). After marker validation in four independent BC1 populations and a diversity panel, we evaluated the distribution of Rf3 in 524 common wheat and 30 European spelt genotypes. In the mapping population, the SNP marker IWB72107 cosegregated with Rf3, whereas IWB14060 was mapped 2.0 cM distal on chromosome 1BS. Surveying the linkage between IWB72107 and Rf3 in the four validation populations revealed map distances that ranged from 0.4 to 2.3 cM. Validation of IWB72107 in the diversity panel showed that it is suitable for marker-assisted selection and related applications. Using this marker, we estimated that 8.8% of the common wheat lines and 66.7% of the spelt cultivars carried the restoring Rf3 allele. We propose that Rf3 explains the restoration capacity of a large proportion of European common wheat lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The shift from line to hybrid breeding in common wheat (Triticum aestivum L.) is currently one of the most debated issues in the wheat breeding community. Whereas the use of hybrids has been tremendously successful in crops such as maize, rice and rye (Crow 1998; Cheng et al. 2007; Geiger and Miedaner 2009), they remain of minor importance in wheat (Koekemoer et al. 2011). However, recent studies confirmed the fundamental assumption that hybrid wheat allows the exploitation of positive commercial heterosis for grain yield (Gowda et al. 2012; Longin et al. 2013). To make use of this advantage, several hybridisation technologies are available, of which cytoplasmic male sterility (CMS) and chemical hybridising agents are the most promising approaches (Whitford et al. 2013).
CMS in wheat was first reported by Kihara (1951) who obtained male-sterile plants by combining the nucleus of T. aestivum with the cytoplasm of Aegilops caudata L. A few years later, male sterility was also observed when the cytoplasms of Aegilops ovata L. (Fukasawa 1953) and Triticum timopheevii Zhuk. (Wilson and Ross 1962) were used (Yen et al. 1969). T. timopheevii rapidly became the most common donor for sterility-inducing cytoplasms in wheat when it was reported that the two Aegilops cytoplasms showed adverse effects (Hayward 1975). Although many species have been used for the development of male-sterile wheat lines, the cytoplasm of T. timopheevii is considered to be the most reliable source to achieve male sterility (Koekemoer et al. 2011). In the present study, we exclusively refer to the CMS system based on this cytoplasm. Schmidt et al. (1962) and Wilson (1962) were the first to report fertility restoration of male-sterile wheat (Yen et al. 1969). Subsequently, restorer genes were found in many species including T. timopheevii (Livers 1964), Triticum spelta L. (Kihara and Tsunewaki 1967), T. aestivum (Oehler and Ingold 1966; Zeven 1967; Sinha et al. 2013), Secale cereale L. (Curtis and Lukaszewski 1993) and Aegilops umbellulata Zhuk. (Ma et al. 1995). The abovementioned genes were consecutively numbered from Rf1 (Livers 1964) to Rf8 (Sinha et al. 2013), with the exception of Rfc3 and Rfc4 (Curtis and Lukaszewski 1993). As these names were often used to refer to loci on different chromosomes, the nomenclature is not definite in the cases of Rf2, Rf3, Rf4 and Rf6 (Tahir and Tsunewaki 1969; Yen et al. 1969; Maan 1985; Ma et al. 1995; Kojima et al. 1997). In addition to restorer genes, environmental factors (Johnson et al. 1967) influence fertility restoration as along with epistatic effects of the genetic background (Maan et al. 1984). Fertility restoration became a crucial aspect in CMS hybrid wheat after it was discovered that the original sources for restorer genes resulted mostly in partially sterile hybrids (Keydel 1973; Hayward 1975). Johnson and Patterson (1977) showed that the combination of different sources of fertility restoration in one line led to an improved restoration capacity.
One of the most effective restorer genes is Rf3, which was discovered in European spelt on chromosome 1B (Tahir and Tsunewaki 1969), but may also control restoration capacity in some common wheat cultivars (Bahl and Maan 1973; Kučera 1982). Although Rf3 was genetically mapped in several studies, there is no molecular marker suitable for the prediction of Rf3 (Ma and Sorrells 1995; Kojima et al. 1997; Ahmed et al. 2001; Zhou et al. 2005). Whether the fertility-restoring allele of Rf3 is fixed in the spelt population and how Rf3 alleles are distributed in common wheat also remained unclear. To fill this knowledge gap, our objectives were to (1) genetically map the restorer gene Rf3 in five biparental populations that involved restorer lines from common wheat and European spelt, and (2) evaluate the distribution of Rf3 in German wheat breeding material and European spelt cultivars using a closely linked molecular marker.
Materials and methods
Plant materials
To identify molecular markers closely linked to the restorer gene Rf3, five BC1 populations were developed. Initially, male-sterile lines were pollinated with the common wheat lines Primepi and PR143, and the spelt cultivars Badenkrone, Badenstern and Schwabenspelz, which were believed to carry fertility-restoring alleles at the Rf3 locus (Tahir and Tsunewaki 1969; Bahl and Maan 1973; Patterson et al. 1996). Whereas Badenstern was combined with CMS-609-73, the other restorer lines were crossed with CMS-Sperber. The F1 hybrids were subsequently backcrossed with the corresponding maintainer lines Sperber and 609-73. To determine the success of each cross, one spike per F1 plant was bagged prior to anthesis and later examined for seed set. The BC1 populations derived from Primepi, PR143, Badenkrone, Badenstern and Schwabenspelz comprised 193, 221, 290, 220 and 288 plants, respectively. The BC1 seeds were sown into multi-pot trays. After vernalisation for 2 months at 5 °C in a growth chamber, seedlings were transferred to 13-cm pots (two plants per pot) and grown in a greenhouse under metal halide lamps at a temperature of 15–18 °C. Whereas the population CMS-Sperber/Primepi//Sperber was split into three subpopulations, with each being exposed to a different greenhouse environment, the other populations were grown in single environments.
To estimate the predictive ability of the markers linked to Rf3, we analysed a diversity panel comprising 29 common wheat and 30 European spelt lines. The accessions were classified based on their restoration capacity and references as described in detail in Table 2. The restoration capacity of the diversity panel was determined by testcrosses with CMS-Sperber and by assessing the seed set of the resulting F1 plants. Common wheat accessions reported to carry the fertility-restoring Rf3 allele were used as positive controls. Common wheat lines served as negative controls either if they were known to possess no restoring allele on chromosome 1B or if they were not able to restore fertility in testcrosses. To expand the diversity panel, we determined the restoration capacity of 30 spelt cultivars. As fertility restoration in T. spelta var. duhamelianum is controlled exclusively by Rf3 (Tahir and Tsunewaki 1969; Kojima et al. 1997), we used accessions of this taxon as positive controls if they were able to restore fertility. Spelt accessions showing no restoration capacity in testcrosses served as negative controls. Fertility restoring spelt lines that might belong to other varieties than var. duhamelianum and that were not shown to carry the restoring Rf3 allele by linkage mapping were excluded from the diversity panel.
The distribution of Rf3 alleles in common wheat and spelt accessions was estimated using 524 German winter wheat breeding lines and 30 European spelt cultivars, of which the latter were also analysed for the diversity panel. To verify whether Rf3 genotypes of the spelt cultivars depend on their relationship to common wheat, we analysed them together with a set of 368 German common wheat cultivars.
The French winter wheat cultivar Primepi and the Belgian winter wheat cultivars Minister and Professeur Marchal were obtained from the gene bank of IPK Gatersleben, Germany. The winter wheat lines PR143 and PR189 were provided by the National Small Grain Collection, ID, USA. Maintainer lines Navojoa and Vorobey were released by CIMMYT, Mexico. The restorer lines R1 and R3 were provided by Sejet Plant Breeding, Denmark. The 524 common wheat breeding lines used to determine the distribution of Rf3 were provided by the German breeding companies Saatzucht Bauer GmbH & Co. KG, Saatzucht Josef Breun GmbH & Co. KG, Limagrain GmbH, SECOBRA Saatzucht GmbH, Saatzucht Streng-Engelen GmbH & Co. KG and Lantmännen SW Seed GmbH. The remaining common wheat and spelt lines were in stock at the germplasm collection of the Bavarian State Research Center for Agriculture.
Assessment of fertility restoration
As a measure for the restoration of male fertility, we determined the seed set of isolated spikes. Before anthesis, one to four emerging spikes of each plant were isolated using glassine bags. After ripening, the bagged spikes were harvested. For each of these spikes, the numbers of spikelets and kernels were recorded. The seed set of a plant was defined as the number of kernels divided by the number of spikelets, averaged over the isolated spikes. A plant was considered male sterile if it contained no seeds, whereas it was considered male fertile if it contained at least one seed. The ratios of fertile and sterile plants of the mapping populations were compared to the expected segregation pattern using Pearson’s χ 2 test (α = 0.05). Modalities of the seed set distributions were analysed using Hartigan’s dip test (α = 0.05). Statistical analysis of the seed set was performed using R (R Core Team 2015).
Genotyping and linkage mapping
Total genomic DNA was extracted from young leaf tissue according to the protocol described by Plaschke et al. (1995). Initially, Rf3 was mapped using the BC1 population CMS-Sperber/Primepi//Sperber. The 193 individuals of the population were genotyped with the simple sequence repeat (SSR) markers Xbarc8, Xbarc128, Xgwm264, Xwmc406 and Xwmc798, which are located on chromosome 1BS (Somers et al. 2004). Primer sequences and amplification conditions were obtained from the GrainGenes database (http://wheat.pw.usda.gov). To increase the marker density in the genomic region of Rf3, we selected individuals that showed recombination for the SSR loci flanking the restorer gene. Recombinant individuals were genotyped by TraitGenetics GmbH (Gatersleben, Germany) using an Illumina® Infinium® 15 k single nucleotide polymorphism (SNP) array based on the 90 k SNP array described by Wang et al. (2014). SNPs linked to Rf3 in this population were converted to cleaved amplified polymorphic sequence (CAPS) markers by aligning multiple DNA sequences obtained from The Triticeae Toolbox (https://triticeaetoolbox.org), CerealsDB (http://www.cerealsdb.uk.net), Unité de Recherche Génomique Info (https://urgi.versailles.inra.fr), GrainGenes (http://wheat.pw.usda.gov) and KOMUGI (http://shigen.nig.ac.jp) using the Clustal Omega program (http://www.ebi.ac.uk). Based on this sequence alignment, we designed polymerase chain reaction (PCR) primers using Primer3web (http://primer3.ut.ee). Restriction enzymes were chosen with the analysis tool SNP2CAPS (IPK, Gatersleben, Germany). The CAPS markers were subsequently validated by genotyping the population CMS-Sperber/Primepi//Sperber. One of the converted markers was used to genotype a sample of each of the populations derived from PR143 (N = 170), Badenkrone (N = 284), Badenstern (N = 87) and Schwabenspelz (N = 87). Products of SSR and CAPS markers were resolved on 5% polyacrylamide gels. For the construction of linkage maps, we used JoinMap® 4.0 (Kyazma B.V., Wageningen, The Netherlands). Linkage groups were established using an independence logarithm of the odds (LOD) value of ≥6.0. Regression mapping was performed using the Kosambi mapping function (Kosambi 1944).
Distribution of Rf3 in common wheat and European spelt
To estimate the distribution of Rf3 in common wheat, a sample of 524 current German breeding lines was genotyped with the Illumina® Infinium® 15 k SNP array. The frequency of Rf3 genotypes was estimated based on the SNP marker linked with Rf3. The distribution of Rf3 in common wheat was evaluated by principal coordinate analysis (PCoA) using modified Roger’s distances between the breeding lines (Wright 1978). To verify whether Rf3 genotypes were homogeneously distributed within the population, we calculated Pearson’s correlation coefficients between binary Rf3 marker genotypes and the principal coordinates. Significance of the correlations was tested using t tests (α = 0.05).
The distribution of Rf3 was additionally estimated in the 30 spelt cultivars also used for the diversity panel. The spelt cultivars were genotyped together with 368 German common wheat cultivars using 33 SSR markers evenly distributed across the genome. We performed PCoA for the spelt lines as well as for the spelt lines together with the 368 wheat cultivars. The distribution of Rf3 in spelt was examined as described for common wheat. To test for a possible relationship between Rf3 genotype classes of the spelt lines and their kinship to common wheat, we calculated the mean genetic distance to common wheat for each spelt cultivar. Mean distances of spelt cultivars with the restoring Rf3 allele were compared to mean distances of spelt cultivars without the restoring Rf3 allele using t tests (α = 0.05). PCoA was performed within the R environment (R Core Team 2015) using the R package “APE” (Paradis et al. 2004).
Results
Seed set of the mapping populations
Segregation into fertile and sterile plants conformed with the expected 1:1 ratios in all populations, indicating a monogenic inheritance of fertility restoration (Table 1). This ratio was also observed for the three subpopulations of CMS-Sperber/Primepi//Sperber (P χ 2 ≥ 0.35). The mean seed sets of the fertile plants ranged from 0.54 to 1.57 seeds per spikelet, with the lowest and highest seed set in CMS-609-73/Badenstern//609-73 and CMS-Sperber/Primepi//Sperber, respectively. At 0.57 seeds per spikelet, the standard deviation of the seed set was higher in CMS-Sperber/Primepi//Sperber than in other populations. Seed set distributions of the fertile plants are shown in Fig. S1. Hartigan’s dip test suggested a unimodal seed set distribution for the fertile plants in each population.
Genotyping and linkage mapping
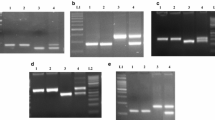
Using the categorical fertility phenotypes (completely sterile or fertile) and SSR genotypes of the BC1 population CMS-Sperber/Primepi//Sperber, we constructed a partial linkage map of chromosome 1BS. The map comprised a single linkage group spanning 33.7 centimorgans (cM). Rf3 was flanked by the SSR loci Xbarc128 and Xwmc406, located 7.2 cM distal and 14.5 cM proximal to Rf3, respectively. To enrich this genomic region with SNP markers, we identified 40 plants that were recombinant between the flanking SSR loci. The selected individuals were genotyped with the 15 k SNP array. After removing markers with >10% missing alleles (N = 625) or a minor allele frequency <10% (N = 8705) as well as monomorphic markers (N = 883), 2793 SNPs remained for linkage mapping. We identified three normally inherited SNP markers closely linked to Rf3, namely IWB14060, IWB72107 and IWB73447. The SNPs IWB14060 and IWB72107 were used to develop CAPS assays, designated CAPS_IWB14060 and CAPS_IWB72107, respectively (Table S1). The development of a CAPS assay for IWB73447 was not successful because it did not affect the recognition site for a restriction enzyme. The CAPS markers were validated by genotyping the population CMS-Sperber/Primepi//Sperber, and the original microarray-based SNP genotypes were reproduced. This validation also confirmed the assumed SNP genotypes of the non-recombinant plants that were not genotyped with the SNP array. The consensus map comprising five SSR and two SNP genotypes of all 193 individuals spanned 33.6 cM (Fig. 1). Whereas IWB72107 cosegregated with Rf3, IWB14060 was mapped 2.0 cM distal to Rf3. Using the CAPS assay CAPS_IWB72107, we mapped Rf3 in the four populations that involved PR143, Badenkrone, Badenstern and Schwabenspelz. In these populations, CAPS_IWB72107 was mapped 0.6 (1 recombinant), 0.4 (1 recombinant), 2.3 (2 recombinants) and 1.2 cM (1 recombinant) from Rf3. There were no instances of deviation from the expected genotype frequencies (Table S2). Map distances between CAPS_IWB72107 and Rf3 indicated that fertility restoration was controlled by the same locus, namely Rf3, in all mapping populations.
Predictive ability of CAPS_IWB72107
The CAPS assay CAPS_IWB72107 based on the SNP IWB72107 was further validated in a diversity panel comprising common wheat and European spelt accessions (Table 2). The marker genotype of Primepi was set as a reference for the prediction of the Rf3 allele associated with fertility restoration. Using this marker, we predicted the correct Rf3 allele in all 29 common wheat accessions. Analysing the restoration capacity of the spelt lines revealed that 20 of 30 spelt accessions restored fertility in testcrosses with CMS-Sperber. The five restoring spelt cultivars Ceralio, Tauro, Titan, Zollernspelz and Züricher Oberländer Rotkorn were excluded from the diversity panel as there was no clear record that they belong to the T. spelta var. duhamelianum taxon. CAPS_IWB72107 predicted the correct Rf3 allele in 23 of the remaining 25 spelt accessions. Only the marker genotypes of Bauländer Spelz and Grey did not correspond to their inability to restore fertility. CAPS_IWB72107 produced the two alleles that were previously observed in the mapping populations as well as null alleles for the negative controls 444-74, 539-74, 563-76, R1, R3 and Samir and Sirino (Fig. S2). These results indicate that the SNP IWB72107 is suitable for marker-assisted selection and related applications.
Distribution of Rf3 alleles in common wheat and European spelt
The frequency of Rf3 alleles in common wheat was estimated using the IWB72107 genotypes of 524 current German breeding lines; 8.8% of the lines carried the SNP allele associated with fertility restoration. Analysis of the population structure with PCoA revealed that IWB72107 alleles were evenly distributed along the first four principal coordinates (P ≥ 0.11), jointly explaining 13.5% of the genotypic variation, thereby indicating a homogeneous distribution of IWB72107, and thus Rf3 alleles in this common wheat population. The first two principal coordinates are depicted in Fig. 2.
The CAPS_ IWB72107 genotypes and the restoration capacity of 30 spelt cultivars indicated that 20 cultivars (66.7%) carried the fertility-restoring allele at the Rf3 locus. The five fertility restoring spelt accessions that were discarded from the diversity panel were included here, since the marker CAPS_ IWB72107 predicted the restoring Rf3 allele for all of them. As the accessions Bauländer Spelz and Grey could not restore fertility in testcrosses, they were classified as carriers of the non-restoring Rf3 allele. The Rf3 genotypes of the spelt cultivars were not significantly correlated to any of the first four principal coordinates (P ≥ 0.06), which together explained 40.8% of the genotypic variation, suggesting that Rf3 alleles are homogeneously distributed within this spelt population (Fig. S3). Mean genetic distances between fertility-restoring spelt cultivars and common wheat were not significantly different to the distances observed for the spelt cultivars without the restoring Rf3 allele (P = 0.052), suggesting that Rf3 genotypes of spelt cultivars were not dependent on their kinship to common wheat (Fig. 3).
Discussion
We genetically mapped the fertility-restoring gene Rf3 using five populations derived from restorer lines of common wheat and European spelt. The segregation of fertility restoration into fertile and sterile plants indicated monogenic inheritance of fertility restoration in all five populations. This is in agreement with the unimodal distributions we observed for the seed set of the fertile plants. Linkage mapping indicated that restoration was controlled exclusively by Rf3, located on chromosome 1BS. Since each population was exposed to a different environment, we could not compare the restoration capacities of the five restorer lines. In CMS-Sperber/Primepi//Sperber, there was a high standard deviation of the seed set compared to the other populations. This can be explained by the fact that each of the three subpopulations was exposed to a different environment, which had a significant effect on the seed set of the fertile plants (data not shown). The monogenic inheritance of fertility restoration we observed in CMS-Sperber/Primepi//Sperber is in accordance with the findings of Ingold (1968), who reported a single restorer gene in Primepi. However, Bahl and Maan (1973) located two restorer genes in Primepi by monosomic analysis: one with higher expressivity located on chromosome 1BS (Rf3), and one with lower expressivity located on chromosome 5D (unnamed). The hypothesis of two restorer genes was confirmed by Miller et al. (1974), who observed a 9:6:1 ratio of fertile, partially fertile and sterile individuals, respectively, in a segregating F2 population involving Primepi. This discrepancy might be due to environmental effects or the genetic background of the CMS lines influencing the expressivity of the restorer gene on chromosome 5D. An alternative explanation may be that different Primepi accessions are polymorphic at this locus. Previous studies have not as yet described the genes controlling the restoration capacity of PR143. PR143 descends from a cross between R3 and Monon/Primepi and was bred by recurrent selection for an optimised restoration capacity (Patterson et al. 1996). According to its pedigree, PR143 could possibly carry alleles for fertility restoration at the Rf1, Rf2 and Rf3 loci, as well as the one on chromosome 5D. In contrast, we observed that Rf3 was the only gene controlling fertility restoration in the population CMS-Sperber/PR143//Sperber. Monogenic inheritance of fertility restoration controlled by Rf3 was also observed in the three populations derived from the European spelt cultivars Badenkrone, Badenstern and Schwabenspelz. This is in accordance with the observations of Tahir and Tsunewaki (1969) and Kojima et al. (1997), who observed that fertility restoration in spelt appears to be solely controlled by Rf3.
The order of SSR and SNP markers in the population CMS-Sperber/Primepi//Sperber is in agreement with the consensus map of Maccaferri et al. (2015), with the exception that Xgwm264 was mapped more distal in the present study. Meaningful validation work in independent mapping populations and a diversity panel suggested that the marker CAPS_IWB72107 and its underlying SNP IWB72107 are suitable for the prediction of Rf3 genotypes in marker-assisted selection. The discrepancy between phenotype and SNP genotype observed for Bauländer Spelz and Grey might be caused by recombination between IWB72107 and Rf3, or by genes suppressing fertility restoration in these lines. The marker CAPS_IWB72107 also produced null alleles, which are often associated with the presence of alien chromatin in host genomes (Brown-Guedira et al. 2003; Landjeva et al. 2006). The null alleles in the lines R1 and R3 might be due to introgressions of T. timopheevii (Bahl and Maan 1973), whereas the T1BL.1RS wheat-rye translocation is most likely responsible for the null alleles in Samir and Sirino (Zeller, personal communication). In addition, the two spelt cultivars also had null alleles for Xpsp3000 and Xgwm18, two other markers located on chromosome 1BS supporting the presence of alien chromatin rather than a normal chromosome 1BS (data not shown). Since the maintainer lines 444-74, 539-74 and 563-76 were derived from wheat-rye translocation lines (Keydel, personal communication), their associated null alleles are probably also due to the T1BL.1RS translocation.
In an initial screening for fertility restoration (data not shown), we observed that about 14% of European common wheat cultivars were able to restore the fertility of lines with T. timopheevii cytoplasm. However, the genes responsible for restoration in these lines remain unknown. In the present study, we used the SNP IWB72107 to survey the frequency of Rf3 genotypes in German breeding material and found that 8.8% of the analysed lines probably contained the fertility-restoring allele for Rf3. This marker frequency is similar to the findings of Zanke et al. (2014) who observed a frequency of 12% for the respective SNP allele in a panel of European wheat cultivars (Korzun, personal communication). Hence, we propose that Rf3 might explain fertility restoration in a large proportion of European wheat cultivars. The observed allele frequency also indicated that the fertility-restoring Rf3 allele probably has no positive effect on fitness and agronomic performance in common wheat. The SNP markers IWB14060 and IWB73447 linked to Rf3 in the population CMS-Sperber/Primepi//Sperber indicated that the Primepi allele was present in 59.0 and 57.3% of the 524 German wheat breeding lines, respectively. Comparing these results to the allele frequency of IWB72107, we concluded that IWB14060 and IWB73447 are not diagnostic, since they would overestimate the presence of the fertility-restoring Rf3 allele. In European spelt, we found that 66.7% of the analysed cultivars carried the fertility-restoring allele at the Rf3 locus. The assumed Rf3 genotypes of the spelt cultivars did not depend on their relationship to common wheat. This observation may support the hypothesis that neither the restoring nor the non-restoring alleles were recently introduced to spelt by crosses with common wheat. Since we found restoring and non-restoring Rf3 genotypes among the two oldest cultivars Altgold and Oberkulmer Rotkorn, respectively, we concluded that both types of Rf3 alleles have existed in European spelts for at least the last six decades. Whether the Rf3 locus is limited to these two alleles and if there are species-specific alleles for common wheat and spelt remains unknown.
Little is also known about a possible relationship between Rf3 and other restorer genes on the homoeologous group 1 chromosomes. Zhang et al. (2003) located a fertility-restoring gene on chromosome 1AS. It is possible that this gene is a homoeolog of Rf3. Moreover, Tsunewaki (2015) and Hohn and Lukaszewski (2016) reported a gene designated Rf multi on chromosome 1BS, that restored male fertility of sterile lines with the cytoplasms of Aegilops kotschyi Boiss., Aegilops mutica Boiss. and Aegilops uniaristata Vis. Further research is required to investigate a possible relationship between the restorer gene on chromosome 1A, Rf multi and Rf3.
References
Ahmed TA, Tsujimoto H, Sasakuma T (2001) QTL analysis of fertility-restoration against cytoplasmic male sterility in wheat. Genes Genet Syst 76:33–38
Bahl PN, Maan SS (1973) Chromosomal location of male fertility restoring genes in six lines of common wheat. Crop Sci 13:317–320
Brown-Guedira GL, Singh S, Fritz AK (2003) Performance and mapping of leaf rust resistance transferred to wheat from Triticum timopheevii subsp. armeniacum. Phytopathology 93:784–789
Cheng SH, Zhuang JY, Fan YY, Du JH, Cao LY (2007) Progress in research and development on hybrid rice: a super-domesticate in China. Ann Bot 100:959–966
Crow JF (1998) 90 years ago: the beginning of hybrid maize. Genetics 148:923–928
Curtis CA, Lukaszewski AJ (1993) Localization of genes in rye that restore male fertility to hexaploid wheat with timopheevi cytoplasm. Plant Breed 111:106–112
Fukasawa H (1953) Studies on restoration and substitution of nucleus of Aegilotricum, I. Appearance of male-sterile durum in substitution crosses. Cytologia 18:167–175
Geiger HH, Miedaner T (2009) Rye breeding. In: Carena MJ (ed) Cereals (handbook of plant breeding), 1st edn. Springer, New York, pp. 157–181
Gowda M, Longin CFH, Lein V, Reif JC (2012) Relevance of specific versus general combining ability in winter wheat. Crop Sci 52:2494–2500
Hayward CF (1975) The status and prospects for hybrid winter wheat. In: Proceedings of the 2nd International Winter Wheat Conference. Zagreb, Yugoslavia
Hohn CE, Lukaszewski AJ (2016) Engineering the 1BS chromosome arm in wheat to remove the Rf multi locus restoring male fertility in cytoplasms of Aegilops kotschyi, Ae. uniaristata and Ae. mutica. Theor Appl Genet. doi:10.1007/s00122-016-2738-7
Ingold M (1968) Male sterility and restorer systems in wheat. Euphytica 17:69–74
Johnson JW, Patterson FL (1977) Interaction of genetic factors for fertility restoration in hybrid wheat. Crop Sci 17:695–699
Johnson VA, Schmidt JW, Mattern PJ (1967) Hybrid wheat in the United States. Plant Food Hum Nutr 14:193–211
Keydel F (1973) Die Restoration der Fertilität in Weizenhybriden. Bayer Landwirtsch Jahrb 50:424–430
Kihara H (1951) Substitution of nucleus and its effects on genome manifestations. Cytologia 16:177–193
Kihara H, Tsunewaki K (1967) Genetic principles applied to the breeding of crop plants. In: Brink RA (ed) Heritage from Mendel. University of Wisconsin Press, Madison, pp. 403–418
Koekemoer FP, Van Eeden E, Bonjean AP (2011) An overview of hybrid wheat production in South Africa and review of current worldwide wheat hybrid developments. In: Bonjean AP, Angus WJ, Van Ginkel M (eds) The world wheat book: a history of plant breeding. Lavoisier, Paris, pp 907–950
Kojima T, Tsujimoto H, Ogihara Y (1997) High-resolution RFLP mapping of the fertility restoration Rf3 gene against Triticum timopheevi cytoplasm located on chromosome 1BS of common wheat. Genes Genet Syst 72:353–359
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eug 12:172–175
Kučera L (1982) Monosomic analysis of fertility restoration in common wheat “Prof. Marchal”. Euphytica 31:895–900
Landjeva S, Korzun V, Ganeva G (2006) Evaluation of genetic diversity among Bulgarian winter wheat (Triticum aestivum L.) varieties during the period 1925–2003 using microsatellites. Genet Resour Crop Evol 53:1605–1614
Livers RW (1964) Fertility restoration and its inheritance in cytoplasmic male-sterile wheat. Science 144:420
Longin CFH, Gowda M, Mühleisen J, Ebmeyer E, Kazman E, Schachschneider R, Schacht J, Kirchhoff M, Zhao Y, Reif JC (2013) Hybrid wheat: quantitative genetic parameters and consequences for the design of breeding programs. Theor Appl Genet 126:2791–2801
Ma ZQ, Sorrells ME (1995) Genetic analysis of fertility restoration in wheat using restriction fragment length polymorphisms. Crop Sci 35:1137–1143
Ma ZQ, Zhao YH, Sorrells ME (1995) Inheritance and chromosomal locations of male fertility restoring gene transferred from Aegilops umbellulata Zhuk. to Triticum aestivum L. Mol Gen Genet 247:351–357
Maan SS, Lucken KA, Bravo JM (1984) Genetic analyses of male-fertility restoration in wheat. I. Chromosomal location of Rf genes. Crop Sci 24:17–20
Maan SS (1985) Genetic analyses of male-fertility restoration in wheat. II. Isolation, penetrance, and expressivity of Rf genes. Crop Sci 25:743–748
Maccaferri M, Ricci A, Salvi S, Milner SG, Noli E, Martelli PL, Casadio R, Akhunov E, Scalabrin S, Vendramin V, Ammar K, Blanco A, Desiderio F, Distelfeld A, Dubcovsky J, Fahima T, Faris J, Korol A, Massi A, Mastrangelo AM, Morgante M, Pozniak C, N’Diaye A, Xu S, Tuberosa R (2015) A high-density, SNP-based consensus map of tetraploid wheat as a bridge to integrate durum and bread wheat genomics and breeding. Plant Biotechnol J 13:648–663
Miller JF, Schmidt JW, Johnson VA (1974) Inheritance of genes controlling male-fertility restoration in the wheat cultivar Primépi. Crop Sci 14:437–438
Oehler E, Ingold M (1966) New cases of male-sterility and new restorer sources in T. aestivum. Wheat Inf Serv 22:1–3
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Patterson FL, Ohm HW, Johnson JW, Wickersham DS (1996) Registration of five wheat pollen fertility restorer germplasm lines: PR143, PR189, PR267, PR270, PR302. Crop Sci 36:1424
Plaschke J, Ganal MW, Röder MS (1995) Detection of genetic diversity in closely related bread wheat using microsatellite markers. Theor Appl Genet 91:1001–1007
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Schmidt JW, Johnson VA, Maan SS (1962) Hybrid-wheat. Neb Exp Sta Quar 9:9
Sinha P, Tomar SMS, Vinod, Singh VK, Balyan HS (2013) Genetic analysis and molecular mapping of a new fertility restorer gene Rf8 for Triticum timopheevi cytoplasm in wheat (Triticum aestivum L.) using SSR markers. Genetica 141:431–441
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Tahir CM, Tsunewaki K (1969) Monosomic analysis of Triticum spelta var. duhamelianum, a fertility-restorer for T. timopheevi cytoplasm. Jpn J Genetics 44:1–9
Tsunewaki K (2015) Fine mapping of the first multi-fertility-restoring gene, Rf multi, of wheat for three Aegilops plasmons, using 1BS-1RS recombinant lines. Theor Appl Genet 128:723–732
Wang S, Wong D, Forrest K, Allen A, Chao S, Huang BE, Maccaferri M, Salvi S, Milner SG, Cattivelli L, Mastrangelo AM, Whan A, Stephen S, Barker G, Wieseke R, Plieske J, International Wheat Genome Sequencing Consortium, Lillemo M, Mather D, Appels R, Dolferus R, Brown-Guedira G, Korol A, Akhunova AR, Feuillet C, Salse J, Morgante M, Pozniak C, Luo M-C, Dvorak J, Morell M, Dubcovsky J, Ganal M, Tuberosa R, Lawley C, Mikoulitch I, Cavanagh C, Edwards KJ, Hayden M, Akhunov E (2014) Characterization of polyploid wheat genomic diversity using a high-density 90,000 single nucleotide polymorphism array. Plant Biotechnol J 12:787–796
Whitford R, Fleury D, Reif JC, Garcia M, Okada T, Korzun V, Langridge P (2013) Hybrid breeding in wheat: technologies to improve hybrid wheat seed production. J Exp Bot 64:5411–5428
Wilson JA (1962) Material prepared through DeKalb Agricultural Assoc. Inc. Wheat Newsl 9:28–29
Wilson JA, Ross WM (1962) Male sterility interaction of the Triticum aestivum nucleus and Triticum timopheevi cytoplasm. Wheat Inf Serv 14:29–30
Wright S (1978) Evolution and the genetics of populations, volume 4. The University of Chicago Press, Chicago
Yen FS, Evans LE, Larter EN (1969) Monosomic analysis of fertility restoration in three restorer lines of wheat. Can J Genet Cytol 11:531–546
Zanke CD, Ling J, Plieske J, Kollers S, Ebmeyer E, Korzun V, Argillier O, Stiewe G, Hinze M, Neumann K, Ganal MW, Röder MS (2014) Whole genome association mapping of plant height in winter wheat (Triticum aestivum L.). PLoS One 9:e113287
Zeven AC (1967) Transfer and inactivation of male sterility and sources of restorer genes in wheat. Euphytica 16:183–189
Zhang C, Wang HY, Shen YZ, Zhao BC, Zhu ZG, Huang ZJ (2003) Location of the fertility restorer gene for T-type CMS wheat by microsatellite marker. Acta Genet Sin 30:459–464
Zhou W, Kolb FL, Domier LL, Wang S (2005) SSR markers associated with fertility restoration genes against Triticum timopheevii cytoplasm in Triticum aestivum. Euphytica 141:33–40
Acknowledgements
We thank Ruth Torrijos, Petra Greim, Sabine Schmidt and the working group Wheat and Oat Breeding Research of the Bavarian State Research Center for Agriculture for their excellent technical assistance. The valuable suggestions of Günther Schweizer and Bianca Büttner are highly appreciated. We also thank the breeding companies for providing their breeding material. The present study was part of the project “CMS-Hybridweizen” (AZ-1066-13) supported by the Bavarian Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 274 kb)
Rights and permissions
About this article
Cite this article
Geyer, M., Bund, A., Albrecht, T. et al. Distribution of the fertility-restoring gene Rf3 in common and spelt wheat determined by an informative SNP marker. Mol Breeding 36, 167 (2016). https://doi.org/10.1007/s11032-016-0592-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-016-0592-6