Abstract
3D culture has allowed the initiation and expansion of organ-like structures, called organoids, from either tissue-resident adult stem cells or pluripotent stem cells. Today, organoids can be grown to resemble a wide variety of organs, exhibiting remarkable similarity to their in vivo counterparts. As successful organoid generation is possible from virtually every patient, organoids hold a great promise for medical research and the development of new treatments. They have already found their way into the clinic, enabling personalized medicine in small patient trials. In this review, we provide an update on current organoid technology and summarize their application in basic research, disease modelling, drug development, personalized treatment and regenerative medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In early 2015, a patient suffering from cystic fibrosis (CF) was treated for the first time based on information from drug-screening tests performed on the patient’s own tissue, cultured ex vivo in the form of organoids. Remarkably, this signalled the technology’s move from bench to bedside in only 4 years: the culture conditions developed for the intestinal organoid cultures used were published in 2011 [1] and by 2013, an assay had been developed to test the function of the disease-causing protein (see Section, “Genetic disease”) [2]. Currently, seven patients have been treated according to the results of this personalized medicine approach in organoids.
The term “organoid” simply means “resembling an organ”. Organoids are defined by three characteristics: self-organization, multicellularity and functionality [3] (Fig. 1). Thus, the cells arrange themselves in vitro into the 3-dimensional (3D) organization that is characteristic for the organ in vivo, the resulting structure consists of multiple cell types found in that particular organ and the cells execute at least some of the functions that they normally carry out in that organ. For example, a prototypical organoid, the mouse intestinal organoid, grows as a single-layered epithelium organized into domains such that it resembles the in vivo intestinal crypt-villus architecture, comprising the different cell types of the intestine (enterocytes, goblet cells, Paneth cells, enteroendocrine cells and stem cells) and surrounding a cystic lumen [4] (Fig. 1).
Organoids are mini-versions of organs. The definition of an organoid includes the 3 characteristics of organization, multicellularity and function. The example shown here is a small intestinal organoid grown from adult stem cells of the mouse. It self-organizes into a 3D structure with small buddings protruding from a central lumen. These buddings contain the cells typically found in the crypts of the small intestine, especially Paneth cells and stem cells. The cystic body contains the cells of the villus region. Within an organoid, cells can execute (part of) the functions that they carry out in vivo, e.g. Paneth cells can provide niche signals for stem cells and, when stimulated, can secrete antimicrobials
The technology utilizes the defining characteristics of stem cells, namely, the clonal expansion capacity and production of daughter cells that can differentiate into multiple cell types (self-renewal and multipotency) [5]. If placed into the right culture conditions, any stem cell should be able to self-renew and generate differentiated offspring, ideally growing into organ- or tissue-like structures. Organoids can now be grown to resemble many tissues or tissue layers, such as the epithelial layer of the gastro-intestinal tract. For each new culture system, researchers have applied knowledge from developmental studies to mimic the molecular cues that guide the cells in vivo. There is a general difference in approach depending on the type of stem cells used to initiate the organoids, namely, either pluripotent stem cells (PSCs), encompassing embryonic stem cells as well as induced pluripotent stem cells, or adult stem cells (ASCs). In order to generate the correct tissue type, PSCs must be taken through a series of carefully choreographed steps using different media cocktails. ASCs are extracted from adult tissues, where they normally reside to regenerate the tissue, and are thus already tissue-specified. Therefore, culture conditions need only mimic the molecular environment in the adult tissue during homeostasis and repair (Fig. 2).
Current organoid techniques. Organoids can be grown via two approaches, either from tissue biopsies containing adult stem cells (ASCs; yellow box), or from pluripotent stem cells (PSCs; blue box). In the ASC approach, researchers mimic the adult stem cell niche to allow natural expansion of the stem cells. In the PSC approach, researchers mimic developmental steps occurring during the generation of a particular organ. A wide range of organs are amenable to either approach (top row). The fact that these include organs of endodermal, mesodermal and ectodermal origin implies that it may be possible to grow all organs “in a dish”
The range of organs this general principle has been applied to is rapidly increasing. Three germ layers are defined during human development: endo-, meso- and ectoderm. The fact that organoids have now been established from organs derived from all three germ layers (Fig. 2) indicates the power of this technology and suggests that the majority of organs are amenable to such modelling. Organoid systems have already been reviewed in detail elsewhere [3, 6–10]. Here, we will first focus on representative organoid systems for each germ layer before summarizing the role that organoids play in basic biomedical research and in the clinic.
Endodermal organoids
The endoderm gives rise to the epithelial lining of the digestive and respiratory tracts and organs such as the lung, liver, gall bladder, pancreas and urinary bladder, amongst others. Organoids have been grown from several organs of endodermal origin; indeed, the first organoids were murine small intestinal organoids derived from ASCs [4]. In the intestine, three molecular gradients converge to create the molecular environment that shapes the epithelium: epidermal growth factor (EGF) and Wnt are highly active in the crypts, whilst bone morphogenetic protein (BMP) is active in the villus. To mimic this environment, Toshiro Sato placed adult epithelial stem cells into a 3D extracellular matrix called “Matrigel” and added three factors: an agonist of the Wnt pathway, R-spondin1, an inhibitor of BMP signalling, noggin, and EGF. Under these conditions, the intestinal stem cells proliferate and form small cysts which grow into 3D structures with a cystic body and small buds protruding outwards into the matrix. These organoids contain all the cell types of the intestine and—upon serial replating—display an apparently infinite expansion capacity [4]. This technique has subsequently been adapted to culture organoids from the human intestine [1], mouse and human stomach [11–13], pancreas [14–16], liver [17], prostate [18, 19], oesophagus [1, 20], gall bladder [21, 22] and taste buds [23]. The development of lung organoids from ASCs has so far been challenging. An early protocol allowed the initial culture of basal cells isolated from the trachea to organoids containing basal and luminal cells but lacking other cell types of the trachea and the culture could not be maintained long-term [24]. Short-term cultures from the alveoli develop into alveolar type I cells [25, 26].
In parallel developments, several endodermal organoid cultures have been generated from PSCs. Currently, human PSCs can be grown into organoids resembling the small intestine [27], lung [28–30], liver [31], thyroid [32, 33] stomach [34, 35] and pancreatic [36] and bile duct tissues [37]. The latest addition to this growing list is human gastric corpus, which present an excellent example to illustrate typical PSC-derived organoid culture [38]. The evolution of the protocol started by defining conditions to grow intestinal organoids from PSCs [27]. To generate gastric organoids, Kyle McCracken in the Wells lab then made use of reports that the murine stomach requires intact retinoic acid signalling to develop [39]. Indeed, adding retinoic acid to the growth cocktail switched cells from a hindgut fate to a foregut fate. Further culture in Matrigel and EGF then gave rise to organoids which resembled the part of the stomach that is closest to the intestine, the gastric antrum [34]. Generating a stomach-specific knockout of beta catenin, McCracken and colleagues could then show that Wnt is necessary for promoting growth of the proximal stomach, the corpus, and in the absence of Wnt signalling corpus specification, and especially the development of the parietal cells (the acid-secreting cells), was impaired [38]. By adding a Wnt agonist (CHIR) to the combined treatment with EGF and the known stomach-specific signalling factor FGF10, organoids were initiated that supported the differentiation of gastric corpus cells, and including, impressively, acid-producing parietal cells [38]. However, these parietal cells were lost with passaging. Thus, the conditions for preservation of parietal cells in long-term expansion remain to be identified [38]. Another exciting new development from the Wells lab is the generation of PSC-derived intestinal organoids with a functional enteric nervous system. For this, the PSC-derived neural crest cells were seeded together with PSC-derived intestinal organoids into Matrigel. The neural cells migrated into the mesenchymal compartment, which surrounds the PSC-derived organoids. When transplanted, they formed ganglionic structures that exhibited spontaneous calcium oscillations and could be stimulated to induce contractions of the intestinal organoids [40].
Mesodermal organoids
The mesoderm forms the mesenchyme, haematopoietic system, muscles, cartilage, bone, kidneys, spleen, gonads and genital ducts. The kidney is a highly complex organ, with more than 20 differentiated cell types whose 3D arrangement is crucial for its function. The two progenitor tissues of the nephron, the ureteric bud and the metanephric mesenchyme induce each other reciprocally. Both originate from the same mesoderm (the intermediate mesoderm, derived from the primitive streak), and both have been generated separately from PSCs [41, 42], but it has long been challenging to generate the two progenitor populations simultaneously. A protocol was developed in the lab of Melissa Little that initially used Activin A and BMP4 to induce a primitive streak identity from PSCs, followed by stimulation with FGF9 to induce an intermediate mesoderm identity. These cells then spontaneously developed into both uretric bud and metanephric mesenchyme [43]. The group then identified the molecular switch that guides the cells between the two fates: the timing and duration of Wnt and FGF9 signalling defines the resulting cell types. The refined protocol, which comprises 4 days of Wnt activity induced by the GSK3β inhibitor CHIR, then 3 days of exposure to FGF9, followed by only a single hour of induced Wnt activity, results in one of the most fascinating and complete organoid structures of multicellular kidney organoids with podocytes and segmented tubules [44]. Following an adapted protocol for ASC-derived cultures, also fallopian tube organoids have been grown from adult tissue [45].
Ectodermal organoids
The (neuro-)ectoderm forms all neural tissues, including the central nervous system and sensory epithelia (e.g., of the eye), the pituitary gland, the tooth enamel, the epidermis and several glands such as the mammary glands and salivary glands. In the lab of Yoshiki Sasai, Mototsugu Eiraku developed a culture in which he seeded ESCs in non-adhesive culture plates and serum-free medium. Under these conditions, ESCs form aggregates that are similar to embryoid bodies [46]. From these aggregates, Sasai and colleagues could generate organoids resembling the optic cup [47, 48], cerebellum [49], hippocampus [50] and adenohypophysis [51], each driven by specific culture conditions. For example, for retinal organoids, transient activin treatment and addition of 2% Matrigel to the suspension culture lead to the development of early optic vesicles marked by expression of the retinal anlage gene, Rax. These vesicles are mechanically cut from the aggregates and cultured in Matrigel-supplemented suspension culture containing serum, retinoic acid and l-taurine in high oxygen. Under these conditions, the vesicles undergo a shape change to form two-walled cups with a stratified epithelium containing photoreceptors, ganglion cells, bipolar cells, horizontal cells, amacrine cells and Müller glia, reminiscent of the early neonatal eye [47]. Using a different approach, Madeline Lancaster in the lab of Juergen Knoblich generated a neuroectoderm from embryoid bodies and embedded it into the Matrigel without the addition of specific growth factors. This allowed the outgrowth of buds that further developed into different brain regions. A single cerebellar organoid may contain many different brain regions, but the population of organoids is heterogeneous. Grown in small bioreactors, these “mini-brains” can become a few millimetres in size [52]. Single cell analysis showed that the cells in these brain organoids resemble human foetal brain [53].
The two ectodermal organoid cultures derived from ASCs, mammary gland and salivary gland organoids highlight the importance of Wnt signalling in maintaining long-term expansion of organoids in culture. Mammary gland organoids have been generated from ASCs in the epithelium, but the initial culture conditions only allowed for two passages [54]. By adding Wnt and neuregulin, the life span of these organoids could be extended to 2.5 months in culture [55]. Similarly, the initial culture conditions for salivary gland organoids only allowed their short-term culture [56] but the addition of Wnt3A and R-spondin1 to these has now enabled their long-term culture [57]. It is remarkable that, as a general rule and regardless of germ layer identity, Wnt signalling appears essential for the establishment and maintenance of organoid systems from ASCs and for the maintenance and expansion of many organoids derived from PSCs.
Applications
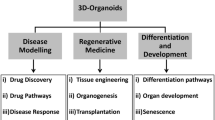
It is evident that organoids hold great promise for basic biomedical research: whilst the establishment of organoid cultures was built on knowledge from developmental studies, this new tool has now enabled researchers to study in vitro the exact cues that govern organogenesis, lineage specification and tissue homeostasis in areas that are inaccessible in vivo using current techniques, e.g. the impact of timed pulses of specific stimuli, as in the case of the kidney. However, organoid technology holds promise for a much wider range of applications and may fill an urgent need for new models in medical research and translational studies (Fig. 3).
Diagnostic and therapeutic potential of organoids. For individual patients, organoids have already been used to identify beneficial treatments, so providing true personalized medicine. They may further serve as autologous material for cell replacement therapies or even future organ transplants, with the possibility to correct disease-causing mutations by CRISPR/Cas9 before transplantation. Organoids grown from groups of patients can be used to model disease, for example in infection biology, but also to understand specific genetic mutations. For drug development, organoid biobanks are a very promising tool for drug discovery. Further, kidney and liver organoids in particular may help in the future to complement or even replace current animal tests for toxicity. Both ASC- and PSC-derived organoids can be used for these areas of biomedicine
Disease modelling
Infectious disease
In contrast to cell lines, which are monotypic and usually transformed, organoids are composed of (ideally all) differentiated cell types of a particular organ. This makes them an attractive tool for the study of infectious diseases, especially of the agents that so far lack a suitable model system, either because they are restricted to humans or because current animal models do not faithfully recapitulate human pathology (reviewed in detail elsewhere [58]). Human gastric organoids can be infected with the gastric pathogen Helicobacter pylori and the model recapitulates the known hallmarks of infection [13, 34]. Differentiation of the organoids has shown that the cellular response to the infection depends on the cell types present in the organoids [13]. ASC-derived intestinal organoids have now also provided the cellular material to grow norovirus, which has previously been refractory to in vitro culture attempts, despite its capacity to consistently and repetitively cause outbreaks of severe gastroenteritis. This is probably due to a tropism of the virus for differentiated primary enterocytes, absent from cell lines but readily produced by organoid technology [59]. Another inspiring example is the recent necessity to develop new models for the emerging Zika virus (ZIKV). ZIKV is particularly dangerous for the developing foetus and causes microcephaly. Since human PSC-derived brain organoids are similar to the foetal brain, they are outstanding candidates for an ideal model. In 2016, three groups demonstrated in parallel that ZIKV can productively infect brain organoids and that the virus exhibits a specific tropism for neural progenitors [60–62]. Early-stage organoids representing first trimester foetal brains are particularly susceptible to destruction by the virus, leading to smaller organoids with a thinner neuronal layer, thus phenocopying the disease [62]. In another study, sequencing unravelled a null mutation in the gene interferon regulatory factor 7, IRF7, in a 7-year-old patient suffering from life-threatening influenza. Lung organoids, grown via iPSCs from the child’s fibroblasts, produced low levels of interferon upon influenza infection allowing the virus to spread and explaining the patient’s condition [63].
Genetic disease
Cystic fibrosis is caused by a variety of mutations in the cystic fibrosis transmembrane conductance regulator gene, CFTR, responsible for ion transport. The site of major complications is the lung but the protein is also functional in other epithelia, such as the intestine. Lung organoids derived from iPSCs from healthy donors express functional CFTR protein, whilst those derived from CF patients with the most common mutation (F508del) display the typical misfolding of the protein, leading to accumulation inside the cell [28]. The function of the lung organoids could be restored if the mutation was corrected by CRISPR/Cas9 genome editing in the iPSCs [64]. Dekkers and colleagues generated ASC-derived organoids from rectal biopsies and developed a microscopic assay to evaluate CFTR function. In this assay, forskolin raises intracellular cyclic AMP and thereby activates CFTR, leading to ion uptake. Subsequent fluid secretion into the lumen of the organoids leads to swelling of the organoids that is quantifiable by microscopic readout (Fig. 4). Intestinal organoids from CF patients show reduced swelling compared to those of healthy controls. The swelling could be restored by drug treatment of the organoids [2] or by correction of the mutation by CRISPR/Cas9 gene editing [65].
Measurement of CFTR function with organoids. Cystic fibrosis (CF) is caused by a large range of possible mutations in the gene CFTR. There are drugs available, but they only work for some of the mutations. Although the site of major complications is the lung, the protein is also expressed in the intestine. Organoids grown from a rectal biopsy allow the in vitro expansion of the patient’s epithelial cells, providing enough material for cost-effective drug testing. In healthy organoids, addition of forskolin leads to CFTR-dependent swelling, and this can be quantified by image analysis. Swelling is impaired in CF organoids and can be restored by treatment with drugs in vitro. This test is now used to identify the patients that most benefit from particular treatments. With the development of living organoid biobanks from larger cohorts of patients, it will now be possible to screen new drugs for efficacy in specific cohorts. The CF biobank in the Netherlands currently includes 300 patients, with numbers growing
Organoids have mirrored the in vivo phenotypes of other genetic diseases, such as multiple intestinal atresia [66], alpha 1-antitrypsin deficiency and Alagille syndrome in the liver [17], microcephaly [52] and even autism [67].
It would seem obvious that the approaches for culturing healthy adult stem cells should be readily applicable to the culturing of their malignant counterparts, i.e. cancer stem cells in the form of cancer organoids, also termed tumoroids. Indeed, organoids have been established from primary cancers of the colon, stomach, prostate and pancreas [1, 13, 15, 36, 68–70], providing unique possibilities for cancer drug testing, but also for better understanding of the influence of specific genetic mutations on cancer progression in vitro. To experimentally generate cancers in organoids, genetic modifications have been introduced into cancer driver genes by shRNA-mediated knockdown [71]. Further, two groups reconstructed the cascade from normal tissue to adenocarcinoma by sequentially altering cancer driver genes by CRISPR/Cas9 gene editing in intestinal organoids [72, 73]. A similar approach used transfection of mutant KRAS or TP53 to generate tumour organoids from PSC-derived pancreatic organoids [36].
Drug development: screening and toxicology
Several organoid biobanks of diseased and healthy control tissues have been established or are currently in the process of being established. Examples are two organoid biobanks derived from various stages of colon cancer and their matching healthy controls [68, 69] and a biobank of intestinal organoids from 71 CF patients [74]. These biobanks cover the range of genetic mutations known from a large-scale sequencing analysis and thus provide the ideal material to screen for new drugs. A proof-of-principle study was performed on a colon cancer biobank, which was used to screen 83 drugs that are currently used in the clinic or in clinical trials for cancer treatment. The screen corroborated known gene-drug associations and thus demonstrated that organoid biobanks are amenable to high-throughput screens. Similarly, a drug screen of known drugs on the CF biobank confirmed previous data from drug responses to two relatively new CF drugs, but furthermore also demonstrated that the screen could identify patients with unusual CFTR mutations that would benefit from a particular treatment [74].
Future screens using these and other biobanks will not only aim to identify new drugs, but also to reveal which patients may benefit from treatment with a certain (existing) drug. In addition, focused tests of potential drugs should identify new leads for the pharmaceutical industry. For example, experimental treatment of PSC-derived CF organoids with a small molecule led to increased surface expression of the receptor [28].
Furthermore, it is envisaged that organoids may be used in the future for toxicology testing to complement, if not in part replace, animal testing. For example, iPSC-derived kidney organoids readily respond to the cancer drug and known nephrotoxin cisplatin by undergoing apoptosis [44]. Hepatocytes derived from an expansion phase as liver organoids will also be a valuable tool for toxicity testing in the future [17].
Regenerative medicine
Material for transplantation is always scarce, and alternative sources are urgently needed. As organoids can be initiated from minuscule amounts of donor cells, expanded and differentiated in vitro, they could provide autologous cells or—in the future—even tissue for transplantation. Organoids have already been transplanted into the murine colon, where they engrafted and retained typical organ features like tissue architecture and cell differentiation status [75, 76]. Similarly, human liver organoids have been engrafted into the mouse liver, and kidney organoids transplanted under the kidney capsule have become vascularized [17, 42, 77]. Future studies need to show whether grafts can execute all functions of the tissue. Autologous organoid transplantations would also allow CRISPR/Cas9-mediated gene correction of disease-causing mutations.
Personalized medicine
Being the miniaturized avatar of a specific patient’s organ, organoids have the potential to identify the ideal treatment for a particular patient. The prime example is cystic fibrosis. Whilst CF as such is fairly common (about 1 in 3000 children are born with CF), some of the mutations in CFTR are rare and patients with rare mutations may not receive the ideal treatment. This was the case for the first CF patient treated on the basis of organoid screening results: the one drug prescribed at that time in the Netherlands was neither prescribed nor reimbursed for patients with this mutation, because it was too rare to have been tested in a clinical trial. Researchers grew organoids from a rectal biopsy from the patient and, using the forskolin-induced swelling assay, identified a positive response to the drug Kalydeco. A second patient followed with the same rare mutation. The treatment was given to the patients, who both improved significantly [74]. After this initial translational success, blinded follow-up studies with larger patient cohorts have now been initiated.
In a similar approach, organoids from cancer patients could not only be used to identify the ideal treatment for a specific patient, but also as cancer organoids retain the genetic heterogeneity of the primary tumour, it is likely that under the application of a specific drug, the same resistant clones may grow out as in vivo, thus predicting the acquisition of drug resistance during treatment. Ongoing studies will have to demonstrate the accuracy of these predictions.
Current limitations
The current version of organoid culture still represents a somewhat reductionist model. Not all organoids contain all cell types of the tissue being modelled; one of many examples is the gastric organoid that cannot maintain parietal cells long-term. Further, a real stomach or intestine is not only more than just the inner epithelial layer, but also has a surrounding mesenchyme, muscular layers, nervous system, vasculature, immune cells and luminal microbiota. The mesenchyme is present in PSC-derived organoids, but the other components are usually missing in our current organoid systems. A good example of the generation of a higher level of tissue complexity is the above-mentioned recent development of human PSC-derived intestinal tissue containing a functional enteric nervous system [40]. This advance represents the only organoid system containing derivatives of all three germ layers: the endodermal intestinal epithelium, mesodermal mesenchyme and ectodermal (neural crest-derived) nervous cells. Similarly, experimental infection of organoids (summarized earlier and elsewhere [58]) adds a layer of complexity to the technology that increases its accuracy as a model system. Future developments in organoid technology and interesting fusions with approaches in tissue engineering will generate ever more complex model systems, combining tissue matrices with organoids [78] or adding other cellular components such as immune cells, thus permitting further insights into disease development. In PSC-derived organoids, current differentiation protocols often mimic the foetal stages of development, but sometimes only recapitulate fully mature cells when the organoid is transplanted in vivo, indicating that some final factors for differentiation are yet to be defined [3].
PSC-derived organoids are typically expanded as stem cells expanded in a specific PSC-state. Their subsequent conversion into defined organoids (e.g. retinal organoids or mini-brains) is incompatible with further expansion. Only where PSC-derived organoids can be cultured in media that were originally developed for ASC-derived organoids (such as mini-guts) can such organoids expand further. Whilst it was previously believed that ASC-derived cells (or organoids) only had a limited life span, a series of examples now exist which refute that dogma. We believe that it will ultimately be possible to develop media that allow the long-term expansion of all epithelial ASCs in the form of organoids.
For regenerative medicine, a current bottleneck is the dependence on Matrigel, which is an extracellular matrix produced by the Engelbreth-Holm-Swarm mouse tumour line, thus precluding its use in humans. Recently, new matrices have been created based on synthetic hydrogel networks that overcome this limitation, at least for intestinal organoids [79]. Tackling these challenges will open new avenues for biomedical research.
Concluding remarks
Organoids can be generated from virtually every patient, either from iPSCs or tissue biopsies containing the ASCs. This allows the study of rare mutations that cause disease. Organoids are also amenable to genetic modification using common tools like lentiviruses or CRISPR/Cas9 and can be generated from single cells to form clonal organoids with the desired genetic changes, either to analyse the effect of a specific mutation or to repair a mutation present in a patient [34, 65, 80]. Lastly, they can be expanded to provide enough material for experimental testing and are amenable to a wide range of standard laboratory techniques including microscopy, RNA-, DNA-, protein- and even proteome [81] analysis as well as many specific functional assays or for example viability assays after drug application. These qualities render organoids a highly promising tool for medical research. For now, organoids are already contributing to basic science in developmental biology, adult stem cell biology and also to disease modelling. In the clinic, the most immediate impact is that of drug testing and personalized medicine. The clinical success obtained with the seven CF patients has convinced Dutch healthcare providers to invest heavily in the test. The CF patient biobank now holds samples of more than 300 patients, with numbers growing steadily. Ultimately, organoid technology may change drug development from testing the cohort with the most prevalent mutations to providing simple and cost-effective tests for all patients to identify those that most benefit from a given treatment.
References
Sato T, Stange DE, Ferrante M, Vries RGJ, van Es JH, van den Brink S, van Houdt WJ, Pronk A, van Gorp J, Siersema PD et al (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141:1762–1772
Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NWM, Bijvelds MJC, Scholte BJ et al (2013) A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19:939–945
Lancaster MA, Knoblich JA (2014) Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345:1247125–1247125
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ et al (2009) Single Lgr5 stem cells build crypt villus structures in vitro without a mesenchymal niche. Nature 459:262–265
Clevers H (2015) What is an adult stem cell? Science 350:1319–1320
Fatehullah A, Tan SH, Barker N (2016) Organoids as an in vitro model of human development and disease. Nat Cell Biol 18:246–254
Clevers H (2016) Modeling development and disease with organoids. Cell 165:1586–1597
Huch M, Koo B-K (2015) Modeling mouse and human development using organoid cultures. Dev Camb Engl 142:3113–3125
Shamir ER, Ewald AJ (2014) Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol 15:647–664
Simian M, Bissell MJ (2017) Organoids: a historical perspective of thinking in three dimensions. J Cell Biol 216:31–40
Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M et al (2010) Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6:25–36
Stange DE, Koo B-K, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH et al (2013) Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155:357–368
Bartfeld S, Bayram T, van de Wetering M, Huch M, Begthel H, Kujala P, Vries R, Peters PJ, Clevers H (2015) In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148:126–136.e6
Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, Sojoodi M, Li VS, Schuijers J, Gracanin A et al (2013) Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J 32:2708–2721
Boj SF, Hwang C-I, Baker LA, Chio IIC, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS et al (2015) Organoid models of human and mouse ductal pancreatic cancer. Cell 160:324–338
Greggio C, Franceschi FD, Figueiredo-Larsen M, Gobaa S, Ranga A, Semb H, Lutolf M, Grapin-Botton A (2013) Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 140:4452–4462
Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MMA, Ellis E, van Wenum M, Fuchs SA, de Ligt J et al (2015) Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160:299–312
Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, van Boxtel R, Wongvipat J, Dowling CM, Gao D, Begthel H, Sachs N et al (2014) Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159:163–175
Chua CW, Shibata M, Lei M, Toivanen R, Barlow LJ, Bergren SK, Badani KK, McKiernan JM, Benson MC, Hibshoosh H et al (2014) Single luminal epithelial progenitors can generate prostate organoids in culture. Nat Cell Biol 16:951–961
DeWard AD, Cramer J, Lagasse E (2014) Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep 9:701–711
Scanu T, Spaapen RM, Bakker JM, Pratap CB, Wu L, Hofland I, Broeks A, Shukla VK, Kumar M, Janssen H et al (2015) Salmonella manipulation of host signaling pathways provokes cellular transformation associated with gallbladder carcinoma. Cell Host Microbe 17:763–774
Lugli N, Kamileri I, Keogh A, Malinka T, Sarris ME, Talianidis I, Schaad O, Candinas D, Stroka D, Halazonetis TD (2016) R-spondin 1 and noggin facilitate expansion of resident stem cells from non-damaged gallbladders. EMBO Rep 17:769–779
Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, Bachmanov AA, Margolskee RF, Jiang P (2014) Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc Natl Acad Sci 111:16401–16406
Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BLM (2009) Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci 106:12771–12775
Desai TJ, Brownfield DG, Krasnow MA (2014) Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507:190–194
Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH, Desai TJ, Krasnow MA, Quake SR (2014) Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature 509:371–375
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM et al (2011) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470:105–109
Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan L-J, Ratjen F, Ellis J, Rossant J (2012) Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol 30:876–882
Dye BR, Hill DR, Ferguson MA, Tsai Y-H, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD et al (2015) In vitro generation of human pluripotent stem cell derived lung organoids. elife 4:e05098
Huang SXL, Islam MN, O’Neill J, Hu Z, Yang Y-G, Chen Y-W, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J et al (2013) Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol 32:84–91
Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang R-R, Ueno Y, Zheng Y-W, Koike N et al (2013) Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. doi:10.1038/nature12271
Kurmann AA, Serra M, Hawkins F, Rankin SA, Mori M, Astapova I, Ullas S, Lin S, Bilodeau M, Rossant J et al (2015) Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell 17:527–542
Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, Shen SS et al (2012) Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10:398–411
McCracken KW, Catá EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai Y-H, Mayhew CN, Spence JR, Zavros Y et al (2014) Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516:400–404
Noguchi TK, Ninomiya N, Sekine M, Komazaki S, Wang P-C, Asashima M, Kurisaki A (2015) Generation of stomach tissue from mouse embryonic stem cells. Nat Cell Biol 17:984–993
Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC et al (2015) Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med 21:1364–1371
Ogawa M, Ogawa S, Bear CE, Ahmadi S, Chin S, Li B, Grompe M, Keller G, Kamath BM, Ghanekar A (2015) Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat Biotechnol 33:853–861
McCracken KW, Aihara E, Martin B, Crawford CM, Broda T, Treguier J, Zhang X, Shannon JM, Montrose MH, Wells JM (2017) Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature 541:182–187
Wang Z, Dollé P, Cardoso WV, Niederreither K (2006) Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev Biol 297:433–445
Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, Chang C-F, Schiesser J, Aubert P, Stanley EG et al (2017) Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med 23:49–59
Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D, Wu M-Z, Dubova I, Esteban CR, Montserrat N et al (2013) Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol 15:1507–1515
Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R (2014) Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14:53–67
Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH (2014) Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16:118–126
Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM et al (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526:564–568
Kessler M, Hoffmann K, Brinkmann V, Thieck O, Jackisch S, Toelle B, Berger H, Mollenkopf H-J, Mangler M, Sehouli J et al (2015) The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun 6:8989
Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y (2008) Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3:519–532
Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472:51–56
Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10:771–785
Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y (2015) Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep 10:537–550
Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, Ohgushi M, Takahashi J, Eiraku M, Sasai Y (2015) Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun 6:8896
Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H et al (2011) Self-formation of functional adenohypophysis in three-dimensional culture. Nature 480:57–62
Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA (2013) Cerebral organoids model human brain development and microcephaly. Nature 501:373–379
Camp JG, Badsha F, Florio M, Kanton S, Gerber T, Wilsch-Bräuninger M, Lewitus E, Sykes A, Hevers W, Lancaster M et al (2015) Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A 112:15672–15677
Linnemann JR, Miura H, Meixner LK, Irmler M, Kloos UJ, Hirschi B, Bartsch HS, Sass S, Beckers J, Theis FJ et al (2015) Quantification of regenerative potential in primary human mammary epithelial cells. Development 142:3239–3251
Jardé T, Lloyd-Lewis B, Thomas M, Kendrick H, Melchor L, Bougaret L, Watson PD, Ewan K, Smalley MJ, Dale TC (2016) Wnt and Neuregulin1/ErbB signalling extends 3D culture of hormone responsive mammary organoids. Nat Commun 7:13207
Nanduri LSY, Baanstra M, Faber H, Rocchi C, Zwart E, de Haan G, van Os R, Coppes RP (2014) Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Rep 3:957–964
Maimets M, Rocchi C, Bron R, Pringle S, Kuipers J, Giepmans BNG, Vries RGJ, Clevers H, de Haan G, van Os R et al (2016) Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Rep 6:150–162
Bartfeld S (2016) Modeling infectious diseases and host-microbe interactions in gastrointestinal organoids. Dev Biol 420:262–270. doi: 10.1016/j.ydbio.2016.09.014
Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng X-L, Qu L et al (2016) Replication of human noroviruses in stem cell–derived human enteroids. Science. doi:10.1126/science.aaf5211
Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, Rana TM (2016) Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell. doi:10.1016/j.stem.2016.04.014
Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK (2016) Zika virus impairs growth in human neurospheres and brain organoids. Science 352:816–818
Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C et al (2016) Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165:1238–1254
Ciancanelli MJ, Huang SXL, Luthra P, Garner H, Itan Y, Volpi S, Lafaille FG, Trouillet C, Schmolke M, Albrecht RA et al (2015) Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science 348:448–453
Firth AL, Menon T, Parker GS, Qualls SJ, Lewis BM, Ke E, Dargitz CT, Wright R, Khanna A, Gage FH et al (2015) Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep 12:1385–1390
Schwank G, Koo B-K, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK et al (2013) Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13:653–658
Bigorgne AE, Farin HF, Lemoine R, Mahlaoui N, Lambert N, Gil M, Schulz A, Philippet P, Schlesser P, Abrahamsen TG et al (2014) TTC7A mutations disrupt intestinal epithelial apicobasal polarity. J Clin Invest 124:328–337
Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, Amenduni M, Szekely A, Palejev D, Wilson M et al (2015) FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162:375–390
Van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor-Weiner A, Kester L et al (2015) Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161:933–945
Fujii M, Shimokawa M, Date S, Takano A, Matano M, Nanki K, Ohta Y, Toshimitsu K, Nakazato Y, Kawasaki K et al (2016) A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell. doi:10.1016/j.stem.2016.04.003
Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora VK et al (2014) Organoid cultures derived from patients with advanced prostate cancer. Cell 159:176–187
Li X, Nadauld L, Ootani A, Corney DC, Pai RK, Gevaert O, Cantrell MA, Rack PG, Neal JT, Chan CW-M et al (2014) Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat Med 20:769–777
Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, Sachs N, Overmeer RM, Offerhaus GJ, Begthel H et al (2015) Sequential cancer mutations in cultured human intestinal stem cells. Nature 521:43–47
Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, Watanabe T, Kanai T, Sato T (2015) Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med. doi:10.1038/nm.3802
Dekkers JF, Berkers G, Kruisselbrink E, Vonk A, de Jonge HR, Janssens HM, Bronsveld I, van de Graaf EA, Nieuwenhuis EES, Houwen RHJ et al (2016) Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med 8:344ra84
Fordham RP, Yui S, Hannan NRF, Soendergaard C, Madgwick A, Schweiger PJ, Nielsen OH, Vallier L, Pedersen RA, Nakamura T et al (2013) Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13:734–744
Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K et al (2012) Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18:618–623
Huch M, Dorrell C, Boj SF, van Es JH, Li VSW, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ et al (2013) In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494:247–250
Chen HJ, Wei Z, Sun J, Bhattacharya A, Savage DJ, Serda R, Mackeyev Y, Curley SA, Bu P, Wang L et al (2016) A recellularized human colon model identifies cancer driver genes. Nat Biotechnol 34:845–851
Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, Lutolf MP (2016) Designer matrices for intestinal stem cell and organoid culture. Nature 539:560–564
Koo B-K, Stange DE, Sato T, Karthaus W, Farin HF, Huch M, van Es JH, Clevers H (2012) Controlled gene expression in primary Lgr5 organoid cultures. Nat Methods 9:81–83
Cristobal A, van den Toorn HWP, van de Wetering M, Clevers H, Heck AJR, Mohammed S (2017) Personalized proteome profiles of healthy and tumor human colon organoids reveal both individual diversity and basic features of colorectal cancer. Cell Rep 18:263–274
Acknowledgements
We thank Chris Hindley for the editing advice and the members of the Bartfeld lab for comments on the text. SB is supported by the Käthe and Josef Klinz Foundation.
H.C. is named as the inventor of several patents related to Lgr5 stem cell-based organoid technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartfeld, S., Clevers, H. Stem cell-derived organoids and their application for medical research and patient treatment. J Mol Med 95, 729–738 (2017). https://doi.org/10.1007/s00109-017-1531-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1531-7