Abstract
Because of sample availability and ethical considerations, the biology of human tissues and organs is challenging to research. However, advancements in stem cell culture make it feasible to generate in vitro three-dimension (3D) tissue that exhibits some of the genuine organoids’ main multicellular, anatomical, and even functional properties. Organoids offer a wide range of uses in fundamental research, drug discovery, and regenerative medicine since they may simulate organ development and illness. Although organoids have some shortcomings in their application, they hold great potential in the future for clinical applications. Methods: For the selection of literature cited, we used the Pubmed database. The keywords used in the MEDLINE research were: organoid, stem cells, disease modelling, 3D culturing. Results: Pluripotent stem cells [(embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs)], neonatal or adult stem/progenitor cells produced in vitro can be used to make organoids. Organoids can be used to stimulate development, homeostasis, regeneration, disease modelling, drug screening and testing, personalised medicine, and regenerative medicine, among other things. Conclusion: Organoids are 3D in vitro tissues with some of the major multicellular, anatomical, and even functional features of real organs, and because of these characteristics, they have been applied in various fields. Despite some drawbacks, organoids hold great potential in the future for further clinical applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Initially, the theory of human organs’ development and function was mostly speculation because most human tissues were inaccessible for research. This understanding of the development and function of human organs has only improved significantly in the last century [1]. This improvement is mainly due to discovering research subjects ranging from Drosophila fruit flies, C. elegans worms to research models of vertebrates such as mice and zebrafish [2]. Although these research models have brought about significant improvements, there are differences between animals and humans that cause studies’ failure to develop effective therapies. Over the decades, developing ex vivo human models has been extremely difficult to deal with due to the accessibility of tissue samples and related ethical issues [2, 3]. Breakthroughs in stem cell culture have enabled the creation of in vitro 3D tissues known as organoids, which have many of the main multicellular, anatomical, and even functional characteristics of true organs [3]. As the name suggests, an organoid means a structure that resembles an organ. Organoids are composed of several organ-specific cell types, can recapitulate several organ-specific functions (e.g. excretion, filtration, neural activity, contraction), and are grouped and arranged spatially organised similar to an organ [4]. The term ‘organoid’ has a broad definition. It has been used to describe many forms of in vitro cultures, ranging from tissue explants to organ-on-chip systems [3]. Here, organoids are defined as 3D structures derived from pluripotent stem cells [(embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs], neonatal or adult stem/progenitor cells grown in vitro, in which the cell spontaneously organised itself into a properly differentiated functional cell type and recapitulates at least some organ function [2, 5, 6].
Deriving Organoids
The key aspects of organoid formation are self-assembly and differentiation [2]. It usually entails the self-organisation of a somewhat homogenous cell population [3]. Even in the presence of a homogeneous signalling environment, a cellular system that lacks an ordered structure can be spatially reorganised by system-autonomous mechanisms. Self-organisation is the process responsible for this. Conceptually, the process of self-organisation may be split into two parts: self-patterning events and morphogenetic rearrangements [7]. Self-patterning is described as the development of cell differentiation patterns in an originally homogenous system due to system-autonomous processes and intracellular communication [7, 8]. The interplay between several different mechanisms has been proposed, and it includes reaction-diffusion mechanisms [9], bistabilities of regulatory networks [10], and asymmetric cell division [7].
On the other hand, Morphogenetic rearrangement is the sorting of various cell types within tissue and the higher-level reorganisation of the system's architecture. Differences in cell adhesion, cortical tension and/or contractility, and cell motility, which facilitates cell sorting, all play a role in the physical contact between various cell types [7, 11]. The system-intrinsic mechanics caused by cell shape changes, cell contraction, cell movement, or differential tissue expansion keeps architectural rearrangements in place [7]. The recapitulation of this process influenced the success in organoid derivation. Besides, it is also influenced by the physical characteristics of the cultural environment; requirements for system autonomous (i.e., endogenous) and/or exogenous signals; and initial cell types and system conditions, which will be explained in further following paragraphs.
Physical Properties of the Culture Environment
To promote the 3D characteristics of organoids, the solid extracellular matrix (ECM) that support cell growth and cell adherence can be used. The most widely used matrix for 3D organoid derivation is Matrigel, a natural ECM purified from Engelbreth-Holm-Swarm rat sarcoma [3]. Some examples of organoids that have been successfully made using Matrigel or similar animal-derived hydrogels that mimic the basement membrane include intestinal, cerebral [12], gastric [13], and mammary gland organoids [3]. Although rare, organoids derivation of the mammary glands and intestines can use a type I collagen matrix [14, 15]. This natural matrix's unique combination of ECM components and growth factors promotes effective cell development and differentiation. However, the diversity and complexity of these compositions makes controlling the cultural milieu challenging and reduces repeatability. To address this, a hydrogel was recently created to sustain intestinal and brain organoid cultures, allowing the metabolic and environmental processes of the culture to be regulated [16, 17]. But they are inherently less bioactive and need to be adapted to the specific requirements of different organoids.
One strategy used to generate optic cup [18], cerebral, cerebellar [19], and hippocampal organoids are the culture of 3D cell aggregates in suspension [20]. The suspension culture method does not employ solid scaffolding for cell embedding to encourage the development of polarised epithelial structures. Low quantities of Matrigel may be applied in some situations [3, 18].
Renal organoids may be created utilising the air-liquid interface technique, which involves the growth of cells in the form of pellets on a thin porous membrane with the cell culture medium only on the basal side of the membrane [21, 22]. The cell pellets then self-organise into a multilayered structure similar to the original kidney's microarchitecture.
The utilisation of particular organoid derivation techniques is currently mostly empirical. There is a dearth of systematic comparison of different procedures for obtaining certain organoids, making it impossible to understand each technique's relative strengths, limitations, and uses [3].
Endogenous and Exogenous Signals
Organoids are generated due to the appropriate developmental signalling pathway being activated and are mostly derived from an initial cell population exposed to certain morphogens at a specific moment in time. If all of the required components are present in the system, these signals can cause self-organisation. Exogenous provision of missing components is required [3].
Some organoids rely nearly completely on endogenous cues to develop. Mouse optic cup organoids generated from mouse PSCs, for example, were collected and grown in a serum-free medium with low growth factor levels. These circumstances promote the development of homogenous neuroepithelium (NE), after which a self-patterning mechanism determines the spatially distinct domains of the neural retina (NR) and retinal pigmented epithelium (RPE). Then, even if no external signal is supplied, morphogenesis can proceed since the starting cell population already has all the components required to arrange itself into an optic cup.
Although the mouse optic cup organoid is nearly entirely reliant on endogenous signalling, most organoid derivation procedures need the addition of particular exogenous signals since the original cellular system lacks all of the necessary components for the intended self-organisation process. In other situations, the exogenous signal is only necessary for the initial cell type to be induced and the remaining self-organising processes to be carried out using the system's autonomous signal. Human PSCs (hPSCs), for example, must be exogenously activated with particular growth factors to generate a mixed population of ureteric epithelial cells and metanephric mesenchyme. The cell population will then arrange themselves into kidney organoids without adding any additional substances to the medium [3, 21, 23].
Many organoid cellular systems, such as stomach organoids generated from hPSC, require stimulation by an appropriate and particular exogenous signal during the derivation process. An exogenously provided factor is necessary to drive definitive hPSC-derived endoderm cells to the posterior foregut destiny [13]. Exogenous stimulation is necessary to control the development, morphogenesis, and differentiation of the cells into functional gastric cell types and to guide them to form the antral or fundic gastric epithelium [13, 24].
Cell Sources, Starting Cell Type, and Initial Culture Condition
The cell source for organoid formation (Fig. 1.1) can be derived from primary tissue or differentiated from pluripotent stem cells, such as embryonic stem cells (ESC) or induced pluripotent stem cells (iPSCs) [3, 25]. iPSCs are easy to obtain and individual-specific. ESC and iPSC can differentiate into almost any type of body tissue [25]. When trying to mimic the complexity of native tissue, the heterogeneity of cell types produced in organoid cultures derived from pluripotent stem cells can be an advantage [24, 26, 27]. However, the unintended heterogeneity of the culture of pluripotent stem cell strains and incomplete knowledge of specific differentiation signals can have unintended consequences for the resulting organoid [25]. This is shown in single-cell transcriptomic studies that iPSC-derived and ESC-derived kidney organoids comprise 10–20% of non-kidney cells, such as brain and muscle cells [28]. Also, organoids derived from pluripotent stem cells may exhibit a gene expression pattern more reminiscent of fetal tissue than from their adult counterparts [13, 29, 30].
In terms of the starting condition of the cell population, the methods utilised in the generation of distinct organoids differ. Depending on the starting circumstances of the cell population, some cells go through all of the self-organisation processes, while others just go through a subset of them. Self-organisation of the cell population requires symmetry-breaking and subsequent patterning to generate spatially distinct domains of the multiple cell types in organoids derived from a single cell type (such as the optic cup or small intestine organoids). The patterned structure is then morphogenetically rearranged to produce the final organoid architecture. In general, beginning from a single cell, organoid derivation methods need an initial stage of cell growth before self-organisation can occur [3]. Some methods call for co-culturing of cell types that have been pre-differentiated independently (for example, PSC-derived liver organoids) [31]. This protocol has mainly established several cell identities. As a result, self-organisation mostly includes cell sorting and subsequent architectural rearrangements.
In addition, the starting circumstances of the cell population will have an impact on the use of organoids as a biological model system. Organoids created by co-culture of individually specified cell types, for example, are less instructive for understanding organogenesis than models in which diverse cell types are grown concurrently. As a result, it is more appropriate to examine the transitory developmental interactions that might occur between distinct progenitors during organoid creation [3]. The starting cell type also influences the characteristics of the final organoid produced. Organoids can be cultured from ASCs (either as isolated cells or from dissected tissue fragments), PSCs [12, 13], or fetal progenitor cells [32, 33]. Neuroectodermal organoids, such as the optic cup and cerebral organoids, and mesodermal renal organoids have been derived only from the PSC [3]. In contrast, organoids from surface ectoderm lineages (especially glandular tissue) are predominantly derived from ASCs or dissociated adult tissue [14, 34, 35]. Most of the endodermal lineage organoids originate from PSCs and ASCs [3].
Different cell types emerge at distinct phases of development and take different paths. As a result, while investigating the factors behind organoid development, choosing the starting cell population is critical. ASCs or adult tissue fragments cultured are thought to create organoids that replicate their original tissue's homeostatic or regenerative circumstances. Thus, stem cells derived from organs with a high renewal rate, such as the epithelium of the small intestine, colon [36] or stomach [37], generate organoids that mimic the homeostatic role of these cells in vivo. Organoids produced from slow turnover tissue, such as the pancreas or liver, in which endogenous stem cells and/or progenitors may play a role only the following damage, on the other hand, are regarded as genuine regeneration models [38, 39].
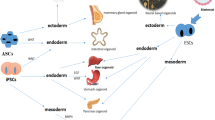
As previously stated, ASC-derived organoids can help researchers address concerns regarding the biology of adult tissues. PSC-derived organoids are primarily utilised to research organogenesis and tissue development [3]. PSC-derived organoids seldom reach the mature tissue stage in vitro. They usually resemble foetal tissue [13]. The restricted development of PSC-derived organoids is most likely owing to progression to more mature cell types, which necessitate continuous culture for a period of time that typically surpasses the capability of the actual culture methods [40]. The several organoids that may be produced from PSCs and the developmental signals [41] are shown in Fig. 1.2.
Various organoids that can be grown from PSCs and the developmental signals that are used (Reproduced from [41])
Next is the embryonic organoid system, which is also called ‘embryoids’ or ‘gastruloids’. These organoids mimic in a very simple way pre-implantation [42] and early post-implantation embryo development [43,44,45], body axis formation [46, 47], gastrulation [46,47,48,49,50,51,52], and neural tube development [53, 54]. Unlike classical organoids, which usually consist of a limited subset of cell types from one germ layer, embryonic organoids contain cells from several germ layers, as in real embryos. These organoids can be used to build a complete development model in vitro and to study the complex interactions between different cell types in the development process [3].
Finally, organoids start directly from the fetal tissue (between the ASC and PSC stages), fetal progenitor-derived organoids [32, 33]. Compared to PSC-derived organoids, fetal organoids can be used to study advanced organogenesis, for example, as has been done to study the enterosphere maturation of fetal intestinal progenitors [32].
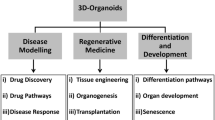
Applications of Organoids
The Use of Organoids as Models for Development, Homeostasis, and Regeneration
In fundamental research, organoids can be utilised to better understand development, homeostasis, and regeneration principles. As simplified and conveniently accessible’ minimal systems,’ Organoids can recreate in vitro some organ biology principles and differentiate the relative contributions of distinct tissue components to complicated morphogenetic processes [3]. Organoids have helped to better understand organogenesis, human development, and adult organ biology due to their ease of access. Organoid cultures can be used to investigate the similarities and differences in the development of humans and other animals. This is critical for understanding human brain development and congenital disorders [2].
The Use of Organoids for Disease Modelling
One of the great potential applications of the organoid model is to analyse human-specific disease mechanisms [2]. Compared to the traditional cell culture of the single-cell type, organoid culture as a disease model can mimic pathology at the organ level [3]. Organoids have been used for modelling various diseases such as genetic diseases [12, 55, 56], diseases involving host-pathogen interactions, and even cancer. This proves that organoids are capable of reproducing certain well-known pathological features. For example, microinjection of the bacterium Helicobacter pylori into human gastric organoids reproduces the typical signs of this bacterial infection [13, 37]. This model is particularly relevant because species-specific gastric characteristics make animal models unsuitable for studying the pathology of the human stomach. H. pylori infection in mice does not develop into ulceration and cancer as in humans [37]. In essence, organoids have been used to study congenital and acquired diseases. The following are some of the diseases that have been studied using organoids.
Congenital Diseases
The first human disease to be mimicked using organoids was cystic fibrosis (CF). Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel, usually expressed on the epithelium of numerous organs, cause this disease [57]. Surface expression of CFTR was missing in iPS-derived lung organoids from CF patients to replicate the in vivo condition in CF [58]. Dekkers and colleagues developed intestinal organoids from CF patients that may imitate the disease in vitro. They developed a swelling experiment in which wild-type organoids respond to cAMP stimulation by importing fluid into the lumen and swelling, but CF organoids do not [55]. This technique is effective for detecting responders to CFTR modulators and has a high predictive value. The Verma lab generated iPS cells from CF patients and utilised CRISPR/Cas9 to repair the mutation. The repaired iPS cells were subsequently converted into mature airway epithelial cells, exhibiting normal CFTR function [59].
Primary microcephaly, a genetic disorder induced by CDK5RAP2 mutations, is another congenital ailment investigated with organoids [12]. The brain organoids derived from patient-derived iPSCs were much smaller and the individual cortical regions were primarily hypoplastic. A series of observations and specific examinations of the orientation of the mitotic spindle during progenitor division revealed that the patient's neural stem cells began to divide asymmetrically and generate neurons prematurely, leading to depletion of the progenitor pool ultimately the decline of overall neurons. Because mice could not properly reproduce the amount of brain shrinkage found in humans, the organoids showed morphological abnormalities that could only be detected in this human-specific model system [57]. Organoids can also be used to simulate idiopathic autism spectrum disease (ASD). Mariani et al. generated iPSC lines from four autistic individuals and four unaffected controls. These were produced first as 3D aggregates, then rosettes were plated. The rosettes were then separated and regrown as 3D aggregates to produce forebrain organoids [60, 61]. Although probands and controls were usually fairly comparable, the ASD organoids had more inhibitory interneurons due to elevated FoxG1, an essential component in forebrain patterning [57].
Acquired Diseases
Apart from modelling the inherited conditions of patient stem cells carrying genetic mutations, organoids can also be used to model acquired diseases such as acquired mutations as in the case of cancer and diseases caused by infectious agents. Organoids can be used to model lung [62], stomach [37, 63, 64], liver [65, 66], pancreatic [66,67,68], colon [36, 63], Van [69], prostate [70], endometrial [71], breast [72], bladder [73, 74], esophageal [36, 75], and brain cancers [76]. These organoids come from tissue resection, biopsy or even circulating tumor cells. Cancer-derived organoids are more likely to retain the genetic and phenotypic features of the original tumor. In this respect, they resemble patient-derived xenografts, but have the advantages of a higher success rate, can be easily expanded in vitro, and can be used for drug screening [77, 78].
Organoids have shown to be a useful model for investigating infectious illnesses and the processes underlying human-specific infectious agents [57]. Models of Helicobacter pylori infection have been developed using gastric organoids [13, 37], whereas influenza virus infection has been mimicked in vitro using pulmonary organoids [79]. Human intestinal organoids can be used to spread coronaviruses in vitro and have enabled the identification of the small intestine as an alternative infection pathway for Middle East respiratory syndrome (MERS) coronaviruses, which cause severe human respiratory infections [80]. With the recent outbreak of the COVID-19 pandemic, substantial efforts have been undertaken to simulate and understand the biology and pathophysiology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [81]. Several investigations utilising organoid models generated from ASCs found that SARS-Cov-2 may infect enterocytes [82], and they revealed that viral replication in enterocytes resulted in viral response gene upregulation and the production of infectious viral particles. In human small intestine enteroids, two mucosa-specific serine proteases, TMPRSS2 and TMPRSS4, facilitate viral entrance and infection of enterocytes [83].
The Use of Organoids in Drug Discovery and Personalised Medicine
Disease-specific organoids are useful in identifying new biomarkers, personalise drug testing, drug screening [3], or toxicology studies, and thus organoids will turn personalised medicine into reality [2]. One of the uses of organoids for drug testing that has been carried out is drug screening for the therapy of Zika virus infection using cortical nerve progenitor cells derived from hPSC and validated parallel in organoid and mouse models [84]. Organoids have also been used in testing drugs for cystic fibrosis (CF), a genetic disease caused by defects in the CFTR gene. Intestinal organoids derived from cystic fibrosis patients who carry CFTR mutations are an example of organoids in personalised medicine [3, 85]. To find out whether existing CF drugs can give a good response, screening is carried out using organoids from patients with CFTR mutations to get the appropriate treatment [86].
Personalised medicine using organoids can also be applied in drug testing for cancer. Organoids derived from various human tumours have demonstrated a response spectrum of conventional and investigational drugs to date [87]. Based on a retrospective cohort study, the patient-derived organoid (PDO) response to tested therapy largely mimicked the initial response of these patients to the same agent [73, 77, 88], [89] [90]. PDO also provides a model for drug development without innate or acquired resistance, and it is particularly relevant in ovarian cancer PDOs in the assessment of DNA repair pathways and the stability of the replication fork [88]. Besides, PDO may also reflect a patient's clinical response to a cytotoxic drug having a narrow therapeutic index in vivo compared to many targeted agents by demonstrating a relative sensitivity response to the cytotoxic drug [77] [89] [90].
Moreover, the creation of organoid biobanks for various pathologies is also very promising. The organoid biobanks will facilitate a robust screening platform that covers a wide variety of population genetics worldwide. With this organoid biobank, most of the spectrum of CFTR mutations in cystic fibrosis and other diseases can be covered [3]. Especially for cancer, a disease with a virtually unlimited number of mutations, making this organoid biobank can be very useful [69, 91]. The creation of cancer organoids can use neoplastic tissue directly or by using normal tissue, which is then genetically modified [87]. Early attempts to create a tumouroid biobank have been made for colon cancer, a very common cancer in humans [69, 91]. In the end, the use of organoids can reduce the experimental animal for research which is following the 3R principle.
The Use of Organoids in Regenerative Medicine
Organoids are a promising alternative in regenerative medicine [3]. Organoids that have the potential to produce human 3D cultures that resemble specific organs have opened up the possibility of using organoids as a source of cell therapy and as an alternative to whole organ transplants [2]. This concept has been proven through experiments in animal models. An example is the transplantation of retinal sheets derived from mouse embryonic stem cells (ESCs) or mouse iPSCs using a modified optical cup organoid protocol in mice with retinal degeneration. The transplanted tissue can give rise to mature photoreceptors and, in some cases, capable of forging synaptic relationships with host cells [92] and restoring light responses [93]. The same was observed in the retinal tissue produced from organoids cultured from human ESCs. When transplanted into mouse and monkey models with retinal degeneration, the resultant tissue can survive, develop, and integrate with the host tissue to some extent [94]. Intestinal organoids derived from dissociated mouse colon epithelium or single stem cells implanted into mice, for example, can repair colonic mucosal damage to various degrees [95]. Animal models have also been used for liver [39, 56] and kidney [96] organoids transplant studies.
Also, organoids can be combined with novel genome-editing tools such as CRISPR/Cas9 to correct affected genes and select appropriate clones before autologous transplantation [2, 3]. CRISPR-Cas9-mediated gene editing was used to correct the most common CFTR mutation in CF. Phenylalanine removal at position 508 on the ISC derived from the patient was then used to produce functional organoids [97]. Although autologous cell therapy transplantation is very promising in the field of organoids, its efficacy, safety, and immunogenicity are still being evaluated [2].
Challenges, Limitations in the Application of Organoids, and Bioengineering Approach to Overcome Limitations
The previously described organoids application is based on creating repeatable organoids that are structurally and functionally comparable to actual organs and may be utilised as appropriate replacements for in vivo research. The primary issue over the next few decades will be bridging the gap with native organs. A frequent drawback is the considerable phenotypic heterogeneity that can occur from all organoid generation procedures. The constraint in many applications is organoid-to-organoid repeatability. This is especially true for translational investigations, such as drug screening, where significant inherent variability may obscure treatment impact. Furthermore, the cellular and architectural intricacy of each organ was reproduced with varying degrees of precision. This is referred to as the organoid capacity to generate all sorts of cells in a certain tissue as well as multiple organ tissues [3]. Intestinal organoids produced from ASCs, for example, are entirely comprised of the intestinal epithelium; nevertheless, intestinal mesenchyme can also emerge from the derivation of intestinal organoids utilising PSCs [30]. Another important limitation is the low level of maturity, especially for PSC-derived organoids, thus hindering their application as a model for adult tissue biology [3]. Other disciplines can help overcome these limitations and will be discussed in the following paragraphs.
Approaches to Improve Organoid Maturity
The limited maturation of cells is the major drawback of PSC-derived organoids. Usually, this type of organoid resembles fetal tissue more than adult tissue [13]. The limited lifespan of organoids can be one reason limiting their ability to reach the next stage of development [3]. The limited lifespan of the organoids can be caused because diffusion cannot supply all cells with sufficient nutrients to support their continued development once a certain size is reached [98]. The use of bioreactors that increase nutrient supply through constant culture spinning is a possible solution to this problem. In tissue engineering, bioreactors are widely used to introduce controlled change in culture conditions, standardisation, and enhancement of tissue production for regenerative medicine [99]. Such bioreactors have successfully extended the duration of brain organoid cultures from several months to 1 year and produce structures more similar to those of the developing human brain [6].
Furthermore, to increase the lifespan of organoids, artificial vascular tissue can also be used. Organoid vascularisation can distribute nutrients via capillaries, as occurs in vivo [3]. Bioengineering approaches have been developed to produce vascular tissue-like structures, namely sacrificial molds [100,101,102] and laser ablation [103], which allow for creating channels in culture scaffolds that can accommodate endothelial cells and form vascular units that can be perfused. An alternative bioprinting method can be used to control the position of endothelial cells in a 3D printed structure [104, 105]. Another technique used to improve the vascularity of developing tissue is to incorporate endothelial cells or their progenitors during organoid development. This method has been used effectively with liver organoids. Human endothelial cells are grown with human mesenchymal stem cells and liver endoderm cells generated from human iPSCs in this technique to form self-organising liver buds with a microvascular network linked to host circulation soon after transplantation into mice [31].
Another possibility of limited organoid maturation is the lack of specific factors in the in vitro environment, so it cannot reach the expected maturation level [3]. For example, sensory stimulation of brain organoids is needed for further maturation to occur. This sensory stimulation contributes to the formation of neural circuits in vivo. For human organoids, a longer culture time is required because it takes longer to mature than mouse organoids at the same stage [18, 106].
Approaches to Improve Organoid Architecture
The organoid 3D microanatomy produced through self-organisation does resemble an in vivo organ, but the overall architecture is different from the actual organ. A stem cell culture scaffold with tissue-appropriate topography can be used to overcome this so that the organoid architecture can be improved and the size can be increased [3]. Micro-collagen gels, for example, that replicate the unique crypt structure of the colonic epithelium have been utilised to cultivate a single layer of self-renewing human colonic cells [107]. Organoid topology can be improved at the microscale by precisely controlling the stem cell interactions and the surrounding ECM [3]. Matrigel, a naturally generated hydrogel matrix, is employed in the majority of the matrices. Because they are ill-defined, have lot-to-lot variability, and do not enable controlled alteration, these matrices are ineffective for directing organoid morphogenesis, despite being very successful at stimulating cell proliferation and self-organisation. This matrix also includes animal-derived goods, which are unsuitable for clinical usage due to the danger of transmitting immunogens and infections [3]. Several synthetic and chemical hydrogels for 3D cell growth have been created to address these constraints, with chemical and physical characteristics that may be manipulated and tuned for specific uses [108, 109]. Cerebral organoids, for example, have been implanted effectively in hyaluronan-based hydrogels [17], and neural tube cysts have been produced in poly (ethylene glycol) (PEG)-based hydrogels [53, 54]. Recently, new hydrogel formulations with spatially and temporally modulable biochemical and biophysical characteristics have been created [16, 110]. Controlling how cells combine might potentially be used to expand control over the organoid's self-organisation. Positioning distinct cell types in conformations that skew cell-type-specific spatial interactions, for example. Furthermore, by controlling diffuse signalling molecules’ geographical and temporal distribution, organoid development may be accelerated [3].
Approaches to Improve Disease Modelling
The primary drawback of organoids in disease modelling applications is their inability to simulate multi-organ diseases. A co-culture approach can help to alleviate some of this [3]. Intestinal organoids and hPSC-derived enteric neurons were used in early attempts in this approach [111]. Furthermore, the present drug testing platform may be improved by merging organoid cultures with organ-on-chip technology to build a 3D system that mimics the interaction between multiple organs. With this technique, the advantages of both systems (the conventional basic organ-on-chip technology and the high in vivo fidelity and functionality of organoids) may be merged [3].
Conclusion
Organoids are 3D in vitro tissues that exhibit some of the key multicellular, anatomical, and even functional properties of actual organs, and they have been used in a variety of disciplines due to these qualities. Despite certain limitations, organoids have much potential for future therapeutic uses.
References
Horder T (2010) History of developmental biology. In: Encyclopedia of life sciences
Huch M, Knoblich JA, Lutolf MP, Martinez-Arias A (2017) The hope and the hype of organoid research. Dev 144:938–941. https://doi.org/10.1242/dev.150201
Rossi G, Manfrin A, Lutolf MP (2018) Progress and potential in organoid research. Nat Rev Genet 19:671–687. https://doi.org/10.1038/s41576-018-0051-9
Lancaster MA, Knoblich JA (2014a) Organogenesisin a dish: Modeling development and disease using organoid technologies. Science (80- ):345:. https://doi.org/10.1126/science.1247125
Huch M, Koo BK (2015) Modeling mouse and human development using organoid cultures. Development
Lancaster MA, Knoblich JA (2014) Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. https://doi.org/10.1038/nprot.2014.158
Sasai Y (2013) Cytosystems dynamics in self-organisation of tissue architecture. Nature
Turner DA, Baillie-Johnson P, Martinez Arias A (2016) Organoids and the genetically encoded self-assembly of embryonic stem cells. BioEssays. https://doi.org/10.1002/bies.201500111
Green JBA, Sharpe J (2015) Positional information and reaction-diffusion: two big ideas in developmental biology combine. Dev. https://doi.org/10.1242/dev.114991
Ferrell JE (2012) Bistability, bifurcations, and Waddington's epigenetic landscape. Curr Biol
Mori H, Gjorevski N, Inman JL et al (2009) Self-organisation of engineered epithelial tubules by differential cellular motility. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.0901269106
Lancaster MA, Renner M, Martin CA et al (2013) Cerebral organoids model human brain development and microcephaly. Nature. https://doi.org/10.1038/nature12517
McCracken KW, Catá EM, Crawford CM et al (2014) Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. https://doi.org/10.1038/nature13863
Linnemann JR, Miura H, Meixner LK et al (2015) Quantification of regenerative potential in primary human mammary epithelial cells. Dev. https://doi.org/10.1242/dev.123554
Sachs N, Tsukamoto Y, Kujala P et al (2017) Intestinal epithelial organoids fuse to form self-organising tubes in floating collagen gels. Dev. https://doi.org/10.1242/dev.143933
Gjorevski N, Sachs N, Manfrin A et al (2016) Designer matrices for intestinal stem cell and organoid culture. Nature. https://doi.org/10.1038/nature20168
Lindborg BA, Brekke JH, Vegoe AL et al (2016) Rapid induction of cerebral organoids from human induced pluripotent stem cells using a chemically defined hydrogel and defined cell culture medium. Stem Cells Transl Med. https://doi.org/10.5966/sctm.2015-0305
Eiraku M, Takata N, Ishibashi H et al (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. https://doi.org/10.1038/nature09941
Muguruma K, Nishiyama A, Kawakami H et al (2015) Self-organisation of polarised cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. https://doi.org/10.1016/j.celrep.2014.12.051
Sakaguchi H, Kadoshima T, Soen M et al (2015) Generation of functional hippocampal neurons from self-organising human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun. https://doi.org/10.1038/ncomms9896
Takasato M, Er PX, Becroft M et al (2014) Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organising kidney. Nat Cell Biol. https://doi.org/10.1038/ncb2894
Takasato M, Er PX, Chiu HS et al (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. https://doi.org/10.1038/nature15695
Takasato M, Little MH (2016) A strategy for generating kidney organoids: Recapitulating the development in human pluripotent stem cells. Dev Biol
McCracken KW, Aihara E, Martin B et al (2017) Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature. https://doi.org/10.1038/nature21021
Kratochvil MJ, Seymour AJ, Li TL et al (2019) Engineered materials for organoid systems. Nat Rev Mater 4:606–622. https://doi.org/10.1038/s41578-019-0129-9
Múnera JO, Sundaram N, Rankin SA et al (2017) Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell. https://doi.org/10.1016/j.stem.2017.05.020
Rankin SA, McCracken KW, Luedeke DM, et al (2018) Timing is everything: reiterative Wnt, BMP and RA signaling regulate developmental competence during endoderm organogenesis. Dev Biol. https://doi.org/10.1016/j.ydbio.2017.11.018
Carcamo-Orive I, Hoffman GE, Cundiff P et al (2017) Analysis of transcriptional variability in a large human iPSC library reveals genetic and non-genetic determinants of heterogeneity. Cell Stem Cell. https://doi.org/10.1016/j.stem.2016.11.005
Forbes TA, Howden SE, Lawlor K et al (2018) Patient-iPSC-derived kidney organoids show functional validation of a ciliopathic renal phenotype and reveal underlying pathogenetic mechanisms. Am J Hum Genet. https://doi.org/10.1016/j.ajhg.2018.03.014
Spence JR, Mayhew CN, Rankin SA et al (2011) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. https://doi.org/10.1038/nature09691
Takebe T, Sekine K, Enomura M et al (2013) Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. https://doi.org/10.1038/nature12271
Fordham RP, Yui S, Hannan NRF et al (2013) Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell. https://doi.org/10.1016/j.stem.2013.09.015
Greggio C, De Franceschi F, Figueiredo-Larsen M et al (2013) Artificial three-dimensional niches deconstruct pancreas development in vitro. Dev. https://doi.org/10.1242/dev.096628
Jamieson PR, Dekkers JF, Rios AC et al (2017) Derivation of a robust mouse mammary organoid system for studying tissue dynamics. Dev. https://doi.org/10.1242/dev.145045
Maimets M, Rocchi C, Bron R et al (2016) Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Rep. https://doi.org/10.1016/j.stemcr.2015.11.009
Sato T, Stange DE, Ferrante M et al (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. https://doi.org/10.1053/j.gastro.2011.07.050
Bartfeld S, Bayram T, Van De Wetering M et al (2015) In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. https://doi.org/10.1053/j.gastro.2014.09.042
Huch M, Bonfanti P, Boj SF et al (2013) Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. https://doi.org/10.1038/emboj.2013.204
Huch M, Dorrell C, Boj SF et al (2013) In vitro expansion of single Lgr5 + liver stem cells induced by Wnt-driven regeneration. Nature. https://doi.org/10.1038/nature11826
Xinaris C, Brizi V, Remuzzi R (2015) Organoid models and applications in biomedical research. Nephron
Clevers H (2016) Modeling development and disease with organoids. Cell 165:1586–1597. https://doi.org/10.1016/j.cell.2016.05.082
Rivron NC, Frias-Aldeguer J, Vrij EJ et al (2018) Blastocyst-like structures generated solely from stem cells. Nature. https://doi.org/10.1038/s41586-018-0051-0
Harrison SE, Sozen B, Christodoulou N, et al (2017) Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science (80- ). https://doi.org/10.1126/science.aal1810
Shao Y, Taniguchi K, Gurdziel K, et al (2017) Self-organised amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat Mater
Shao Y, Taniguchi K, Townshend RF et al (2017) A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat Commun. https://doi.org/10.1038/s41467-017-00236-w
Baillie-Johnson P, van den Brink SC, Balayo T et al (2015) Generation of aggregates of mouse embryonic stem cells that show symmetry breaking, polarisation and emergent collective behaviour in vitro. J Vis Exp. https://doi.org/10.3791/53252
Van Den Brink SC, Baillie-Johnson P, Balayo T et al (2014) Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Dev. https://doi.org/10.1242/dev.113001
Etoc F, Metzger J, Ruzo A et al (2016) A balance between secreted inhibitors and edge sensing controls gastruloid self-organization. Dev Cell. https://doi.org/10.1016/j.devcel.2016.09.016
Martyn I, Kanno TY, Ruzo A et al (2018) Self-organisation of a human organiser by combined Wnt and Nodal signaling. Nature. https://doi.org/10.1038/s41586-018-0150-y
Morgani SM, Metzger JJ, Nichols J et al (2018) Micropattern differentiation of mouse pluripotent stem cells recapitulates embryo regionalised cell fate patterning. Elife. https://doi.org/10.7554/eLife.32839
Tewary M, Ostblom J, Prochazka L et al (2017) A stepwise model of reaction-diffusion and positional information governs self-organised human peri-gastrulation-like patterning. Dev. https://doi.org/10.1242/dev.149658
Warmflash A, Sorre B, Etoc F et al (2014) A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Meth. https://doi.org/10.1038/nMeth.3016
Meinhardt A, Eberle D, Tazaki A et al (2014) 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Rep. https://doi.org/10.1016/j.stemcr.2014.09.020
Ranga A, Girgin M, Meinhardt A et al (2016) Neural tube morphogenesis in synthetic 3D microenvironments. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1603529113
Dekkers JF, Wiegerinck CL, De Jonge HR et al (2013) A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. https://doi.org/10.1038/nm.3201
Huch M, Gehart H, Van Boxtel R et al (2015) Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. https://doi.org/10.1016/j.cell.2014.11.050
Lancaster MA, Huch M (2019) Disease modelling in human organoids. DMM Dis Model Mech 12. https://doi.org/10.1242/dmm.039347
Wong AP, Bear CE, Chin S et al (2012) Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol 30. https://doi.org/10.1038/nbt.2328
Firth AL, Menon T, Parker GS et al (2015) Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep 12. https://doi.org/10.1016/j.celrep.2015.07.062
Mariani J, Coppola G, Zhang P et al (2015) FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162. https://doi.org/10.1016/j.cell.2015.06.034
Mariani J, Simonini MV, Palejev D et al (2012) Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci U S A 109. https://doi.org/10.1073/pnas.1202944109
Sachs N, Papaspyropoulos A, Zomer‐van Ommen DD, et al (2019) Long‐term expanding human airway organoids for disease modeling. EMBO J 38. https://doi.org/10.15252/embj.2018100300
Li X, Nadauld L, Ootani A et al (2014) Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat Med 20. https://doi.org/10.1038/nm.3585
Seidlitz T, Merker SR, Rothe A et al (2019) Human gastric cancer modelling using organoids. Gut 68. https://doi.org/10.1136/gutjnl-2017-314549
Broutier L, Mastrogiovanni G, Verstegen MMA et al (2017) Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med 23. https://doi.org/10.1038/nm.4438
Nuciforo S, Fofana I, Matter MS et al (2018) Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep 24. https://doi.org/10.1016/j.celrep.2018.07.001
Seino T, Kawasaki S, Shimokawa M et al (2018) Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell 22. https://doi.org/10.1016/j.stem.2017.12.009
Tiriac H, Bucobo JC, Tzimas D et al (2018) Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy sampling for personalised cancer treatment. Gastrointest Endosc 87. https://doi.org/10.1016/j.gie.2017.12.032
Van De Wetering M, Francies HE, Francis JM et al (2015) Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. https://doi.org/10.1016/j.cell.2015.03.053
Gao D, Vela I, Sboner A et al (2014) Organoid cultures derived from patients with advanced prostate cancer. Cell 159. https://doi.org/10.1016/j.cell.2014.08.016
Turco MY, Gardner L, Hughes J et al (2017) Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol 19. https://doi.org/10.1038/ncb3516
Sachs N, de Ligt J, Kopper O et al (2018) A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172. https://doi.org/10.1016/j.cell.2017.11.010
Lee SH, Hu W, Matulay JT et al (2018) Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. https://doi.org/10.1016/j.cell.2018.03.017
Mullenders J, de Jongh E, Brousali A et al (2019) Mouse and human urothelial cancer organoids: a tool for bladder cancer research. Proc Natl Acad Sci USA 116. https://doi.org/10.1073/pnas.1803595116
Li X, Francies HE, Secrier M et al (2018) Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat Commun 9. https://doi.org/10.1038/s41467-018-05190-9
Hubert CG, Rivera M, Spangler LC et al (2016) A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res 76. https://doi.org/10.1158/0008-5472.CAN-15-2402
Pauli C, Hopkins BD, Prandi D et al (2017) Personalised in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-16-1154
Weeber F, Ooft SN, Dijkstra KK, Voest EE (2017) Tumor organoids as a pre-clinical cancer model for drug discovery. Cell Chem Biol 24
Zhou J, Li C, Sachs N et al (2018) Differentiated human airway organoids to assess infectivity of emerging influenza virus. Proc Natl Acad Sci USA 115. https://doi.org/10.1073/pnas.1806308115
Zhou J, Li C, Zhao G et al (2017) Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci Adv 3. https://doi.org/10.1126/sciadv.aao4966
Azar J, Bahmad HF, Daher D et al (2021) The use of stem cell-derived organoids in disease modeling: an update. Int J Mol Sci 22. https://doi.org/10.3390/ijms22147667
Lamers MM, Beumer J, Vaart J Van Der et al (2020) SARS-CoV-2 productively infects human gut enterocytes. Science (80- ) 369. https://doi.org/10.1126/science.abc1669
Zang R, Castro MFG, McCune BT et al (2020) TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol 5. https://doi.org/10.1126/sciimmunol.abc3582
Zhou T, Tan L, Cederquist GY et al (2017) High-content screening in hPSC-neural progenitors identifies drug candidates that inhibit Zika Virus infection in fetal-like organoids and adult brain. Cell Stem Cell. https://doi.org/10.1016/j.stem.2017.06.017
Dekkers JF, Berkers G, Kruisselbrink E et al (2016) Characterising responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aad8278
Saini A (2016) Cystic fibrosis patients benefit from mini guts. Cell Stem Cell. https://doi.org/10.1016/j.stem.2016.09.001
Tuveson D, Clevers H (2019) Cancer modeling meets human organoid technology. Science (80- ) 364:952–955. https://doi.org/10.1126/science.aaw6985
Hill SJ, Decker B, Roberts EA et al (2018) Prediction of DNA repair inhibitor response in short-term patient-derived ovarian cancer organoids. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-18-0474
Tiriac H, Belleau P, Engle DD et al (2018) Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-18-0349
Vlachogiannis G, Hedayat S, Vatsiou A, et al (2018) Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science (80- ). https://doi.org/10.1126/science.aao2774
Fujii M, Shimokawa M, Date S et al (2016) A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell. https://doi.org/10.1016/j.stem.2016.04.003
Assawachananont J, Mandai M, Okamoto S et al (2014) Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Rep. https://doi.org/10.1016/j.stemcr.2014.03.011
Mandai M, Fujii M, Hashiguchi T et al (2017) iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice. Stem Cell Rep. https://doi.org/10.1016/j.stemcr.2016.12.008
Shirai H, Mandai M, Matsushita K et al (2016) Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1512590113
Yui S, Nakamura T, Sato T et al (2012) Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5 + stem cell. Nat Med. https://doi.org/10.1038/nm.2695
Taguchi A, Kaku Y, Ohmori T et al (2014) Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. https://doi.org/10.1016/j.stem.2013.11.010
Schwank G, Koo BK, Sasselli V et al (2013) Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. https://doi.org/10.1016/j.stem.2013.11.002
Qian X, Nguyen HN, Song MM et al (2016) Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. https://doi.org/10.1016/j.cell.2016.04.032
Zhao J, Griffin M, Cai J, et al (2016) Bioreactors for tissue engineering: an update. Biochem. Eng J
Miller JS, Stevens KR, Yang MT et al (2012) Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. https://doi.org/10.1038/nmat3357
Tocchio A, Tamplenizza M, Martello F et al (2015) Versatile fabrication of vascularizable scaffolds for large tissue engineering in bioreactor. Biomaterials. https://doi.org/10.1016/j.biomaterials.2014.12.031
Wang XY, Jin ZH, Gan BW et al (2014) Engineering interconnected 3D vascular networks in hydrogels using molded sodium alginate lattice as the sacrificial template. Lab Chip. https://doi.org/10.1039/c4lc00069b
Brandenberg N, Lutolf MP (2016) In situ patterning of microfluidic networks in 3D cell-laden hydrogels. Adv Mater. https://doi.org/10.1002/adma.201601099
Zhang YS, Arneri A, Bersini S et al (2016) Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. https://doi.org/10.1016/j.biomaterials.2016.09.003
Zhu W, Qu X, Zhu J et al (2017) Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials. https://doi.org/10.1016/j.biomaterials.2017.01.042
Nakano T, Ando S, Takata N et al (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. https://doi.org/10.1016/j.stem.2012.05.009
Wang Y, Kim R, Gunasekara DB et al (2018) Formation of human colonic crypt array by application of chemical gradients across a shaped epithelial monolayer. CMGH. https://doi.org/10.1016/j.jcmgh.2017.10.007
Phelps EA, Enemchukwu NO, Fiore VF et al (2012) Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv Mater. https://doi.org/10.1002/adma.201103574
Tsurkan MV, Chwalek K, Prokoph S et al (2013) Defined polymer-peptide conjugates to form cell-instructive starpeg-heparin matrices in situ. Adv Mater. https://doi.org/10.1002/adma.201300691
Mosiewicz KA, Kolb L, Van Der Vlies AJ et al (2013) In situ cell manipulation through enzymatic hydrogel photopatterning. Nat Mater. https://doi.org/10.1038/nmat3766
Workman MJ, Mahe MM, Trisno S et al (2017) Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. https://doi.org/10.1038/nm.4233
Acknowledgements
Ahmad Faried is supported by Grants-in-Aid from the Ministry of Research and Technology/National Research and Innovation Agency Republic of Indonesia for basic research (No. 8/E1/KPT/2021). The authors would like to thank Nararian Padma Dewi as technical assistants.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Faried, A., Hermanto, Y., Amalia, P.R., Bolly, H.M.B. (2022). The Organoids: Derivations and Applications. In: Yahaya, B.H. (eds) Organoid Technology for Disease Modelling and Personalized Treatment . Stem Cell Biology and Regenerative Medicine, vol 71. Humana, Cham. https://doi.org/10.1007/978-3-030-93056-1_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-93056-1_1
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-93055-4
Online ISBN: 978-3-030-93056-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)