Abstract
Key message
qFS07.1 controlling fiber strength was fine-mapped to a 62.6-kb region containing four annotated genes. RT-qPCR and sequence of candidate genes identified an LRR RLK gene as the most likely candidate.
Abstract
Fiber strength is an important component of cotton fiber quality and is associated with other properties, such as fiber maturity, fineness, and length. Stable QTL qFS07.1, controlling fiber strength, had been identified on chromosome 7 in an upland cotton recombinant inbred line (RIL) population from a cross (CCRI35 × Yumian1) described in our previous studies. To fine-map qFS07.1, an F2 population with 2484 individual plants from a cross between recombinant line RIL014 and CCRI35 was established. A total of 1518 SSR primer pairs, including 1062, designed from chromosome 1 of the Gossypium raimondii genome and 456 from chromosome 1 of the G. arboreum genome (corresponding to the QTL region) were used to fine-map qFS07.1, and qFS07.1 was mapped into a 62.6-kb genome region which contained four annotated genes on chromosome A07 of G. hirsutum. RT-qPCR and comparative analysis of candidate genes revealed a leucine-rich repeat protein kinase (LRR RLK) family protein to be a promising candidate gene for qFS07.1. Fine mapping and identification of the candidate gene for qFS07.1 will play a vital role in marker-assisted selection (MAS) and the study of mechanism of cotton fiber development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cotton is the world’s most widely cultivated fiber crop. Upland cotton (Gossypium hirsutum L., 4n = 4x = 52) dominates the production of cotton fiber, accounting for about 95% of the world’s total production (Chen et al. 2007). Modern textile mills are adopting more efficient spinning technologies that rely on high speed and automation to achieve higher performance (Bradow and Davidonis 2000). Pursuing a high yield of good quality fiber is the ultimate goal of cotton breeding (Arpat et al. 2004; Lee et al. 2007), but it is still a challenging job for cotton breeders because of the complex genetic foundation governing fiber development.
Fiber quality traits are genetically complex quantitative traits, controlled by multiple genes and easily affected by environment, so it takes a long time to improve fiber quality through the traditional genetic improvement methods. Marker-assisted selection (MAS) is a more economical and efficient method than the traditional breeding based on phenotypic selection (Lande and Thompson 1990). Researchers have expended a great deal of effort on mapping quantitative trait loci (QTL). A recent meta-QTL analysis suggested that 1075 QTL in intraspecific G. hirsutum and 1059 QTL in interspecific (G. hirsutum × G. barbadense) populations has been detected for yield, fiber quality, seed quality, and stress tolerance (Said et al. 2015). Several new reports have added new cotton fiber quality QTL (Tang et al. 2015; Tan et al. 2015; Fang et al. 2014a; Shang et al. 2015).
Currently, only a few studies of fine-mapping cotton fiber quality QTL have been reported. Su et al. (2013) fine-mapped a fiber strength QTL QFS-D11-1 into an interval of 0.6 cM on chromosome 21 using introgressed lines from the cross between TM-1 (G. hirsutum L.) × H102 (G. barbadense L.). Cao et al. (2015) anchored qFL-chr.7 for fiber length and qFS-chr.7 for strength to a 0.36-cM interval, and anchored qFM-chr.7 for fiber micronaire to a 0.44-cM interval using a G. barbadense introgressed line. Islam et al. (2016) used an upland cotton intraspecific F3 population to validate qFBS-c3, qSFI-c14, qUHML-c14, and qUHML-c24 related to fiber bundle strength, short fiber index, and fiber length, respectively, and spanned 4.4, 1.8, and 3.7 Mb of physical distance in the G. raimondii reference genome. Liu et al. (2016) identified a major QTL controlling multiple fiber quality traits using an upland cotton intraspecific population and mapped the QTL into 0.28 cM interval with a 5.3 Mb physical distance on chromosome A06 in G. hirsutum. Yang et al. (2016) detected and validated a stable fiber strength QTL (qFS-c9-1) within a ~2.98-Mbps genome region through four interspecific hybridization populations. However, all these QTL identified had confidence intervals too large for marker-assisted selection (MAS) and map-based cloning. Therefore, it is important to fine-map fiber quality QTL and identify QTL candidate genes in cotton.
Cotton fibers are seed trichomes that derive from individual cells of the epidermal layer of the seed coat. Fiber development consists of four overlapping stages: fiber initiation, cell elongation, secondary wall deposition, and maturation (Kim and Triplett 2001). Many studies have highlighted the stage-specific transcription of genes involved in fiber initiation, elongation, and secondary cell wall formation. For example, GhMYB25 (Machado et al. 2009), GhMYB25-like (Walford et al. 2011), GhSusA1 (Jiang et al. 2012), GbPDF1 (Deng et al. 2012), GhHD-1 (Walford et al. 2012), GhFLA1 (Huang et al. 2013), GhVIN1 (Wang et al. 2014), GhCFE1A (Lv et al. 2015), and Gh14-3-3 (Zhou et al. 2015) have been demonstrated to have definite roles in cotton fiber initiation, and GhPIP2 (Li et al. 2013), GhHOX3 (Shan et al. 2014), GhCaM7 (Tang et al. 2014), GhPAG1 (Yang et al. 2014), and GhCPC (Liu et al. 2015a) play roles in controlling cotton fiber elongation. Fiber strength is a key component of fiber quality in cotton, and it is closely related to secondary cell wall synthesis (Fang et al. 2014b). So far, several genes related to secondary cell wall synthesis have been reported, such as GhMYB7 (Huang et al. 2016), GbEXPATR (Li et al. 2016), GhMYB1 (Sun et al. 2015), GhCesA4 (Kim et al. 2011), GbTLP1 (Munis et al. 2010), and GhADF1 (Wang et al. 2009). However, only a few genes directly related to fiber strength have been reported. Therefore, an effective strategy to unravel the relationship between fiber quality traits and genes remains to be developed.
In our previous study, a major QTL for fiber strength, qFS07.1, was identified on chromosome 7 in five environments in upland cotton, with the favorable allele contributed by Yumian1 (Tan et al. 2015). To fine-map qFS07.1, we crossed CCRI35 with recombinant inbred line RIL014 with the Yumian1 allele for the qFS07.1 region to produce a fine-mapping population, and designed SSR primers according to the G. raimondii and G. arboreum genomes (Paterson et al. 2012; Li et al. 2014). RT-qPCR and molecular cloning were utilized to analyze gene expression and gene structure, identifying candidate genes related to fiber quality.
Materials and methods
Plant materials and fiber quality measurement
In our previous studies, CCRI35 and Yumian1 were chosen to establish a recombinant inbred line (RIL) population (Tan et al. 2015). CCRI35 is a high yielding and disease resistant cultivar bred by the Chinese Academy of Agricultural Sciences (Tan et al. 2015). Yumian1, a high fiber quality cultivar, especially characterized by high fiber strength, was developed from a multiple-cultivar intermating program (Zhang et al. 2009).
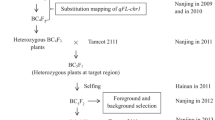
To fine-map qFS07.1 on chromosome 7, we selected one recombinant line, RIL014, characterized by superior fiber quality as one parent. For RIL014, the QTL region on chromosome 7 is derived from Yumian1 whereas the other regions with alleles affecting fiber quality are derived from CCRI35. We crossed CCRI35 with RIL014 in the summer of 2012 at Southwest University, Chongqing, China. F1 plants were self-pollinated to obtain F2 seeds in the winter of 2012 in Sanya, Hainan, China. The F2 population and parents were grown in Chongqing, in the summer of 2013. F2:3 individuals derived from F2 recombinant individual plants were planted in the summer of 2014 at Southwest University, Chongqing, China.
All naturally opened bolls from the F2/F2:3 individual plants and parents were hand-harvested to gin fiber. Fiber samples were evaluated for fiber quality traits using the HVI (High Volume Instrument) system at the Supervision Inspection and Testing Cotton Quality Center, Anyang, China. Data were collected on fiber elongation (FE, %), fiber upper half mean length (FL, mm), fiber micronaire reading (FM), fiber strength (FS, cN/tex), and fiber length uniformity ratio (FU, %).
Marker development
Cotton genomic DNA was extracted from young leaves of F2/F2:3 individuals and parents, using a modified CTAB method (Zhang et al. 2005). To fine-map qFS07.1, a total of 1062 SSR primer pairs were designed from chromosome 1 of G. raimondii (Paterson et al. 2012) and 456 SSR primer pairs were designed from chromosome 1 of G. arboreum (Li et al. 2014). These primer pairs were named ‘SWU’ and were synthesized by Invitrogen Co. Ltd. (Shanghai, China) and Beijing Genomics Institute Genomics Co. Ltd. (Beijing, China).
The newly developed SSR markers were first screened for polymorphism between the mapping parents and those showing clear polymorphism were used to genotype the fine-mapping population. PCR amplification and product testing were performed according to Zhang et al. (2005). Clear polymorphic DNA bands on the gels were used for scoring and genotyping. Loci detected were named with the primer name.
QTL analysis
The genetic linkage map of the QTL region was constructed using JoinMap 4.0 (Van Ooijen 2006). Recombination values were converted into Kosambi genetic distances (cM) (Kosambi 1944). The interval mapping method of MapQTL6.0 (Van Ooijen 2009) was used to estimate additive effects and phenotypic variance explained. Linkage groups were created using MapChart 2.2 (Voorrips 2002). The precise position of QTL was determined by comparing the phenotypic means of F2:3 recombinant plants within the QTL region.
Validation of candidate genes by real-time quantitative RT-qPCR
Total RNA was extracted from the developing cotton fibers [10-, 15-, 20-, and 25-day post anthesis (DPA)] of CCRI35 and Yumian1 using the RN38 EASYspin Plus plant RNA kit (Aidlab Biotechnologies Co., Ltd) according to the manufacturer’s protocol. First strand cDNA was synthesized from total RNA by priming with oligodT primer using Thermoscript Reverse Transcriptase (Invitrogen, Carlsbad, CA). RT-qPCR reactions were carried out in quantitative real-time PCR kits (Bio-Rad), with a final volume of 20 ul containing 10 ul iTaq™ SYBR® Green Supermix with ROX (Bio-Rad Laboratories), 1 mM forward and reverse primers, and 0.1 mM cDNA template. Reactions were performed using an iQTM 5 Multicolor Real-Time Quantitative PCR Detection System (Bio-Rad, USA). The values from triplicate reactions were averaged, and the Ct values were determined and compared with the 2−ΔΔCt method (Livak and Schmittgen 2001).
Cloning and sequence analyses for candidate genes
Genomic DNA from parents CCRI35 and Yumian1 was used to amplify the DNA sequences of candidate genes. PCR reactions were performed in a total volume of 25 μl according to the PrimerSTAR® Max DNA Polymerase manufacturer’s instructions. The products were electrophoresed through 1% agarose gels and extracted using a gel extraction kit (GeneMark). The purified PCR products were then used for “+A base” reactions with EasyTaq® DNA polymerase (#AP111, Transgen Biotech). The 50-μl reaction volume included 2.5 U Taq polymerase, 0.2 mM of dNTP, 5 μl of 10 × Buffer (KCl), and the purified PCR products. The mixture was heated to 72 °C for 30 min, and the PCR products were subsequently used for TA cloning using TAKARA kits. Single bacterial colonies were validated by PCR, and triplicate positive clones of each cotton line were sequenced by Invitrogen Co. Ltd. (Shanghai, China) and Beijing Genomics Institute (BGI) Co. Ltd. (Beijing, China).
All DNA sequences related to upland cotton line TM-1 were downloaded from the Phytozome website (https://phytozome.jgi.doe.gov/pz/portal.html). Gene structural profiles were determined based on alignment analysis of DNA (NCBI, http://www.ncbi.nlm.nih.gov/spidey/spideyweb.cgi) as well as structural prediction from the FGENESH website. DNA and amino acid sequences were aligned with the DNAMAN program.
Results
Phenotypic variation for fiber quality traits in parental and mapping populations
Basic statistics describing fiber quality parameters for F2 and F2:3 populations were presented in Table S1. Fiber quality traits showed near-normal phenotypic distributions in F2 and F2:3 populations (Fig. S1). Based on 1 year of data collected from 2484 individuals, significant correlations were detected among the five fiber quality traits (p = 5%), except between fiber elongation and fiber micronaire (fineness) (Table 1).
High-density genetic map
All polymorphic SSR primer pairs were used to genotype the F2 population of 2484 individuals (Table S2). Combined with the original primers mapped on chromosome 7, we constructed a high-density genetic map of the QTL region with 75 loci, spanning 28.3 cM (Fig. 1).
Genetic map and LOD score for qFS07.1 for fiber strength on cotton chromosome 07, based on 2484 F2 plants in 2013. The nearest marker of qFS07.1 is SWU8784, LOD score 25.47. The confidence interval for the position of the QTL from the peak LOD to less than 1, qFS07.1, was mapped into the interval of 2.83 cM flanked by SWU2662 and C2-0114
To check the accuracy and precision of the local genetic map, we compared it with the physical maps of the corresponding chromosome segments. The physical positions of SSR markers were based on aligning the primer sequences to A07 and D07 of G. hirsutum (Zhang et al. 2015) reference genomes (Table S3). The locus order on the local genetic map is completely consistent with the order on the physical maps from A07 and D07 of G. hirsutum (Fig. 2), which indicated that the local genetic map is accurate and suitable for QTL mapping.
Comparison of genetic and physical maps in the qFS07.1 QTL region. Chr07_QTL_region is the QTL region genetic map on chromosome 07 constructed in this study; Gh_A07 and Gh_D07 are the physical maps, based on the physical positions of SSR markers, aligning the primer sequences to A07 and D07 of G. hirsutum (Zhang et al. 2015) reference genomes, respectively
QTL mapping of fiber quality in F2 and F2:3 populations
Based on the fiber quality traits of the F2 population with 2484 individuals and the local genetic map, qFS07.1 was mapped into an interval of 2.83 cM flanked by SWU2662 and C2-0114. The additive effect of qFS07.1 was 1.17 cN/tex, and the phenotypic variation explained by qFS07.1 was 10.2% (Fig. 1; Table 2). The favorable allele of qFS07.1 was contributed by RIL014, whose QTL region on chromosome 7 is derived from Yumian1.
SSR markers within the interval flanked by SWU2662 and C2-0114 were used to further genotype 2571 F2:3 individuals derived from 207 F2 recombinant plants. Out of 2571 F2:3 individuals, 904 individuals with more than 15 gram fiber were measured for fiber property and QTL analysis mapped qFS07.1 into a 0.33-cM interval flanked by SWU8775 and SWU2707 within the fine-map linkage group Gh_A07. The additive effect of qFS07.1 reached to 1.50 cN/tex and the phenotypic variation explained by qFS07.1 was 16.5% (Table 2; Fig. 3a).
Fine-mapping and substitution mapping of the qFS07.1 QTL region. a qFS07.1 fine-mapping based on 2571 F2:3 individual plants in 2014. b Graphical genotypes and fiber strength for F2:3 recombinants derived from 2571 individuals. The white, black, and gray bars represent genotypes of RIL014, CCRI35, and heterozygotes, respectively. The nearest marker of qFS07.1 is SWU8789, LOD score 35.09. The confidence interval for the position of the QTL from the peak LOD to less than 1, qFS07.1, was mapped into a 0.33-cM interval flanked by SWU8775 and SWU2707
QTL substitution mapping
Out of 904 F2:3 individuals tested for fiber quality traits, 149 plants each had one recombinant event which took place between marker SWU8775 and SWU2707. These recombinants were grouped into nine classes based on their breakpoints (Fig. 3b). Comparing the genotypes of recombinant classes with those of parents, G1 to G5 had lower fiber strength, not distinguishable from CCRI35. Classes G6 to G8 had higher fiber strength, not distinguishable from RIL014. The recombinant groups G3, G4, G5, G6, and G8 delineated qFS07.1 to a 0.17 cM region between SWU8775 and SWU8789.
Predicted genes in the QTL region
The 0.17-cM region between SWU8775 and SWU8789 corresponds to 62.6-kb genome region on chromosome A07 of the G. hirsutum genome. According to the chromosome A07 of G. hirsutum reference genome annotation (Zhang et al. 2015), the 62.6-kb genome region contains four predicted genes: histidine-containing phosphotransfer factor 5 (AHP5, Gh_A07G1748), leucine-rich repeat protein kinase family protein (LRR RLK, Gh_A07G1749), receptor-like protein 45 (RLP45, Gh_A07G1750), and nudix hydrolase homolog 23 (NUDIX23, Gh_A07G1751) (Fig. 4). Coding sequence comparisons between chromosomes of polyploid G. hirusutum revealed some asymmetry between genomes. Gh_A07G1748 and Gh_A07G1751 exist only in the A genome of G. hirsutum and the D genome reference sequence contained no homoeologous counterparts. The D genome reference sequence of G. hirsutum only contained Gh_D07G1953 (homoeologous to GhA07G1749) and Gh_D071954 (homoeologous to GhA07G1750) in the QTL region.
Annotated genes within the 62.6-kb qFS07.1 QTL region on A07 and D07 of G. hirsutum. Gh_A07G1748 (histidine-containing phosphotransfer factor 5, AHP5); Gh_A07G1749 (leucine-rich repeat protein kinase family protein; LRR RLK); Gh_A07G1750 (receptor-like protein 45, RLP 45); Gh_A07G1751 (nudix hydrolase homolog 23, NUDIX 23). Gh_D07G1953 (leucine-rich repeat transmembrane protein kinase, LRR RLK); Gh_D07G1954 (leucine-rich repeat transmembrane protein kinase, LRR RLK)
RT-qPCR analyses of the genes within the QTL region
The four annotated genes within the QTL region were tested for expression levels by RT-qPCR using fiber tissue from four developmental stages (10, 15, 20, and 25 DPA) in Yumian1 and CCRI35. Through the sequence alignment of A genome and D genome of G. hirsutum (Zhang et al. 2015), single-locus specific primers of the four candidate genes were designed at the divergent coding region (Table S4). The relative expression levels in both parents Yumian1 and CCRI35 were shown in Fig. 5. The expression levels of AHP5 did not show significant differences between Yumian1 and CCRI35 during the four developmental stages tested. LRR RLK showed gradually increasing expression from 10 DPA to 25 DPA, especially in CCRI35 which experienced about a three-fold increase compared with Yumian1 in the 20 and 25 DPA stages. The gene RLP45 showed a variable expression pattern in CCRI35 across the four fiber development stages, whereas it showed stable expression in Yumian1 through the four stages. NUDIX23 showed significantly differential expression at 10 DPA and 25 DPA between Yumian1 and CCRI35.
RT-qPCR expression assay of genes in the QTL region during fiber development. a Histidine-containing phosphotransfer factor 5 (AHP5, Gh_A07G1748), b leucine-rich repeat protein kinase family protein (LRR RLK, Gh_A07G1749), c receptor-like protein 45 (RLP45, Gh_A07G1750), and d nudix hydrolase homolog 23 (NUDIX23, Gh_A07G1751). The grey bars are CCRI35, and the black bars are Yumian1. All data were normalized to the expression level of actin. Error bars indicate the standard deviation of three biological replicates. * and ** Denote significantly differential expression at p < 0.05 and p < 0.01, respectively
Comparative analysis of candidate genes within the QTL region
To investigate the gene structure of candidate genes in the QTL region, primer pairs (Table S4) were designed to amplify the genomic DNA sequences and the gene upstream region (~2000 bp) from CCRI35 and Yumian1. Genomic DNA sequences from CCRI35, Yumian1, and TM-1 were then used for the analysis of putative amino acid (aa) sequences deduced from the genomic interval containing these candidate genes (Fig. 6). The DNA sequences were also aligned and described in Fig S2. Annotation of AHP5, LRR RLK, RLP45, and NUDIX23 from “TM-1” indicated that there were three, one, one, and five exons in the coding regions, respectively. The alignment of AHP5 DNA sequence revealed a missense mutation in the second exon. Comparison of the nucleotide sequences of LRR RLK alleles from CCRI35 and Yumian1 revealed a 1-bp deletion resulting in a premature stop codon in Yumian1. The alignment of RLP45 DNA sequences revealed multiple single base mutations, which caused missense mutations of aa sequences in Yumian1. The alignment of NUDIX23 DNA sequences in the five exons was consistent among the three cotton lines. The ~2 kb genome sequences in upstream region were obtained and compared between CCRI35, Yumian1, and TM-1 (Fig S3). In addition to some single-base differences in four candidate genes between CCRI35 and Yumian1, a 7 bp deletion of “CATGCTG” was detected locating at 250 bp upstream of the LRR RLK start codon in Yumian1 which was likely to be involved in the differential gene expression in Yumian1 and CCRI35. Although RLP45 cannot be excluded completely the possibility, LRR RLK more likely contributed to the different fiber quality traits between CCRI35 and Yumian1.
Discussion
Mapping of complex traits using an efficient strategy
Fiber quality traits are unarguably the most important traits in commercial cotton production. The elucidation of specific favorable alleles related to fiber quality traits promotes more effective plant breeding. The increasing availability of genome sequences for species in the Gossypium genus (Paterson et al. 2012; Wang et al. 2012; Li et al. 2014, 2015; Zhang et al. 2015; Liu et al. 2015b; Yuan et al. 2015) has permitted anchoring of genetic maps and positioning of QTL on physical maps. Despite some positional cloning successes in plants, there is room for complementary approaches (Monclus et al. 2012). Integrating genome annotation and QTL position to prioritize candidate genes (Price 2006) has been used in Populus spp. (Monclus et al. 2012), Glycine max L. (Bolon et al. 2010), and Triticum aestivum L. (Barrero et al. 2015).
In the present study, to uncover key genes related to superior fiber traits in Yumian1, we integrated structural and functional genomics analyses. First, we mapped qFS07.1 to a 2.38-cM interval using an F2 population. Second, using an F2:3 population derived from F2 recombinant individual plants, we mapped qFS07.1 by substitution mapping to a 0.17-cM interval containing four genes. Finally, we combined RT-qPCR for detecting expression patterns and gene cloning to determine alteration of genes structure. The results revealed an LRR RLK as a potential candidate gene responsible for superior fiber strength in upland cotton cultivar Yumian1. Improvement of upland cotton genome annotation and the reduction of QTL confidence intervals might help to refine this strategy of candidate gene discovery.
Stable QTL for fiber quality traits on Chr.07
In our previous studies, Tan et al. (2015) identified qFS07.1, qFL07.1, qFM07.1, qFU07.1, and qFE07.1 flanked by SSR makers DPL0757 and BNL1604 across multiple environments through the (CCRI35 × Yumian1) RIL population. Zhang et al. (2012) used a three-parent composite population in upland cotton (G. hirsutum L.) to identified QTL for fiber quality traits on Chr.07 between HAU1367 and HAU2282. In addition, Wang et al. (2013a) identified qFL-C7-1, qFU-C7-1, qFE-C7-1, and qFS-C7-1 flanked by SSR markers SHIN1447 and DPL0757 on Chr.07 using F2 and F2:3 family lines derived from a cross between Luyuan (LY343) and Lumianyan (LMY22). Sun et al. (2012) identified qFS-C7-1, qFM-C7-1, and qFL-C7-1 in F2, F2:3, and RIL populations derived from an upland cotton cross between strains 0–153 and sGK9708. All these QTL are located in the interval that overlapped with that of qFS07.1 identified in the current study, based on the shared markers DPL0757, DC40182, and BNL1604. Therefore, qFS07.1 is a QTL of widespread importance for superior fiber strength in upland cotton.
Functional annotation of genes in the QTL region
In this study, qFS07.1 was fine-mapped to a 62.6-kb region on chromosome A07 of the G. hirsutum genome, which contained five annotated genes (AHP5, LRR RLK, RLP45, and NUDIX23). Histidine-containing phosphotransfer factors, designated AHPs, are involved in signal transduction mechanisms, by which plant cells appear to respond to certain hormonal stimuli, including cytokinin. Compared to wild-type plants, AHP2-overexpressing plants showed inhibitory effects on root and hypocotyl elongation (Suzuki et al. 2002). AHP2, AHP3, and AHP5 are very closely related to each other and showed similar expression profiles (Suzuki et al. 2000). Receptor-like kinases (RLKs) play crucial roles in various signaling processes during plant growth and development, hormone perception, self-incompatibility, symbiont recognition, and pathogen responses (Morris and Walker 2003). The largest subfamily of these genes is the leucine-rich repeat (LRR) receptor-like kinases (LRR-RLKs) (Li et al. 2005), comprising extracellular LRR domain, a transmembrane region, and a kinase domain that have recently been reported as a novel signaling pathway that regulates plant cell wall integrity maintenance (Hamann 2015). Two LRR RLKs, FEI1 and FEI2, have been reported to play a vital role in cellulose deposition during elongation of root tips and seeds in Arabidopsis (Harpaz-Saad et al. 2011; Xu et al. 2008). Other LRR RLKs are suggested as regulators of secondary cell wall (SCW) formation in Arabidopsis (Wang et al. 2013b) and poplar (Song et al. 2011). The amino acid sequences of receptor-like proteins (RLPs) contain the LRR domain, typically, which differ from RLKs in that they lack the cytoplasmic kinase domain and only have a short cytoplasmic tail. In several plant species, RLPs have been found to play a role in disease resistance and plant development (Wang et al. 2008). Nudix proteins are postulated to control the cellular concentration of these compounds (Kraszewska 2008). Nudix hydrolases in Arabidopsis control a variety of metabolites and are pertinent to a wide range of physiological processes (Ogawa et al. 2008).
The functional role of RLKs in fiber development
In this study, sequence alignment showed that an LRR RLK (Gh_A07G1749) has a 1-bp deletion resulting in a premature stop codon of the Yumian1 allele that has superior fiber, and significantly lower expression (3.4- and 3.0-fold at 20 and 25 DPA, respectively) of this mutant allele was also observed. Zhao et al. (2013) revealed that GbRLK is involved in the drought and high salinity stresses pathway by activating or participating in the ABA signaling pathway. However, the previous literature indicated that the first peak of ABA content of the fiber occurred during fiber elongation and the second during secondary wall thickening (Davis and Addicott 1972; Gokani et al. 1998). Yang et al. (2001) also suggested that in vitro studies, ABA (50 μmol L− 1) markedly enhanced the accumulation of dry matter and cellulose in the fiber cell wall during secondary wall thickening. Niu et al. (2016) revealed that six genes from the CrRLK1L family, which is a subgroup of the receptor-like kinase (RLK), were highly associated with fiber development. Li et al. (2005) reported that a plasma membrane bound cotton LRR RLK named GhRLK1 was induced during the active SCW synthesis stage. Islam et al. (2016) revealed that one RLK gene (thaumatin-like protein 3) has a premature stop codon and significantly higher expression (3.3- and 5.8-fold in 15 and 20 DPA, respectively) in MD90ne with inferior fiber, which may indicate feedback regulation that attempts to compensate for the lack of a functional protein.
This study fine-mapped a QTL to small genomic regions containing four candidate genes. Of those genes, only LRR RLK (Gh_A07G1749) had sequence differences in the promoter and coding region. Either the promoter (a 7 bp deletion presumably causing lower expression of Yumian1 allele) or the coding sequence (a premature stop codon attenuating its transcription by a feedback loop or attenuating its function by omitting part of an active domain to translation) appeared to be the cause of the superior fiber of RIL014 compared to that of CCRI35. It could be that both genetic factors could play a role in the phenotype, and they each deserve further study. Regardless of the causative genetic lesion, these findings suggest RLK signaling pathways to be engaged in mediating a combination of cell elongation and SCW biosynthesis during cotton fiber development.
The potential of qFS07.1 in MAS
Stable and major QTL for fiber quality are important to molecular breeding. The present study mapped qFS07.1 controlling fiber strength into a 0.17-cM interval with a 62.6-kb physical distance on chromosome 7 in G. hirsutum, and the presence of qFS07.1 led to an increase in fiber strength of approximately 3.0 cN/tex. Compared with the previously studies about fine-mapped QTL for fiber quality traits (Su et al. 2013; Cao et al. 2015; Islam et al. 2016; Liu et al. 2016; Yang et al. 2016), it thus appears that qFS07.1 fine-mapped in this study has the largest, single effect, and the smallest confidence interval within the genome. Therefore, qFS07.1 for fiber strength would have the potential to improve cotton fiber qualities through MAS and promote the investigation of the genetic mechanisms of high fiber strength.
Author contribution statement
ZSZ conceived the experiment, designed SSR markers, analyzed the data, and revised the manuscripts. XMF contributed to genotyping, RT-PCR, clone, data analysis, and writing. XYL and XQW assisted in SSR genotyping, RT-PCR, and clone. WWW analyzed the sequencing data. DXL, JZ, DJL, and ZHT made crosses and conducted field tests. ZYT, FL, FJZ, MCJ, and XLJ contributed to DNA and RNA extraction. JWZ and JHY measured fiber properties. All authors reviewed and approved this submission.
References
Arpat AB, Waugh M, Sullivan JP, Gonzales M, Frisch D, Main D, Wood T, Leslie A, Wing RA, Wilkins TA (2004) Functional genomics of cell elongation in developing cotton fibers. Plant Mol Biol 54:911–929
Barrero JM, Cavanagh C, Verbyla KL, Tibbits JFG, Verbyla AP, Huang BE, Gubler F (2015) Transcriptomic analysis of wheat near-isogenic lines identifies PM19-A1 and A2 as candidates for a major dormancy QTL. Genom Biol 16(1):1
Bolon YT, Joseph B, Cannon SB, Graham MA, Diers BW, Farmer AD, May GD, Muehlbauer GJ, Specht JE, Tu ZJ, Weeks N, Xu WW, Shoemaker RC, Vance CP (2010) Complementary genetic and genomic approaches help characterize the linkage group I seed protein QTL in soybean. BMC Plant Biol 10:41
Bradow JM, Davidonis GH (2000) Quantitation of fiber quality and the cotton production-processing interface: a physiologist’s perspective. J Cotton Sci 4:34–64
Cao ZB, Zhu XF, Chen H, Zhang TZ (2015) Fine mapping of clustered quantitative trait loci for fiber quality on chromosome 7 using a Gossypium barbadense introgressed line. Mol Breed 35:215
Chen ZJ, Scheffler BE, Dennis E, Triplett BA, Zhang TZ, Guo WZ, Chen XY, Stelly DM, Rabinowicz PD, Town CD, Arioli T, Brubaker C, Cantrell RG, Lacape JM, Ulloa M, Chee P, Gingle AR, Haigler CH, Percy R, Saha S, Wilkins T, Wright RJ, Deynze AV, Zhu YX, Yu SX, Abdurakhmonov I, Katageri I, Kumar PA, Rahman M, Zafar Y, Yu JZ, Kohel RJ, Wendel JF, Paterson AH (2007) Toward sequencing cotton (Gossypium) genomes. Plant Physiol 145:1303–1310
Davis LA, Addicott FT (1972) Abscisic acid: correlation with abscission and with development in the cotton fruit. Plant Physiol 49:644–648
Deng FL, Tu LL, Tan JF, Li Y, Nie YC, Zhang XL (2012) GbPDF1 is involved in cotton fiber initiation via the core cis-element HDZIP2ATATHB2. Plant Physiol 158:890–904
Fang L, Tian RP, Chen JD, Wang S, Li XH, Wang P, Zhang TZ (2014b) Transcriptomic analysis of fiber strength in Upland cotton chromosome introgression lines carrying different Gossypium barbadense chromosomal segments. PLoS One 9:e94642
Fang DD, Jenkins JN, Deng DD, McCarty JC, Li P, Wu J (2014a) Quantitative trait loci analysis of fiber quality traits using a random-mated recombinant inbred population in Upland cotton (Gossypium hirsutum L.). BMC Genom 15:397
Gokani SJ, Kumar R, Thaker VS (1998) Potential role of abscisic acid in cotton fiber and ovule development. J Plant Growth Regul 17:1–5
Hamann T (2015) The plant cell wall integrity maintenance mechanism-a case study of a cell wall plasma membrane signaling network. Phytochem 112:100–109
Harpaz-Saad S, McFarlane HE, Xu S, Divi UK, Forward B, Western TL, Kieber JJ (2011) Cellulose synthesis via the FEI2 RLK/ SOS5 pathway and cellulose synthase 5 is required for the structure of seed coat mucilage in Arabidopsis. Plant J 68:941–953
Huang JF, Chen F, Wu SY, Li J, Xu W (2016) Cotton GhMYB7 is predominantly expressed in developing fibers and regulates secondary cell wall biosynthesis in transgenic Arabidopsis. Sci China. Life Sci 59(2):194–205
Huang GQ, Gong SY, Xu WL, Li W, Li P, Zhang CJ, Li DD, Zheng Y, Li FG, Li XB (2013) A fasciclin-like arabinogalactan protein, GhFLA1, is involved in fiber initiation and elongation of cotton. Plant Physiol 161:1278–1290
Islam MS, Zeng LH, Thyssen GN, Christoher DD, Kim HJ, Li P, Fang DD (2016) Mapping by sequencing in cotton (Gossypium hirsutum) line MD52ne identified candidate genes for fiber strength and its related quality attributes. Theor Appl Genet 129(6):1071–1086
Jiang YJ, Guo WZ, Zhu HY, Ruan YL, Zhang TZ (2012) Overexpression of GhSusA1 increases plant biomass and improves cotton fiber yield and quality. Plant Biotech J 10:301–312
Kim HJ, Murai N, Fang DD, Triplett BA (2011) Functional analysis of Gossypium hirsutum cellulose synthase catalytic subunit 4 promoter in transgenic Arabidopsis and cotton tissues. Plant sci 180(2):323–332
Kim HJ, Triplett BA (2001) Cotton fiber growth in planta and in vitro: models for plant cell elongation and cell wall biogenesis. Plant Physiol 127:1361–1366
Kosambi D (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Kraszewska E (2008) The plant Nudix hydrolase family. Acta Biochim Pol 55(4):663–671
Lande R, Thompson R (1990) Efficiency of marker-assisted selection in the improvement of quantitative traits. Genetics 124(3):743–756
Lee JJ, Woodward AW, Chen ZJ (2007) Gene expression changes and early events in cotton fiber development. Ann Bot (Lond) 100(7):1391–1401
Li FG, Fan GY, Lu CR, Xiao GH, Zou CS, Kohel RJ, Ma ZY, Shang HH, Ma XF, Wu JY, Liang XM, Huang G, Percy RG, Liu K, Yang WH, Chen WB, Du XM, Shi CC, Yuan YL, Ye XL, Liu X, Zhang XY, Liu WQ, Wei HL, Wei SJ, Huang GD, Zhang XL, Zhu SJ, Zhang H, Sun FM, Wang XF, Liang Jie, Wang JH, He Q, Huang LH, Wang J, Cui JJ, Song GL, Wang KB, Xu X, Yu JZ, Zhu YX, Yu SX (2015) Genome sequence of cultivated Upland cotton (Gossypium hirsutumTM-1) provides insights into genome evolution. Nat biotech 33(5):524–530
Li FG, Fan GY, Wang KB, Sun FM, Yuan YL, Song GL, Li Q, Ma ZY, Lu CR, Zou CS, Chen WB, Liang XM, Shang HH, Liu WQ, Shi CC, Xiao GH, Gou CY, Ye WW, Xu X, Zhang XY, Wei HL, Li ZF, Zhang GY, Wang JY, Liu K, Kohel R, Percy RG, Yu JZ, Zhu YX, Wang J, Yu SX (2014) Genome sequence of the cultivated cotton Gossypium arboreum. Nat Genet 46:567–572
Li DD, Ruan XM, Zhang J, Wu YJ, Wang XL, Li XB (2013) Cotton plasma membrane intrinsic protein 2 s (PIP2s) selectively interact to regulate their water channel activities and are required for fiber development. New Phytol 199:695–707
Li YL, Sun J, Xia GX (2005) Cloning and characterization of a gene for an LRR receptor-like protein kinase associated with cotton fiber development. Mol Genet Genom 273:217–224.
Li Y, Tu LL, Pettolino FA, Ji SM, Hao J, Yuan DJ, Deng FL, Tan JF, Hu HY, Wang Q, Llewellyn DJ, Zhang XL (2016) GbEXPATR, a species-specific expansin, enhances cotton fiber elongation through cell wall restructuring. Plant biotech J 14(3):951–963.
Liu DX, Zhang J, Liu XY, Wang WW, Liu DJ, Teng ZH, Fang XM, Tan ZY, Tang SY, Yang JH, Zhong JW, Zhang ZS (2016) Fine mapping and RNA-Seq unravels candidate genes for a major QTL controlling multiple fiber quality traits at the T 1 region in upland cotton. BMC genom 17(1):1
Liu BL, Zhu YC, Zhang TZ (2015a) The R3-MYB gene GhCPC negatively regulates cotton fiber elongation. PloS One 10(2): e0116272.
Liu X, Zhao B, Zheng HJ, Hu Y, Lu G, Yang CQ, Chen JD, Chen JJ, Chen DY, Zhang L, Zhou Y, Wang LJ, Guo WZ, Bai YL, Ruan JX, Shangguan XX, Mao YB, Shan CM, Jiang JP, Zhu YQ, Jin L, Kang H, Chen ST, He XL, Wang R, Wang YZ, Chen J, Wang LJ, Yu ST, Wang BY, Wei J, Song SC, Lu XY, Gao ZC, Gu WY, Deng X, Ma D, Wang S, Liang WH, Fang L, Cai CP, Zhu XF, Zhou BL, Chen ZJ, Xu SH, Zhang YG, Wang SY, Zhang TZ, Zhao GP, Chen XY (2015b) Gossypium barbadense genome sequence provides insight into the evolution of extra-long staple fiber and specialized metabolites. Sci Rep 5:1413
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2– ∆∆CT method. Methods 25:402–408
Lv FN, Wang HH, Wang XY, Han LB, Ma YP, Wang S, Feng ZD, Niu XW, Cai CP, Kong ZS, Zhang TZ, Guo WZ (2015) GhCFE1A, a dynamic linker between the ER network and actin cytoskeleton, plays an important role in cotton fiber cell initiation and elongation. J Exp Bot 66(7):1877–1889
Machado A, Wu YR, Yang YM, Llewellyn DJ, Dennis ES (2009) The MYB transcription factor GhMYB25 regulates early fiber and trichome development. Plant J 59:52–62
Monclus R, Leplé JC, Bastien C, Bert PF, Villar M, Marron N, Brignolas F, Jorge V (2012) Integrating genome annotation and QTL position to identify candidate genes for productivity, architecture and water-use efficiency in Populus spp. BMC Plant Biol 12:173
Morris ER, Walker JC (2003) Receptor-like protein kinases: the keys to response. Curr Opin Plant Biol 6(4):339–342
Munis MF, Tu L, Deng F, Tan J, Xu L, Xu S, Long L, Zhang X (2010) A thaumatin-like protein gene involved in cotton fiber secondary cell wall development enhances resistance against Verticillium dahliae and other stresses in transgenic tobacco. Biochem Biophys Res Commu 393:38–44
Niu EL, Cai CP, Zheng YJ, Shang XG, Fang L, Guo WZ (2016) Genome-wide analysis of CrRLK1L gene family in Gossypium and identification of candidate CrRLK1L genes related to fiber development. Mol Genet Genom: 1–18
Ogawa T, Yoshimura K, Miyake H, Ishikawa K, Ito D, Tanabe N, Shigeoka S (2008) Molecular characterization of organelle-type Nudix hydrolases in Arabidopsis. Plant Physiol 148(3):1412–1424
Paterson AH, Wendel JF, Gundlach H, Guo H, Jenkins J, Jin D, Llewellyn D, Showmaker KC, Shu S, Udall J, Yoo MJ, Byers R, Chen W, Doron-Faigenboim A, Duke MV, Gong L, Grimwood J, Grover C, Grupp K, Hu G, Lee TH, Li J, Lin L, Liu T, Marler BS, Page JT, Roberts AW, Romanel E, Sanders WS, Szadkowski E, Tan X, Tang H, Xu C, Wang J, Wang Z, Zhang D, Zhang L, Ashrafi H, Bedon F, Bowers JE, Brubaker CL, Chee PW, Das S, Gingle AR, Haigler CH, Harker D, Hoffmann LV, Hovav R, Jones DC, Lemke C, Mansoor S, ur Rahman M, Rainville LN, Rambani A, Reddy UK, Rong JK, Saranga Y, Scheffler BE, Scheffler JA, Stelly DM, Triplett BA, Van Deynze A, Vaslin MF, Waghmare VN, Walford SA, Wright RJ, Zaki EA, Zhang T, Dennis ES, Mayer KF, Peterson DG, Rokhsar DS, Wang X, Schmutz J (2012) Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibers. Nature 492:423–427
Price AH (2006) Believe it or not, QTLs are accurate! Trends Plant Sci 11(5):213–216
Said JI, Song MZ, Wang HT, Lin, ZX, Zhang XL, Fang DD, Zhang JF (2015). A comparative meta-analysis of QTL between intraspecific Gossypium hirsutum and interspecific G. hirsutum × G. barbadense populations. Mol Genet Genom 290(3), 1003–1025
Shan CM, Shangguan XX, Zhao B, Zhang XF, Chao LM, Yang CQ, Wang LJ, Zhu HY, Zeng YD, Guo WZ, Zhou BL, Hu GJ, Guan XY, Chen ZJ, Wendel JF, Zhang TZ, Chen XY (2014) Control of cotton fiber elongation by a homeodomain transcription factor GhHOX3. Nat commu 5:5519
Shang LG, Liang QZ, Wang YM, Wang XC, Wang KB, Abduweli A, Ma LL, Cai SH, Hua JP (2015) Identification of stable QTLs controlling fiber traits properties in multi-environment using recombinant inbred lines in Upland cotton (Gossypium hirsutum L.). Euphytica 205:877–888
Song D, Xi W, Shen J, Bi T, Li L (2011) Characterization of the plasma membrane proteins and receptor-like kinases associated with secondary vascular differentiation in poplar. Plant Mol Biol 76:97–115
Su CF, Wang W, Qiu XM, Yang L, Li S (2013) Fine-mapping a fiber strength QTL QFS-D11-1 on cotton chromosome 21 using introgressed lines. Plant Breed 132:725–730
Sun X, Gong SY, Nie XY, Li Y, Li W, Huang GQ, Li, XB (2015) A R2R3-MYB transcription factor that is specifically expressed in cotton (Gossypium hirsutum) fibers affects secondary cell wall biosynthesis and deposition in transgenic Arabidopsis. Physiol Plant 154:420–432
Sun FD, Zhang JH, Wang SF, Gong WK, Shi YZ, Liu AY, Li JW, Gong JW, Shang HH, Yuan YL (2012) QTL mapping for fiber quality traits across multiple generations and environments in Upland cotton. Mol Breed 30:569–582
Suzuki T, Ishikawa K, Yamashino T, Mizuno T (2002) An Arabidopsis histidine-containing phosphotransfer (HPt) factor implicated in phosphorelay signal transduction: overexpression of AHP2 in plants results in hypersensitiveness to cytokinin. Plant Cell Physiol 43(1):123–129
Suzuki T, Sakurai K, Imamura A, Nakamura A, Ueguchi C, Mizuno T (2000) Compilation and characterization of histidine-containing phosphotransmitters implicated in His-to-Asp phosphorelay in plants: AHP signal transducers of Arabidopsis thaliana. Biosci Biotech Bioch 64(11):2486–2489
Tan ZY, Fang XM, Tang SY, Zhang J, Liu DJ, Teng ZH, Li L, Ni HJ, Zheng FM, Liu DX, Zhang TF, Paterson AH, Zhang ZZ (2015) Genetic map and QTL controlling fiber quality traits in Upland cotton (Gossypium hirsutum L.). Euphytica 203:615–628
Tang SY, Teng ZH, Zhai TF, Fang XM, Liu F, Liu DX, Wang SF, Zhang K, Shao QS, Tan ZY, Paterson AH, Zhang ZZ (2015) Construction of genetic map and QTL analysis of fiber quality traits for Upland cotton (Gossypium hirsutum L.). Euphytica 201:195–213
Tang WX, Tu LL, Yang XY, Tan JF, Deng FL, Hao J, Guo K, Lindsey K, Zhang XL (2014) The calcium sensor GhCaM7 promotes cotton fiber elongation by modulating reactive oxygen species (ROS) production. New Phytol 202:509–520
Van Ooijen JW (2006) JoinMap 4.0: software for the calculation of genetic linkage maps in experimental populations. Kyazma BV, Wageningen
Van Ooijen J (2009) MapQTL 6, software for the mapping of quantitative trait loci in experimental populations of diploid species. Kyazma BV, Wageningen
Voorrips R (2002) MapChart: software for the graphical presentationof linkage maps and QTLs. J Hered 93:77–78
Walford SA, Wu YR, Llewellyn DJ, Dennis ES (2011) GhMYB25-like: a key factor in early cotton fibre development. Plant J 65:785–797
Walford SA, Wu YR, Llewellyn DJ, Dennis ES (2012) Epidermal cell differentiation in cotton mediated by the homeodomain leucine zipper gene, GhHD-1. Plant J 71:464–478
Wang L, Cook A, Patrick JW, Chen XY, Ruan YL (2014) Silencing the vacuolar invertase gene GhVIN1 blocks cotton fiber initiation from the ovule epidermis, probably by suppressing a cohort of regulatory genes via sugar signaling. Plant J 78:686–696
Wang G, Ellendorff U, Kemp B, Mansfield JW, Forsyth A, Mitchell K, Bastas K, Liu CM, Woods-Tör A, Zipfel C, de Wit PJ, Jones JD, Tör M, Thomma BP (2008) A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis. Plant Physiol 147(2):503–517
Wang HY, Wang J, Gao P, Jiao GL, Zhao PM, Li Y, Wang GL, Xia GX (2009) Down-regulation ofGhADF1gene expression affects cotton fiber properties. Plant biotech J 7(1):13–23
Wang KB, Wang ZW, Li FG, Ye WW, Wang JY, Song GL, Yue Z, Cong L, Shang HH, Zhu SL, Zou CS, Li Q, Yuan YL, Lu CR, Wei HL, Gou CY, Zheng ZQ, Yin Y, Zhang XY, Liu K, Wang B, Song C, Shi N, Kohel RJ, Percy RG, Yu JZ, Zhu YX, Wang J, Yu SX (2012) The draft genome of a diploid cotton Gossypium raimondii. Nat genet 44(10):1098–1103
Wang FR, Xu ZZ, Sun R, Gong YC, Liu GD, Zhang JX, Wang LM, Zhang CY, Fan SJ, Zhang J (2013a) Genetic dissection of the introgressive genomic components from Gossypium barbadense L. that contribute to improved fiber quality in Gossypium hirsutum L. Mol breed 32(3):547–562
Wang JH, Kucukoglu M, Zhang LB, Chen P, Decker D, Nilsson O, Jones B, Sandberg G, Zheng B (2013b) The Arabidopsis LRR-RLK, PXC1, is a regulator of secondary wall formation correlated with the TDIF-PXY/TDR-WOX4 signaling pathway. BMC Plant Biol 13:94
Xu SL, Rahman A, Baskin TI, Kieber JJ (2008) Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell 20:3065–3079
Yang X, Wang Y, Zhang G, Wang X, Wu L, Ke H, Liu H, Ma Z (2016) Detection and validation of one stable fiber strength QTL on c9 in tetraploid cotton. Mol Genet Genom 291(4):1625–1638
Yang MY, Xu CN, Wang BM, Jia JZ (2001) Effects of plant growth regulators on secondary wall thickening of cotton fibers. Plant Growth Regul 35(3):233–237
Yang ZR, Zhang CJ, Yang XJ, Liu K, Wu ZX, Zhang XY, Zheng Wu, Xun QQ, Liu CL, Lu LL, Yang ZE, Qian YY, Xu ZZ, Li CF, Li J, Li FG (2014) PAG1, a cotton brassinosteroid catabolism gene, modulates fiber elongation. New Phytol 203:437–444
Yuan DJ, Tang ZH, Wang MJ, Gao WH, Tu LL, Jin X, Chen LL, He YH, Zhang L, Zhu LF, Li Y, Liang QQ, Lin ZX, Yang XY, Liu N, Jin SX, Lei Y, Ding YH, Li GL, Ruan XA, Ruan YJ, Zhang XL (2015) The genome sequence of SeaIsland cotton (Gossypium barbadense) provides insights into the allopolyploidization and development of superior spinnable fibres. Sci Rep 5(6):17662
Zhang TZ, Hu Y, Jiang WK, Fang L, Guan XY, Chen JD, Zhang JB, Saski CA, Scheffler BE, Stelly DM, Hulse-Kemp AM, Wan Q, Liu BL, Liu CX, Wang S, Pan MQ, Wang YK, Wang DW, Ye WX, Chang LJ, Zhang WP, Song QX, Kirkbride RC, Chen XY, Dennis E, Llewellyn DJ, Peterson DG, Thaxton P, Jones DC, Wang Q, Xu XY, Zhang H, Wu HT, Zhou L, Mei GF, Chen SQ, Tian Y, Xiang D, Li XH, Ding J, Zuo QY, Tao LN, Liu YC, Li J, Lin Y, Hui YY, Cao ZS, Cai CP, Zhu XF, Jiang Z, Zhou BL, Guo WZ, Li RQ, Chen ZJ (2015) Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat. Biotech 33:531–537
Zhang ZS, Hu MC, Zhang J, Liu DJ, Zheng J, Zhang K, Wang W, Wan Q (2009) Construction of a comprehensive PCR based marker linkage map and QTL mapping for fiber quality traits in Upland cotton (Gossypium hirsutum L.). Mol Breed 24:49–61
Zhang ZS, Xiao YH, Luo M, Li XB, Luo XY, Hou L, Li DM, Pei Y (2005) Construction of a genetic linkage map and QTL analysis of fiber-related traits in Upland cotton (Gossypium hirsutum L.). Euphytica 144:91–99
Zhang K, Zhang J, Ma J, Tang SY, Liu DJ, Teng ZH, Liu DX, Zhang ZZ (2012) Genetic mapping and quantitative trait locus analysis of fiber quality traits using a three-parent composite population in Upland cotton (Gossypium hirsutum L.). Mol breed 29(2):335–348
Zhao J, Gao Y, Zhang Z, Chen TZ, Guo WZ and Zhang TZ. 2013. A receptor-like kinase gene (GbRLK) from Gossypium barbadense enhances salinity and drought-stress tolerance in Arabidopsis. BMC plant Biol 13(1):1
Zhou Y, Zhang ZT, Li M, Wei XZ, Li XJ, Li BY, Li XB (2015) Cotton (Gossypium hirsutum) 14-3-3 proteins participate in regulation of fiber initiation and elongation by modulating brassinosteroid signaling. Plant Biotech J 13(2):269–280
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 31271770 and 31571720) and National science and technology major projects of China (No. 2016ZX08005003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors declare that this study complies with the current laws of the countries in which the experiments were performed.
Additional information
Communicated by Joshua A Udall.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fang, X., Liu, X., Wang, X. et al. Fine-mapping qFS07.1 controlling fiber strength in upland cotton (Gossypium hirsutum L.). Theor Appl Genet 130, 795–806 (2017). https://doi.org/10.1007/s00122-017-2852-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-017-2852-1