Abstract
Generally, ethanol is the alcohol commonly used in the SO2-alcohol-water (SAW) fractionation process. In this study, Eldar pine (Pinus eldarica) was fractionated with different alcohols (methanol, isopropanol, and 2-methyl-2-propanol, in comparison to ethanol) at 135 °C, duration (60–120 min.), and liquor composition (SO2:alcohol:water = 12:44:44, w/w%) to achieve a consistent kappa number (∽25). The field emission scanning electron microscopy (FE-SEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FTIR) were used to analyze the changes in physical and chemical structure characteristics of selected pulps. The use of isopropanol, as opposed to ethanol (common alcohol in SAW fractionation), showed a beneficial effect on the delignification rate. Meanwhile, both methanol and 2-methyl-2-propanol reduced the delignification rate. Moreover, isopropanol pulp required fewer beating revolutions to achieve a similar freeness (∽385 mL CSF) compared to other alcohols. Handsheets produced from isopropanol fractionation exhibited superior characteristics, including air permeability, apparent density, and tensile, tear, and burst indexes, when compared to those obtained from other alcohols. Overall, isopropanol is a highly suitable alternative to ethanol in SAW fractionation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The kraft, sulfite, soda, and soda-antraquinone (AQ) processes are the most commonly used methods for industrial-scale pulping (Jahan et al. 2021). However, in recent years, despite the many disadvantages of the mentioned processes, the demand for pulp has increased. Therefore, the development of the pulping industry in the world requires further work and research on novel and emerging processes with high performance. The development of novel processes will allow access to advanced biorefineries and the production of a new generation of cellulose pulps (Huang et al. 2019; Survase et al. 2019; Yadollahi et al. 2019; Iakovlev et al. 2020a, b).
The fractionation method used for lignocellulosic biorefineries should produce high yields, low inhibitors, and no sticky lignin precipitates, while converting lignocellulosic to cellulosic and hemicellulosic sugars, and finally sugar-derived products (Iakovlev et al. 2020a). Various pretreatment and fractionation methods have been developed, including different configurations of acidic (Dagnino et al. 2013), alkaline (Tang et al. 2023), oxidative (Zhou et al. 2023), hydrothermal (Hou et al. 2023), enzymatic, organosolv (Vergara et al. 2019; Gutierrez et al. 2023), high-energy radiation (Fei et al. 2020; Anoopkumar et al. 2023), green bio-based aprotic solvents (Li et al. 2023a), biphasic solvent (Li et al. 2023b), and combination pretreatment (Meenakshisundaram et al. 2021). Nevertheless, acid-based pretreatments are more feasible for industrial use because the pre-hydrolyzate can be directly utilized for biorefinery goals after undergoing certain treatments. Consequently, the development of a practical acid-based pretreatment to achieve the thorough removal of hemicellulose and lignin is crucial for the production of sugars from lignocellulosic biomass (Huang et al. 2018).
Fractionation processes based on alcohol, SO2, and water were first introduced under the name of “SO2–ethanol–water (SEW)” (Iakovlev et al. 2010; Yamamoto et al. 2011; Iakovlev and van Heiningen 2012a). Nowadays, due to the use of other alcohols, the name of this process has been changed from SEW to “SO2-alcohol-water (SAW)”. SAW fractionation is a favorably suitable process for future integrated lignocellulosic-based biorefineries (Sharazi and Van Heiningen 2017). High flexibility for raw material selection, simple and efficient fractionation chemical recovery are some of its governing advantages (Iakovlev et al. 2014). The SEW method results in a higher proportion of carbohydrates remaining in the spent pretreatment liquid, leading to an enhancement in both the overall recovery of carbohydrates and their conversion in downstream processes (Huang et al. 2018). Fractionation using SAW is comparable to acid sulfite (AS) and organosolv pulping (Iakovlev et al. 2009). AS and SEW processes share similar mechanisms for pulping. Lignin removal occurs through acid-catalyzed sulfonation of propane’s carbon atoms, which produce lignosulfonic acid groups. These groups are highly hydrophilic and facilitate polymeric lignin dissolution in ethanol-water solution (Iakovlev and Van Heiningen 2011).
The type of solvent in the SAW process is an effective factor in delignification and is important in pulping studies (Sharazi et al. 2018). Methanol and ethanol are commonly used as low molecular weight aliphatic alcohols in organosolv processes. Due to their low cost, these substances are particularly useful in commercial applications (Oliet et al. 2002). In organosolv-based pulping processes, methanol is used as a solvent in “alkaline sulfite anthraquinone methanol (ASAM)” (Mertoglu-Elmas et al. 2012; Moradbak et al. 2016) and autocatalyzed methanol pulping (Oliet et al. 2002). The Alcell, Organocell, and ASAM processes are well-known and commercially tested organosolv processes that use either methanol or ethanol, but they were industrially tested and never commercially successful (Shatalov and Pereira 2008; Huijgen et al. 2014; Mertoglu-Elmas and Ozden 2019). Due to its low boiling point and the formation of a small amount of methanol (1.02–1.33 g/L) from lignocellulosic materials during the delignification process, methanol has advantages such as simple recovery and low cost (Zhu et al. 1999). In addition to its increased toxicity and flammability, methanol is less desirable for pulping. One disadvantage of ethanol and methanol is that they require high process pressure during fractionation, which leads to high investment costs. Methanol is less expensive and volatile than ethanol.
Methanol has a lower solubility of lignin than ethanol or isopropanol, but ethanol and isopropanol produce better delignification and have fewer safety regulations than methanol (Sharazi et al. 2018). When the total yield of pulp is considered, ethanol and methanol show similar selectivity, but in pulping using ethanol, the screened pulp yield is higher. To date, the utilization of isopropanol in the pulping process has not been widely prevalent. Nonetheless, previous findings have demonstrated that the introduction of isopropanol into sulfite cooking liquor yields notable improvements in pulping outcomes. To elucidate, the supplementation of 40–50% (by volume) isopropanol to magnesium bisulfite liquor has exhibited enhancements in pulp strength, increased pulp yield, and augmented delignification (Sakai and Uprichard 1987).
According to our knowledge to date, there is no comprehensive study on the effects of the type of alcohol on the properties of papers obtained in the SAW fractionation process. In this study, the effects of using different alcohols including methanol, isopropanol, and 2-methyl-2-propanol in comparison to ethanol on the pulping and papermaking properties in SAW fractionation of Eldar pine (Pinus eldarica) were investigated. Therefore, a notable research gap exists concerning the comparative analysis of SAW fractionation with previous fractionation methods applied to lignocellulosic resources. Existing literature has primarily focused on the kinetics of SAW fractionation, yet a comprehensive understanding of how SAW performance (especially in papermaking) against conventional fractionation techniques remains underexplored. This study deficiency hinders the industry’s ability to make informed decisions regarding the most suitable and sustainable fractionation method for optimizing lignocellulosic resources. Addressing this research gap is crucial for advancing the knowledge base in sustainable wood processing and biorefining practices.
2 Materials and methods
2.1 Raw materials
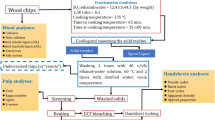
Fresh Eldar pine (Pinus eldarica) was obtained from the Shastkalate educational and research forest located in Golestan province, Iran. To prepare the chips for pulping, each log was transformed into a disc with a thickness of 10 cm at breast height. Subsequently, the discs were manually stripped of bark and chipped using a chisel, resulting in pieces measuring 25 × 15 × 3 mm in size, which were suitable for the pulping process. Air-dried wood chips (dry matter content 91.5%) were stored in polythene bags at room temperature for further use. The physicochemical properties of the solvents (Merck and Co. Inc., Darmstadt, Germany) used are provided in Table 1. High-purity sulfur dioxide (SO2) gas, with a purity exceeding 99%, was obtained from Farayand Gas Co. in Tehran, Iran. In the case of all solutions used, deionized water was employed during their preparation.
2.2 Chemical composition analysis of wood
The quantification of lignin, acetone soluble extractives, and ash content was performed by TAPPI test methods T 222 om-02, T 204 cm-97, and T 211 om-02, respectively. The amount of cellulose was measured according to the Kürschner-Hoffer method and the amount of holocellulose was calculated according to the following Equation 1 (Zoia et al. 2017):
2.3 SAW fractionation conditions
The 20.0 g air-dried chips (dry matter content 91.5%) and liquors at a liquor-to-wood ratio of 6 L kg-1 were placed into 500 mL bombs. Fractionation white liquor was prepared by directly injecting SO2 gas into an ethanol-water solution. In all the experiments, the cooking liquor composition and charge were SO2:alcohol:water = 12:44:44, by weight (Iakovlev et al. 2009, 2010; Iakovlev and Van Heiningen 2011; Yamamoto et al. 2014; Yadollahi et al. 2018). The maximum temperature (135 ± 1 °C) was kept constant, while the cooking times in the maximum temperature (60–120 min) were varied. The time to maximum temperature for all the cooking experiments was 65 min. Fractionation was conducted using a laboratory glycol bath (Frank-PTI, Austria) equipped with an electric heating system. Subsequently, the bombs were cooled in cold water after the fractionation procedure. The collection of the spent liquor was accomplished by exerting pressure on the pulp suspension within washing bags, resulting in the extraction of the liquor. According to the method of Iakovlev and Van Heiningen (2011), the solid residue in each bomb was washed twice with a 40% v/v alcohol-water solution at 60 °C and finally washed twice with 500 mL of distilled water at room temperature. The pulps underwent natural air drying at the prevailing ambient temperature, followed by their storage in polythene bags. Specific details regarding the fractionation conditions are provided in Fig. 1.
2.4 Characterization
2.4.1 Field emission scanning electron microscopy (FESEM)
Sample morphology was examined using FESEM. A thin platinum coating with a thickness of less than 0.2 nm was deposited onto the samples in a vacuum environment utilizing a sputter coater machine (Quorum, Q150R ES, UK). The coated samples were subsequently examined using a FESEM (IRA3 TESCAN-XMU model, Czech Republic,) with an accelerating voltage of 10 kV. Furthermore, the analysis also involved Energy-Dispersive X-ray spectroscopy (EDX) using the same instrument, MIRA3 TESCAN-XMU.
2.4.2 X-ray diffraction (XRD)
X-ray Diffraction (XRD) is a commonly employed technique for evaluating the crystallinity of cellulose (French 2014). From directions perpendicular to the surface of the sample, a diffractometer XRD instrument (Unisantis XMD300 model, Singapore) was used to irradiate the specimens with Cu-Kα radiation at 50 kV and 30 mA. The X-ray characterization was performed with a sampling width of 0.02°, and scans were conducted within the range of 10–60° (2θ). According to Eq. 2, the crystalline index of cellulose in selected pulps was determined.
where I200 is the maximum intensity of the [200] lattice diffraction, which is typically in the range 2θ = 21°–23° and Iam is the intensity diffraction at 2θ = 18° (Segal et al. 1959).
2.4.3 Fourier transform infrared spectroscopy (FTIR)
The samples were analyzed using FTIR to identify their functional groups. FTIR spectrometer (Perkin-Elmer, Spectrum RX I) in the range of 4000 − 500 cm-1 was used.
2.4.4 Analysis of pulps
Based on TAPPI T 412 cm-02, moisture content was determined in the pulps. The measurement of the pulp yield obtained through screening was conducted following the guidelines outlined in TAPPI T 210 cm-03. Additionally, the wet screening process was utilized to determine the yield of the rejected pulp, which refers to the particles unable to pass through 0.84 mm slots corresponding to mesh No. 20. The measurement of the rejected pulp yield was carried out using Eqs. 3 and 4. The total pulp yield was calculated by combining the reject yield with the screened yield, as shown in Eq. 5. A methodology described in TAPPI T 236 cm-99 was used to determine the kappa number of the pulps.
2.4.5 Beating and handsheet making
To conduct a comparison of SAW pulps, the characteristics of pulps having a similar kappa number of approximately ∽25 were assessed. Prior to the processing stage, each pulp underwent a soaking period of 24 h in deionized water. The pulps were treated using a pulp disintegrator (PTI, Paper Testing Instruments GmbH, Austria) according to TAPPI T 218 sp-02 at 15,000 revolutions. The determination of pulp freeness was conducted by employing a Canadian Standard Freeness (CSF) tester apparatus (PTI, Paper Testing Instruments GmbH, Austria). This measurement was carried out by the guidelines outlined in TAPPI T 227 om-04. In accordance with TAPPI T 248 sp-00, the beating was performed in a PFI mill machine. To determine pulp and paper properties, pulps obtained from SAW fractionation were beaten to achieve a freeness of ∽385 mL CSF. In the next step, handsheet samples (with a grammage of 60 ± 1 g/m2) were formed in a sheet former (200-1 PTI, Austria) in accordance with TAPPI T 205 sp-02.
2.4.6 Analysis of handsheet properties
Prior to evaluating the mechanical characteristics of the handsheets, including the tensile index (TAPPI T 494 om-96), burst index (TAPPI T 403 om-97), and tear index (TAPPI T 414 om-98), they were subjected to a conditioning period of 24 h at a controlled temperature of 23 ± 1 °C and relative humidity of 50 ± 2%. Additionally, the air permeability of the handsheets was assessed using the measurement method outlined in TAPPI T 460 om-96.
2.5 Statistical analysis
Data were recorded as mean ± standard deviation for each experiment conducted in triplicate. Analysis of variance (ANOVA) was used to determine the differences within each mean value. Statistically significant differences were determined by Duncan multiple range tests (DMRT) using SPSS 21 (IBM Corp. Armonk, NY, USA).
3 Results and discussion
3.1 Compositional analysis of raw material
Table 2 presents the recorded values for the chemical composition of Eldar pine (Pinus eldarica) wood utilized in the context of this study. These characteristics have been reported differently depending on the geographical conditions, climate, species, and age of the tree (Akgül et al. 2007; Sable et al. 2012; Barman et al. 2020).
3.2 Effect of cooking time and alcohol type on delignification
The conditions for beating and handsheet making of selective pulps with the same kappa number (∽25) are presented in Table 3. The results showed that the cooking time for isopropanol (80 min) is shorter than for other alcohols to achieve the same kappa number, with times of 90, 100 and 120 min for ethanol, 2-methyl-2-propanol, and methanol, respectively. Additionally, at the same cooking time (60 min), the relationship for kappa number is isopropanol < ethanol < methanol < 2-methyl-2-propanol. Furthermore, the pulp yield obtained from fractionation with 2-methyl-2-propanol is lower than that obtained from the other alcohols at the same kappa number (∽25). In SAW fractionation, carbohydrate peeling-off reactions are absent, and hemicelluloses are better retained due to the relatively short pulping duration (Iakovlev et al. 2009). Based on the relationships and the results in the table, it can be concluded that 2-methyl-2-propanol is relatively less efficient for delignification in the SAW fractionation process. These findings are consistent with earlier reports. Sharazi et al. (2018) reported that in the SAW process, the residual lignin content in fractionated pulps with isopropanol was lower than that with ethanol and methanol. Ethanol and isopropanol are commonly used solvents in the organosolv pulping for the production of high-quality cellulose fibers (Iakovlev and van Heiningen 2012b; Sharazi et al. 2018). Oliet et al. (2002) in their study on “solvent effects in autocatalyzed alcohol–water pulping” reported that alcohols can impact the properties of pulp, depending on the type and the amount used. Methanol and 2-methyl-2-propanol are also solvents that can be used in the pulp and paper industry for the production of cellulose fibers. However, unlike ethanol and isopropanol, these solvents can decrease the mechanical properties of the cellulose fibers if not used appropriately (Muurinen 2000). In a study, Jiménez et al. (1999) investigated the use of butanol-water solutions for the production of pulp from wheat straw. They reported that obtaining pulp that contains a high amount of α-cellulose requires a long cooking time and high temperature, along with a low concentration of butanol. By increasing cooking time, total carbohydrate, lignin, extractives, pH, and yield are more sensitive than when butanol concentrations are decreased.
3.3 XRD analysis
The examination of the crystalline structure of materials is effectively carried out through the utilization of X-ray diffraction (XRD), which serves as a potent methodology for analysis. Overall, the XRD peaks of SAW fractionated cellulose pulp can provide important information on the crystal structure and morphology of the cellulose. These peaks can be useful in understanding the properties and behavior of the pulp in various applications. In general, a higher crystallinity index (Crl) indicates a higher degree of crystallinity, which can affect the mechanical properties and other functional properties of the cellulose. Figure 2 shows the XRD of pulps obtained from different alcohols (ethanol, methanol, isopropanol, and 2-methyl-2-propanol. The Crl of pine wood chips was lower (48.52%) than that of SAW pulps, indicating an increase in pulps crystallinity during SAW fractionation compared to pine wood chips. It appears that pulping removed lignin and hemicelluloses as amorphous biopolymers, leaving a higher proportion of semi-crystalline cellulose. Similar results were reported by Socha et al. (2013), Park et al. (2010), Priyadarshinee et al. (2015), and Yousefi et al. (2011, 2018). It seems that the difference in the crystallinity index is due to the effect of the nature of the solvent in the dissolution of lignin and the removal of amorphous regions.
The XRD pattern of pulp obtained from SAW fractionation typically shows peaks at 2θ values of 15°, 16.4°, 22.6°, and 35.5° corresponding to the (1–10), (110), (200), and (004) planes of cellulose Iβ structure, respectively. The intensity of the (200) peak is typically high, indicating the high degree of crystallinity of the cellulose pulp. The XRD pattern of cellulose pulp prepared by ethanol fractionation generally shows peaks at 2θ angles of approximately 14°, 16°, and 22°. These peaks reflect the presence of the crystalline cellulose Iβ phase, which is the most stable and frequently found in natural cellulose. The intensity and location of these XRD peaks can provide information on the degree of crystallinity, which can reflect the efficiency of the SEW process. A higher degree of crystallinity means more efficient fractionation and removal of non-cellulosic components. The fractionation with isopropanol results in a higher CrI (65.65%) due to the removal of amorphous portions from the pulp. Therefore, the interpretation of the XRD peaks of cellulose pulp prepared by isopropanol fractionation is that the process has increased the CrI of the cellulose, resulting in more distinct and intense diffraction peaks in the XRD pattern.
3.4 FTIR analysis
The FTIR analysis can help to provide further insight into the chemical structure of the cellulose pulp, which can be used to optimize the cellulose pulp production process for various applications. In general, the FTIR spectrum of cellulose pulp prepared by the SEW method can provide insights into the chemical composition, purity, degree of polymerization, crystallinity, and functional groups of the cellulose, and can be used as a diagnostic tool for quality control and process optimization in pulp and paper industry. Table 4 and Fig. 3 represent the FTIR transmittance spectra of pulps obtained from different alcohols (ethanol, methanol, isopropanol, and 2-methyl-2-propanol).
The bands at 1000 and 1200 cm-1 are related to cellulose and hemicellulose structural features. The peak at 3302 cm-1 represents OH groups in H-bonded bonds. This showed that hemicelluloses are removed in the SAW process. The peak observed at 1030 cm-1 is attributed to the stretching of the C–O bonds in cellulose and hemicellulose, as well as the deformation of the C–H bonds in the guaiacyl component of lignin (Darwish et al. 2013; Gallio et al. 2018), which has disappeared in Eldar pine wood. The signals of the ether bond at 1253 cm− 1 (Barman et al. 2020) became weaker. Many absorption bands are observed in the fingerprint region of 900–1800 cm-1 associated with lignin functional groups. The peak at 2360 cm-1 corresponding to lignin (Puntambekar et al. 2016) is observed in pulp samples, due to the use of alcohol in the fractionation process. Therefore, this peak is not observed in pine wood. There is a band around 890 cm-1 that indicates aromatic C-H out of plane deformation in all FTIR spectra, but the intensity is lower for pulp obtained from fractionation with isopropanol, suggesting that lignin can be easier removed by this alcohol. The broad bond at 3700 − 3100 cm-1 is attributed to the O–H stretching from carboxylic acids (Rosu et al. 2010). However, the specific bands and their intensities varied depending on the alcohol used for fractionation.
3.5 Beatability of pulps
Table 5 shows the minimum number of beating revolutions required to produce pulps with similar freeness (∽385 mL CSF). The results indicated that the required number of beating revolutions for the pulp prepared with isopropanol was less than for other alcohols. The pulp obtained from 2-methyl-2-propanol alcohol required the highest number of beating revolutions (10,000), while the pulp obtained from isopropyl required the lowest (5,000).
The beating process of pulp requires approximately 100–500 kWh/t of energy. This value represents 40% of the mill’s total electricity consumption. However, cellulose pulp with a low beating degree can be used to manufacture a large number of paper products (Małachowska et al. 2020). It is expected that SAW pulp will exhibit similar properties to AS pulp due to similar chemistry (pH, temperature, and SO2 delignification agent) (Iakovlev et al. 2010). Dehghani Firouzabadi and Tatari (2023) reported that the required number of beating revolutions for produced pulps using SEW fractionation was lower than those of Kraft pulps. Iakovlev et al. (2010) reported that the beating of SEW pulps requires a lower number of beating revolutions and is carried out faster than alkaline pulps.
The obtained pulps from fractionation with four different alcohols were observed using the FESEM technique and the micrographs of unbeaten and beaten pulps are provided in Fig. 4. These micrographs indicate that the different alcohols significantly changed the structure of fibers. More fiber-fiber bonding (due to less porosity) is observed in beaten pulps resulting from fractionation with isopropanol than with other alcohols.
3.6 EDX and chemical composition analysis after fractionation
The EDX analysis is an analytical method used to analyze the structural or chemical properties of a sample. EDX analysis of handsheet samples is presented in Fig. 5. The amounts of residual sulfur in the pulps obtained from ethanol, methanol, isopropanol, and 2-methyl-2-propanol were 1.65%, 3.98%, 2.66%, and 2.65%, respectively. These values in the final handsheets were 1.18, 1.64, 2.35, and 2.04%, respectively. The reason for the decrease of some elements in handsheets in comparison to pulps can be related to the leaching of water-soluble elements. Sharazi et al. (2018) reported that the amount of residual sulfur in pulps obtained from fractionation with methanol was higher. In addition, the amounts of residual sulfur in pulps obtained from fractionation with ethanol and isopropanol were similar. It was reported by You et al. (2017) that 0.9–1.2 and 48–53 g of SO2 per o.d. kg of sugarcane straw is bound to pulp and SEW liquor; these correspond to 0.09–0.15 mol S/mol C9 for pulp and 0.8–0.9 mol S/mol C9 for dissolved lignin, respectively. Iakovlev and van Heiningen (2012b) reported that the amount of sulfur in the pulp obtained from SEW fractionation of spruce (wood meal) and its degree of sulfonation under similar fractionation conditions is 0.068 (% on wood) and 0.122, respectively. Considering the lower amount of residual sulfur in the pulp and paper obtained from the SEW fractionation process, it seems that the efficiency of sulfur recovery in fractionation with ethanol is higher than the other three alcohols.
The chemical composition analysis results of pulps after the SAW fractionation using different solvents, including ethanol, methanol, isopropanol, and 2-methyl-2-propanol are provided in Table 6. Holocellulose (cellulose + hemicelluloses), also shows relatively consistent values across the different solvents, with percentages ranging from 95.64 to 96.46%. This suggests that the holocellulose fraction is largely retained during the SAW fractionation. During SEW fractionation, two phases are involved in removing hemicellulose. In the first phase (called the “initial” phase), about 50–75% of the mannose and xylose are removed from the solid phase, whereas in the second phase (called the “bulk” phase), the removal is much slower and is the first order for mannan and xylan. As a result of the morphology of this residual fraction, glucomannan and xylan were removed at a lower rate during the second phase (Iakovlev 2011). During the SAW process, there are no carbohydrate peeling-off reactions, so cellulose is retained in the pulps. Due to its resistance to acid hydrolysis and the random nature of the hydrolytic cleavage reaction, cellulose is believed to be retained in the pulp. In acidic conditions, protons randomly attack glycosidic bonds along cellulose chains, resulting in a rapid decline in the average polymerization degree (DP). Nevertheless, as the degree of polymerization of the cellulose chains that have been degraded remains around 1000, the cellulose is preserved in large quantities in the pulp (Iakovlev et al. 2009). There is a slight difference between the four solvents in terms of lignin content, including acid-insoluble lignin (AIL) and acid-soluble lignin (ASL). The AIL content ranges from 3.32 to 4.08%, while ASL is not detected in any of the solvent fractionation methods. This indicates that the SAW process effectively removes the acid-soluble lignin from the pulps. The table also reports the absence of acetone-soluble extractives in the pulps after fractionation with all solvents, indicating the successful removal of these extractives during the SAW fractionation. Additionally, the ash content of the pulps after fractionation ranges from 0.22 to 0.28% across the different solvents, suggesting small change in mineral content.
3.7 Effect of alcohol type on physical and mechanical properties of handsheets
Figure 6 indicates the physical and mechanical properties (air permeability, apparent density, caliper, tensile index, burst index, and tear index) of handsheets. The handsheets obtained from isopropanol fractionation had higher tensile index (61.56 Nm/g), burst index (3.93 kPa.m2/g), and tear index (2.69 mN.m2/g) than those of handsheets obtained from other alcohols (Fig. 6a,b). In addition, the air permeability (Fig. 6c) and the apparent density (Fig. 6d) of handsheets made from isopropanol fractionation were significantly higher than other handsheets. Higher density can be attributed to factors such as greater specific surface area and higher hydrogen bonding or relative bonded area (RBA). The bonding ability of pulps obtained from fractionation with isopropanol alcohol can be confirmed by the results of FESEM (see Sect. 3.3) and caliper results (Fig. 6d).
Table 7 provides a comparative analysis of handsheet properties resulting from SO2-ethanol-water (SEW) pulping processes for various wood and non-wood materials. In this study, specific handsheet properties for Eldar pine are detailed under defined fractionation conditions, while other research focuses on different species and their corresponding handsheet properties. The table outlines specific fractionation conditions, including SO2:ethanol:water ratios, kappa numbers, temperature, cooking time, and freeness, offering a comprehensive overview of the experimental setup. The comparison encompasses results from previous studies, including the current study, Dehghani Firouzabadi and Tatari (2023), Iakovlev et al. (2010), and Tatari et al. (2017). Additionally, spruce and sugarcane bagasse demonstrate distinct handsheet characteristics (tear and burst indexes) under specific SEW fractionation parameters.
Many reasons and explanations can be proposed to improve the properties of paper obtained from SAW fractionation with isopropanol compared to other alcohols. Firstly, considering that the performance of isopropanol is better than other alcohols in lignin removal, cooking time is also effectively reduced. This preserves the inherent properties of the fibers partially because the less the fibers are exposed to the acidic condition of SAW, the less their inherent properties are affected, and their bonding ability is improved. Sharazi and Van Heiningen (2017) and Sharazi et al. (2018) also reported greater solubility of lignin in isopropanol than in ethanol and methanol under the same conditions. Secondly, evidence obtained from FESEM micrographs also confirms the improvement of fiber-fiber bonds in pulps obtained from fractionation with isopropanol compared to other alcohols (see Sect. 3.5). Although parameters such as fiber length and fiber coarseness were not addressed in this study, it seems that the fiber length and fiber coarseness of the obtained pastes were directly influenced by the alcohols used. In a similar study, Iakovlev et al. (2010) reported that internal bond (Scott-bond) strength developed faster during the beating of SEW pulps. This result indicates that SEW pulp has a higher inter-fiber bonding than Kraft with the same beating energy. They also reported that the coarseness of SEW and Kraft pulps is 0.217 and 0.172 mg/m, respectively, which is equivalent to a 26.2% increase in SEW pulps. Dehghani Firouzabadi and Tatari (2023) also reported similar results regarding the mechanical properties of papers obtained from SEW fractionation.
Ethanol and isopropanol can improve the cellulose fiber by selectively removing hemicelluloses and lignin from the pulp, which can improve the physical and chemical properties of fibers. By selectively removing hemicelluloses and lignin from the pulp, ethanol and isopropanol can improve the quality of the resulting cellulose fibers. Pulp brightness can be increased by removing lignin. In contrast, the removal of hemicelluloses can reduce the amount of impurities in the pulp and improve the fibers strength and flexibility. The utilization of ethanol and isopropanol as solvents in the production of pulp and paper can minimize the environmental consequences by reducing the quantity of chemicals necessary for the pretreatment stage (Muurinen 2000). Lignocellulosic materials are quickly and completely impregnated by fractionation liquor with ethanol due to surface tension differences during transport (so-called Marangoni effect) (Iakovlev 2011); this can improve cellulose fiber bonding and paper strength. A pretreatment liquor containing ethanol may be able to protect the β-O-4 substructure from breaking and reduce lignin condensation (Yao et al. 2022).
4 Conclusion
The use of isopropanol in comparison to ethanol accelerated SAW delignification in the 45 to 60% yield range, but methanol and 2-methyl-2-propanol decreased the delignification rate compared to isopropanol. To beat pulps to the same freeness, isopropanol required fewer beating revolutions than other alcohols. Handsheets obtained from fractionation with isopropanol had superior characteristics than other alcohols used. SAW pulps can be recommended for a variety of paper/tissue and viscose production applications based on their fractionation data. In general, it is concluded that the isopropanol has suitable potential for the SAW fractionation process compared to other alcohols.
Data availability
The data provided in this study is available with the corresponding author and can be presented on considerable request.
References
Akgül M, Çöpür Y, Temiz S (2007) A comparison of kraft and kraft-sodium borohydrate brutia pine pulps. Build Environ 42:2586–2590
Anoopkumar AN, Reshmy R, Aneesh EM et al (2023) Progress and challenges of microwave-assisted pretreatment of lignocellulosic biomass from circular bioeconomy perspectives. Bioresour Technol 369:128459
Barman DN, Haque MA, Hossain MM et al (2020) Deconstruction of pine wood (Pinus sylvestris) recalcitrant structure using alkali treatment for enhancing enzymatic saccharification evaluated by Congo red. Waste Biomass Valoriz 11:1755–1764
Dagnino EP, Chamorro ER, Romano SD et al (2013) Optimization of the acid pretreatment of rice hulls to obtain fermentable sugars for bioethanol production. Ind Crops Prod 42:363–368. https://doi.org/10.1016/j.indcrop.2012.06.019
Darwish SS, Hadidi EL, Mansour N M (2013) The effect of fungal decay on ficus sycomorus wood. Int J Conserv Sci 4:271–282
Dehghani Firouzabadi M, Tatari A (2023) SO2-ethanol–water (SEW) and Kraft pulp and paper properties of Eldar pine (Pinus eldarica): a comparison study. Biomass Convers Biorefinery 1–9. https://doi.org/10.1007/s13399-023-03785-x
Fei X, Jia W, Wang J et al (2020) Study on enzymatic hydrolysis efficiency and physicochemical properties of cellulose and lignocellulose after pretreatment with electron beam irradiation. Int J Biol Macromol 145:733–739
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896
Gallio E, Zanatta P, Ribes DD et al (2018) Fourier transform infrared spectroscopy in treated woods deteriorated by a white rot fungus. Maderas Cienc Y Tecnol 20:479–488
Gutierrez S, Mangone F, Vergara P et al (2023) Lignocellulosic biomass pre-treatments by diluted sulfuric acid and ethanol-water mixture: a comparative techno-economic analysis. Bioresour Technol Reports 101514
Hou Y, Wang S, Deng B et al (2023) Selective separation of hemicellulose from poplar by hydrothermal pretreatment with ferric chloride and pH buffer. Int J Biol Macromol 251:126374
Huang C, Ma J, Liang C et al (2018) Influence of sulfur dioxide-ethanol-water pretreatment on the physicochemical properties and enzymatic digestibility of bamboo residues. Bioresour Technol 263:17–24
Huang C, Sun R, Chang H et al (2019) Production of dissolving grade pulp from tobacco stalk through SO2-ethanol-water fractionation, alkaline extraction, and bleaching processes. BioResources 14:5544–5558
Huijgen WJJ, Telysheva G, Arshanitsa A et al (2014) Characteristics of wheat straw lignins from ethanol-based organosolv treatment. Ind Crops Prod 59:85–95
Iakovlev M (2011) SO2-ethanol-water (SEW) fractionation of lignocellulosics. In: Doctoral Dissertation, Aalto University, Finland
Iakovlev M, Van Heiningen A (2011) SO2-ethanol-water (SEW) pulping: I. Lignin determination in pulps and liquors. J Wood Chem Technol 31:233–249
Iakovlev M, van Heiningen A (2012a) Efficient fractionation of spruce by SO2-ethanol-water treatment: closed mass balances for carbohydrates and sulfur. Chemsuschem 5:1625–1637
Iakovlev M, van Heiningen A (2012b) Kinetics of fractionation by SO2–ethanol–water (SEW) treatment: understanding the deconstruction of spruce wood chips. RSC Adv 2:3057–3068
Iakovlev M, Pääkkönen T, Van Heiningen A (2009) Kinetics of SO2-ethanol-water pulping of spruce. Holzforschung 63:779–784
Iakovlev M, Hiltunen E, van Heiningen A (2010) Paper technical potential of spruce SO2-ethanol-water (SEW) pulp compared to kraft pulp. Nord pulp Pap Res J 25:428–433
Iakovlev M, You X, van Heiningen A, Sixta H (2014) SO2–ethanol–water (SEW) fractionation process: production of dissolving pulp from spruce. Cellulose 21:1419–1429
Iakovlev M, Survase S, Hill L et al (2020a) Pilot scale sulfur dioxide-ethanol-water fractionation of recycled wood to sugars, bioethanol, lignin and lignosulfonates: carbohydrate calance. Bioresour Technol 123240
Iakovlev M, Survase S, Segers P et al (2020b) Sulfur dioxide-ethanol-water fractionation platform for conversion of recycled wood to sugars, lignin and lignosulfonates. Bioresour Technol 300:122652
Jahan MS, Rahman MM, Ni Y (2021) Alternative initiatives for non-wood chemical pulping and integration with the biorefinery concept: a review. Biofuels Bioprod Biorefining 15:100–118
Jiménez L, Maestre F, Pérez I (1999) Use of butanol-water mixtures for making wheat straw pulp. Wood Sci Technol 33:97–109
Li J, Li T, Wang Y et al (2023a) Green bio-based aprotic solvents for efficient fractionation of hemicellulose and targeted value addition of lignin. Ind Crops Prod 205:117453
Li R, Zheng Y, Zhao X et al (2023b) Recent advances in biomass pretreatment using biphasic solvent system. Green Chem 25: 2505–2523
Małachowska E, Dubowik M, Lipkiewicz A et al (2020) Analysis of cellulose pulp characteristics and processing parameters for efficient paper production. Sustainability 12:7219
Meenakshisundaram S, Fayeulle A, Leonard E et al (2021) Fiber degradation and carbohydrate production by combined biological and chemical/physicochemical pretreatment methods of lignocellulosic biomass–a review. Bioresour Technol 331:125053
Mertoglu-Elmas G, Ozden O (2019) Alkaline sulfite anthraquinone and methanol (ASAM) pulping of hybrid poplar (P. Deltoides). Fresenius Environ Bull 28:1126–1131
Mertoglu-Elmas G, Gunaydin K, Ozden O (2012) Environmental friendly alkaline sulfite anthraquinone-methonal (ASAM) pulping with Rumex crispus plant extract of woody materials. J Environ Biol 33:941
Moradbak A, Tahir PM, Mohamed AZ et al (2016) Effects of Alkaline Sulfite anthraquinone and methanol pulping conditions on the mechanical and Optical Paper properties of Bamboo (Gigantochloa Scortechinii). BioResources 11:5994–6005
Muurinen E (2000) Organosolv pulping- a review and distillation study related to peroxyacid pulping, Doctoral dissertation, University of Oulu
Oliet M, Garcıa J, Rodrıguez F, Gilarrranz MA (2002) Solvent effects in autocatalyzed alcohol–water pulping: comparative study between ethanol and methanol as delignifying agents. Chem Eng J 87:157–162
Park S, Baker JO, Himmel ME et al (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:1–10
Priyadarshinee R, Kumar A, Mandal T, Dasguptamandal D (2015) Improving the perspective of raw eucalyptus kraft pulp for industrial applications through autochthonous bacterial mediated delignification. Ind Crops Prod 74:293–303
Puntambekar R, Pydimalla M, Dinda S, Adusumalli RB (2016) Characterization of Eucalyptus heartwood and sapwood pulp after kraft cooking. J Indian Acad Wood Sci 13:8–15
Rosu D, Teaca C-A, Bodirlau R, Rosu L (2010) FTIR and color change of the modified wood as a result of artificial light irradiation. J Photochem Photobiol B Biol 99:144–149
Sable I, Grinfelds U, Jansons A et al (2012) Properties of wood and pulp fibers from lodgepole pine (Pinus contorta) as compared to scots pine (Pinus sylvestris). BioResources 7:1771–1783
Sakai K, Uprichard JM (1987) Isopropanol-sulphite pulping studies on radiata pine. Appita 40:193–200
Segal L, Creely JJ, Martin AE Jr, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794
Sharazi AM, Van Heiningen A (2017) Kinetics of sulfur dioxide-alcohol-water (SAW) pulping of sugarcane straw (SCS). TAPPI J 16:314–329
Sharazi AM, van Heiningen ARP, Sumerskii I, Bacher M (2018) Sugarcane straw lignin obtained by sulfur dioxide-alcohol-water (SAW) fractionation: effect of solvent. Ind Crops Prod 115:235–242
Shatalov AA, Pereira H (2008) Arundo donax L. reed: new perspectives for pulping and bleaching. 5. Ozone-based TCF bleaching of organosolv pulps. Bioresour Technol 99:472–478
Socha AM, Plummer SP, Stavila V et al (2013) Comparison of sugar content for ionic liquid pretreated Douglas-fir woodchips and forestry residues. Biotechnol Biofuels 6:1–10
Survase SA, Zebroski R, Bayuadri C et al (2019) Membrane assisted continuous production of solvents with integrated solvent removal using liquid-liquid extraction. Bioresour Technol 280:378–386
Tang W, Ling Z, Huang C, He Y-C (2023) Utilizing cetyltrimethylammonium bromide assisted alkali pretreatment to improve monosaccharide production of wheat straw by intensifying lignin extraction. Fuel 352:129002
Tatari A, Dehghani Firouzabadi M, Saraiyan A, Aryaimonfared M (2017) Comparative study of the characteristics of pulp and paper prepared by Sulfur dioxide-ethanol-water (SEW) and soda from bagasse fiber. J Wood for Sci Technol 24:221–240
Vergara P, García-Ochoa F, Ladero M et al (2019) Liquor re-use strategy in lignocellulosic biomass fractionation with ethanol-water mixtures. Bioresour Technol 280:396–403
Yadollahi R, Dehghani Firouzabadi M, Resalati H et al (2018) SO2–ethanol–water (SEW) and kraft pulping of giant milkweed (Calotropis procera) for cellulose acetate film production. Cellulose 25:3281–3294
Yadollahi R, Dehghani Firouzabadi M, Mahdavi H et al (2019) How properties of cellulose acetate films are affected by conditions of iodine-catalyzed acetylation and type of pulp. Cellulose 26:6119–6132
Yamamoto M, Iakovlev M, van Heiningen A (2011) Total mass balances of SO2-ethanol-water (SEW) fractionation of forest biomass. Holzforschung 65:559–565
Yamamoto M, Iakovlev M, van Heiningen A (2014) Kinetics of SO2-ethanol-water (SEW) fractionation of hardwood and softwood biomass. Bioresour Technol 155:307–313
Yao L, Cui P, Chen X et al (2022) A combination of deep eutectic solvent and ethanol pretreatment for synergistic delignification and enhanced enzymatic hydrolysis for biorefinary process. Bioresour Technol 350:126885
You X, van Heiningen A, Sixta H, Iakovlev M (2017) Sulfur balance of sulfur dioxide-ethanol-water fractionation of sugarcane straw. Bioresour Technol 241:998–1002
Yousefi H, Faezipour M, Nishino T et al (2011) All-cellulose composite and nanocomposite made from partially dissolved micro-and nanofibers of canola straw. Polym J 43:559–564
Yousefi H, Azari V, Khazaeian A (2018) Direct mechanical production of wood nanofibers from raw wood microparticles with no chemical treatment. Ind Crops Prod 115:26–31
Zhou Z, Ouyang D, Liu D, Zhao X (2023) Oxidative pretreatment of lignocellulosic biomass for enzymatic hydrolysis: Progress and challenges. Bioresour Technol 367:128208
Zhu JY, Chai XS, Dhasmana B (1999) Formation of volatile organic compounds (VOCs) during pulping. J pulp Pap Sci 25:256–263
Zoia L, Salanti A, Tolppa EL et al (2017) Valorization of side-streams from a SSF biorefinery plant: wheat straw lignin purification study. BioResources 12:1680–1696
Acknowledgements
The content presented in this article has been extracted from the doctoral dissertation of Mr. Aliasghar Tatari. The authors gratefully acknowledge support from the Gorgan University of Agricultural Sciences and Natural Resources (9723214101).
Funding
Authorship, publication, and/or research performed by the authors were not supported financially.
Author information
Authors and Affiliations
Contributions
AT: Data curation, software, resources, formal analysis, writing the original draft, supervision, and visualization. MDF: Conceptualization, methodology, validation, investigation. AG: Project administration and methodology. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Humans or animals are not the subjects of this study. No animals or humans have been studied in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tatari, A., Firouzabadi, M.D. & Ghasemian, A. SO2-alcohol-water (SAW) fractionation of Eldar pine (Pinus eldarica): effects of alcohol type on pulp and paper properties. Eur. J. Wood Prod. 82, 833–847 (2024). https://doi.org/10.1007/s00107-024-02051-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-024-02051-9