Abstract
Purpose
The changes of pine wood structure were evaluated based on Congo red (CR) adsorption in this study. Cellulose free –OH group act as electron acceptor and CR amino or azo group as electron donor that interacts each other to form H–bonds. This interaction of CR with cellulose is considered in this study to analyze the structural changes of pine wood.
Methods
Pine wood samples were treated with different concentrated NaOH solution at 130 °C for 30 min. CR adsorption of treated samples were assayed in terms of absorbance decrease of CR solution measured by spectrophotometer. Field emission scanning electron microscopy (FE-SEM), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) were used to evaluate the microstructural changes of treated samples. Finally enzymatic saccharification was carried out using commercial cellulase enzyme.
Results
Treatment of pine wood with alkali degrades the intra and/or intermolecular H– bonds of cellulose leading to an exposure of free –OH groups. These free –OH groups bind with CR molecule while in contact with CR solution. Structural change of pine wood caused by different NaOH (0 to 20%) treatment at 130 °C for 30 min was evaluated in terms of CR adsorption. Scanning electron microscopy and Fourier transform infrared spectroscopy studies showed that 10% NaOH treated sample exposed more cellulose fibers compared to other treatments leading to more CR binding. The cellulose crystalline index was increased with increasing NaOH–treatments and lowered by the 20% NaOH–treatment due to degradation of cellusose fibres. Moreover, after 72 h, reducing sugar yield was 76.5% and 70.7% using enzyme loading of 15FP U/g and 30CB U/g from 10% and 20% NaOH treated pine wood samples, respectively. Reducing sugar yield was decreased from samples while treating more than 10% NaOH solution.
Conclusion
10% NaOH treatment can be considered as an effective concentration for pine wood treatment at 130 °C for 30 min. These results suggest that CR approach is supposed to be helpful for selecting the treatment condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
So far we concern, this is the first study to evaluate the structural changes of NaOH treated biomass using CR and treatment condition selection using CR will open up a novel approach for the researcher.
Introduction
The depletion of fossil fuel reserves has led to increasing interest in liquid bio-fuel from renewable biomass [1]. Biomass-based fuels could be generated from lignocellulosic biomass, such as woody crops, agricultural residues, and wastes. Among the lignocellulosic biomass, wood is a suitable benchmark fuel, because there is often obtained high bio-oil yields with wood and the potential of bioethanol production from Jabon wood has been evaluated [2]. During the last decades chemical modification of wood cellulose structure has come into view as it is a promising source of alternative energy such as bioethanol. Wood is the most abundant lignocellulosic resources on the world. Pine are evergreen, coniferous, and fast-growing resinous trees which are widely grown in most temperate regions in relatively dense stands and can be a promising source for lignocelluloses for bioethanol [3]. However, carbohydrates in lignocellulosic residues are found within a strong reticular network [4], therefore pretreatment of lignocelluloses materials are necessary to reduce the recalcitrance by removing its lignin shell for bioethanol production. Due to its resistant structure native lignocelluloses cannot be degraded by cellulase. Therefore, an appropriate pretreatment is needed to improve the accessibility of the cellulose by breaking down its tight and recalcitrant matrix [5]. Several classes of organic solvents and ligninolytic pretreatment using enzyme were reported for swelling of compressed fibers and wood. Compared to other pretreatment methods; alkali pretreatment uses lower temperature and pressure [6,7,8]. However, very few reports have been published regarding the alkali treatment of pinewood. Pretreatment with alkali catalyst hydrolyzes ester bonds that cross-link lignin to hemicellulose making the polysaccharides more accessible to enzymes for hydrolysis [9]. Cellulose is the most abundant biopolymer on earth and it holds great potential as an alternative source of valuable chemicals and fuels. Although the cellulose chain contains numerous H–bonding hydroxyl groups, this polysaccharide is completely insoluble in water under normal conditions [10]. It is crucial to improve the solubility of cellulose for efficient saccharification. Sodium hydroxide is a simple chemical that can swell cellulose in a certain concentration. The dissolution mechanism is that soda hydrates penetrate the amorphous region of cellulose, and destruct the nearby crystalline regions by destroying inter and intra-hydrogen bonds between cellulose molecules [11]. Recently, cellulose microfibril swelling enzyme which enhances cellulose hydrolysis has been isolated from Bacillus sp. AY8 [12]. Moreover, recombinant swollenin had been used for disrupting the H-bonds that caused amorphogenesis of cellulose [13]. In the semicrystalline polymers, dyeing is possible at the amorphous areas and the surfaces of the crystalline phase [14]. Since Congo red (CR) molecule is smaller, about 2.5 nm, than the micropore diameter, therefore it can be absorbed into micropores, macropores, and to the external surfaces of the fibers of wood pulp, microfibrilated cellulose pulp from wood and bacterial cellulose samples. Consequently, CR was used to determine the specific surface area (SSA) of cellulose microfibrils [15]. Besides, CR adsorption technique was used to measure the amount of cellulose on woody biomass [16]. In addition, CR had been useful for the assay of cellulase enzymes for its binding affinity with cellulose [17]. Cellulase degrades the cellulose β (1–4) glycosidic chain. As a consequence, the adsorbed CR released and formed a clear hollow zone on agar media plate [18]. Meanwhile, Haft et al. reported the application of CR for quantitative measurement of cellulose degradation [19].
There are various ways CR may interact with surface of cellulose fibers, for instance: by electrostatic forces through the sulfonic groups, by H–bonding through the azo and amino groups, and by hydrophobic interactions through the conjugated Π-electron system [20]. This dye could be adsorbed on the surface of cellulose by electrostatic interaction between –SO3 groups of CR and –OH groups of cellulose and hydrogen bonding of –NH2 group of CR with ether oxygen atom of cellulose [21]. According to Venkataraman, CR binds mainly to primary alcohol groups of cellulose and does not react with aldehyde groups and H–bond plays an important role in the CR adsorption on cellulose fibers [22, 23].
In particular, cellulose with free –OH group in the amorphous area, generated by degrading the H–bonds of crystalline area, may act as electron acceptor and CR amino or azo group as electron donor to make the H–bonds. This interaction of CR with cellulose through H–bond formation is considered in this study to analyze the structural changes of pine wood caused by alkali treatment. The pine wood is treated with varying concentrations of NaOH at 130 °C for 30 min. The treated samples were characterized in terms of microstructural changes by FTIR and XRD and compared those with untreated one and morphological changes were observed by using SEM. Enzymatic saccharification was also carried out for the treated samples using commercial enzymes to determine the efficiency of CR approach. So far we concern, this is the first study about the structural changes of wood evaluated by CR. Therefore, this study opens up a novel approach for easy determination of treatment condition for biomass using CR.
Materials and Methods
Materials
Commercial chips of pine tree were purchased from local market, Korea. The chips were dried and milled with a Thomas Wiley (model 4) mill and sieves to give particles of 1 mm in diameter and then stored at room temperature before NaOH treatment. The sodium hydroxide is procured from Wako Pure Chemicals Industries, Ltd. (Chuo-ku, Osaka, Japan). Congo red powder is bought from Sigma–Aldrich Chemical (St. Louis, MO, USA). All other chemicals used in this study are analytical grade.
NaOH Treatment of Pine Wood
The treatment was carried out in an autoclave at 130 °C and at 15 psi for 30 min. Pine wood samples were treated with water and different NaOH (2.5%, 5%, 7.5%, 10%, 12.5%, 15%, 17.5% and 20%) solutions at a solid loading of 10% mass concentration. A 100 mL glass bottle with a fitted cap was used as a container for each biomass solution during the treatment. The cooling and ramping time of the autoclave was approximately 15 min. Each treated sample was separated into solid and liquid fractions by using a Buchner funnel and GF-A glass fiber filter (Whatman, USA). The treated samples were washed several times with deionized water until neutral pH had been achieved and stored in plastic bags at room temperature.
Congo Red Adsorption on Treated Materials
For each sample, 1 mg is weighed and kept in test tube. Three replicates of test tubes are prepared for each single treatment. After that, 1 mL of CR stock solution (10 µM) is added in the test tube and the samples are soaked gently. After 5 min, 600 µL of supernatant is taken out and kept in amount of 200 µL in three consecutive micro well in the 96 well plate. The absorbance is measured at 530 nm using a SpectraMaxM3Multi-Mode Microplate reader (Molecular device, USA). The average optical density (OD) of each sample is deducted from the average OD of CR stock solution (10 µM). The difference of the OD between CR stock solution and sample solution is the OD of adsorbed CR.
Field Emission Scanning Electron Microscopy (FE-SEM)
The morphology of natural and treated wood samples was characterized using a field emission scanning electron microscope (FE-SEM). Prior to the FE-SEM evaluation, the treated sample was coated with a thin layer of gold to avoid the sample becoming charged under the electron beam. An FE-SEM-XL-30SFEG instrument (Phillips, Netherlands) was used to obtain surface micrographs of the NaOH treated, water-treated, and raw samples by using an accelerated voltage of 15 kV.
Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR spectra of the samples were recorded from a KBr disc containing 1% finely ground sample using a Vertex 80v powder FTIR spectrometer (Bruker Optics, Germany) in the range of 2000 − 800 cm−1.
X-ray Diffraction (XRD) Analysis
The crystallinity of the untreated and treated ground wood was analyzed by XRD, using a D8 Discover powder X-ray diffractometer with GADDS (Bruker, Germany). The diffraction patterns were measured from 2θ = 10° to 30° in steps of 0.02° with a time interval 2.99928 s using Cu Kα radiation (λ = 0.15418 nm) at 40 kV and 40 mA. The crystallinity index was calculated according to the following formula proposed by Segal et al. [24].
where CrI is the crystallinity index, I002 is the maximum intensity of the I002 peak at 2θ = 22°, and Iam is the intensity at 2θ = 18°.
Chemical Component Analysis
Cellulose, hemicelluloses and lignin contents in wood samples were determined by TAPPI methods [25,26,27]. The extractives of each solid sample was analyzed according to the method described by Lin et al. [28]. This method is adapted from Blasi et al. [29].
Enzymatic Saccharification
The enzymatic hydrolysis was performed in batch system. The total batch volume was 3 mL with enzyme and dry powder of wood samples concentration of 10 mg/mL. The mixture was buffered with 50 mM acetic acid sodium acetate, pH 4.8. Enzymatic hydrolysis was carried out at 50 °C for a fixed time in a reciprocating shaker bath. The reaction was monitored by withdrawing samples from the supernatant after 72 h and measuring release of soluble reducing sugars by the DNS assay using D-glucose as a standard [30]. Total reducing sugars which can form aldehydes or ketones (including disaccharide, monosaccharide) can be measured by the DNS method. Six different types of substrates (raw, water treated, 7.5%, 10%, 12.5% and 20% NaOH treated wood) were investigated for enzymatic hydrolysis using an enzyme loading of 15FP U/g (Celluclast 1.5 L, Novozymes) and 30CB U/g (Novozyme 188, Novozymes). All assays were performed in triplicate. Yield of reducing sugars from wood was calculated as follows [31]:
Statistical Analysis
The effect of alkali concentration on wood samples for lignin and hemicellulose removal and reducing sugar yield were determined using the analysis of variance (ANOVA) method and significant differences of means were compared using the Tukey HSD test at 5% significant level using SPSS software (version 12).
Results and Discussion
Congo Red Adsorption
Figure 1 shows the CR adsorption pattern of raw, water treated and different NaOH treated wood. CR adsorption is increased with increasing the NaOH concentration used in treatment and the highest adsorption was observed at 10% NaOH treated sample. After that increasing the NaOH concentration cause reduction of CR adsorption and the least adsorption was observed at 20% NaOH treated sample. The increase of CR adsorption is due to the fact that, NaOH treatment removes the lignin layer from the wood samples and exposes cellulose. The more lignin is removed the more cellulose will be exposed. As a consequence more CR will get chance to bind with exposed cellulose. However, NaOH concentration more than 10% renders reduced CR adsorption. This might be due to the loss of some cellulose fibers which is evidenced by the SEM images of treated samples (discussed in next sections). Since the accessible areas for dyeing are mainly the amorphous area, the trend of CR adsorption enhancement is due to the increased exposure of free –OH groups on amorphous areas of treated samples [14]. During adsorption, most of the H–bonds occur via the sulfonate, amino, and azo groups of CR and hydroxyl groups of C2 and C6 carbon atom of each glucosyl ring of cellulose [32]. Therefore, the increment of CR adsorption might be due to the enhanced H–bonding of CR with the exposed hydroxyl groups of cellulose.
Morphological Characterization of Pine Wood Treated with NaOH
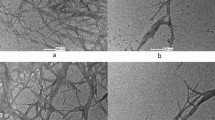
SEM was used to determine changes in surface structure of pine wood after NaOH treatment. We took SEM images of raw pine and 7.5, 10, 12.5, and 20% NaOH treated wood samples. Based on CR adsorption assay, we did not consider all samples because the samples, treated with just below and upper of the 10% NaOH would provide better comparison among different treatments effect on pine wood morphological structure. Figure 2 shows a noticeable change in the different samples morphology. SEM images showed that the surface of untreated wood was smooth due to the coating of cellulose by lignin (Fig. 2a). Smoothness decreased with increasing NaOH concentration as is evidenced by the water treated (Fig. 2b) and 7.5% NaOH treated sample and internal structure was exposed (Fig. 2c). More exposure of internal structure was observed at 10% NaOH treated sample and some hollow region appeared (Fig. 2d). This indicates the removal of lignin from surface of 10% NaOH treated sample. However, further increment of NaOH concentration at 12.5% resulted in degradation of wood surface (Fig. 2e). This implies that exposed cellulose is degraded by higher NaOH concentration. The highest degradation was found at 20% NaOH treated sample (Fig. 2f). This qualitative result supports our CR adsorption result, which showed 10% NaOH treated samples adsorb the highest CR compared to other samples due to its low lignin content and more exposed cellulose. In contrast, due to some degradation of cellulose caused by higher concentration of NaOH (more than 10%), relatively less CR molecules were adsorbed. Wang et al. reported that untreated pinewood had a fibrous structure, while pretreated materials with NaOH and H2SO4 had a loose and porous structure [33].
Chemical Composition Analysis of Pine Wood
In order to determine the effect of chemical composition of the wood samples on CR adsorption the amount of cellulose, hemicellulose, lignin and extractives was determined. The results for the chemical composition are shown in Table 1. The lignin and extractives are higher in raw samples and gradually reduced for samples treated with higher NaOH concentration. The same trend was observed by You et al. [34] who found that, lignin content of the corn stover decreased gradually according to the NaOH pretreatment seviarity and a higher removal rate was achieved at higher NaOH concentration. Chilari et al. also found that about 31.9% lignin was removed from cotton stalks while pretreated at 10% NaOH at 121 °C for 60 min [35]. The removal of lignin was increased significantly (p < 0.05) with increasing concentration of NaOH from 2.5 to 10%. Non significant removal was observed while increasing NaOH concentration from 10 to 12.5%. Lignin acts as a physical barrier, restrict cellulase access to cellulose and, thus, reduce the activity of the enzyme through nonproductive binding [36]. Excessive lignin might be a cause for less amount of CR binding in raw samples while binding was maximum at 10% treated sample, even though 10% treated sample have lignin content greater than 12.5% and 20% treated samples. This is because some cellulose content was degraded in 12.5% and 20% NaOH treated samples, therefore we got lower cellulose content compared to 10% NaOH treated sample (Table 1). Low level of cellulose might be responsible for less CR binding. Our findings are in good agreement with Wang et al. who found that NaOH at higher concentration can dissolve cellulose [11].
Like lignin, hemicellulose content was also reduced with increasing NaOH concentration. The removal of hemicellulose was increased significantly (p < 0.05) with increasing concentration of NaOH from 2.5 to 10%. However, non significant removal was observed while increasing NaOH concentration from 10 to 12.5%. The amorphous and branched structure of hemicellulose is easier to break down than the semicrystalline cellulose, which makes hemicellulose more susceptible to alkali attack. Hemicellulose interacts with cellulose, thus forms a physical barrier to enzyme attack. Effective hemicellulose solubilization can help enhancing digestibility of cellulose that increases enzymatic conversion efficiency during hydrolysis [37]. Therefore, the less the hemicelluloses the more exposed cellulose in biomass. As in 10% NaOH treated sample contains higher cellulose fibers compared to 12.5% and 20% NaOH treated samples where some cellulose are degraded, the highest CR adsorption was found in 10% treated sample. Therefore, 10% NaOH is supposed to be an effective alkali concentration in these treatment conditions for pine tree.
Microstructural Changes of Pine Wood
For better comparison among samples we conducted FTIR and XRD on four samples; raw, water treated, 10% NaOH treated sample (highest CR adsorbing) and 20% NaOH treated sample (lowest CR adsorbing).
Fourier Transform Infrared Spectroscopy (FTIR) of Pine Wood
The structural differences of biomass before and after treatment can be evaluated by Fourier transform infrared spectroscopy [38]. NaOH acts on lignocellulosic biomass and breaks the ester bonds between cellulose and lignin. Therefore, pine wood treated with NaOH are degraded into cellulose and lignin are shown in Fig. 3. This Figure is modified from Soundarrajan et al. [39]. Figure 4 shows the FTIR spectrum for elucidating the chemical changes occur after treating the pine wood samples with NaOH. The modification of the chemical structure and functional group of biomass compositions influences the enzymatic digestibility. The FTIR spectrum of water treated pine wood was similar to the untreated ones, suggesting water treatment hardly decomposed the pine wood. The signals of the ether bond at 1253 cm−1 [40, 41] became weaker, and the peaks for ester bond at 1734 cm−1 [40, 42] were disappeared for 10% and 20% NaOH treated samples. Also the peaks around 1516 cm−1 and 1640 cm−1 represents aromatic ring of lignin and residual adsorbed water (H–O–H) on cellulose [57], respectively, which were found abundantly in raw pine sample and markedly declined after 10% NaOH treatment. This indicated that NaOH treatment might cleave the ether and ester linkages between lignin and carbohydrates of pine wood or reduce the lignin percentage in the biomass. A small sharp band at 896 cm−1 demonstrates the presence of predominant β-glycosidic linkages between the sugar units in cellulose and hemicellulose [43]. The decrease of this peak for NaOH–treated pine suggested the change of linkages between sugar units and intermolecular degradation in the hemicellulose structure. But this band signal is almost similar between 10% and 20% NaOH treated samples suggesting 10% treatment might be sufficient to remove hemicelluloses. Furthermore, sharp stretching at 1163 cm−1 representing a β-(1–4) glucoside linkage was apparent in raw samples and around 1057 cm−1 are associated with typical cellulose were found in all samples [44, 45]. For raw pine sample, these peaks were abundant compared with other treated samples as this sample was not washed and higher intensities might come from free cellulosic fractions [46]. Moreover, the intensity of the peaks around 1373 cm−1 and 1432 cm−1 that are associated with the CH2 vibration of cellulose [31] are higher for 10% NaOH treated sample compared to water and 20% NaOH treated sample. It is important to note that the cellulosic O–H absorbance peak intensity at 1024 cm−1 [47] was also higher for 10% NaOH treated sample compared to water and 20% NaOH treated sample.
More importantly, peaks at 1057 cm−1 are more pronounced for 10% NaOH treated sample compared to 20% NaOH treated sample suggesting some cellulose fibers might be degraded and washed out. Since more free –OH groups of cellulose are exposed after removal of the lignin, pectin, fat, and hemicelluloses in 10% NaOH–treated samples, the –OH groups invites more Congo red for H–bonding on the cellulose structure and as the cellulose degradation or structural deformations are occurred after 20% NaOH treatment, less free –OH groups of cellulose are observed in the FTIR micrograph [12, 48]. Therefore a reduced CR adsorption was observed in 20% NaOH treated samples compared to 10% NaOH treated samples.
X-ray Diffraction (XRD) Analysis of Pine Wood
The crystallinity of the biomass material has been considered a major factor that affects enzymatic hydrolysis [49]. The crystallinity is influenced by the composition of the biomass because among biomass components, only cellulose is crystalline, while hemicellulose and lignin are both amorphous [36]. Figure 5 represents the X-ray diffraction patterns and the CrI of the raw and treated samples are calculated as shown in Table 2. The CrI is 48% in raw pine wood whereas slightly increases to 49.9% in water treated samples. However, 10% NaOH treated samples show the highest CrI, 53.9% but drops to 43.8% in case of 20% NaOH treated samples. The increase in CrI was due to the removal amorphous lignin and hemicellulose from which in turn increased the proportion of cellulose, resulting in an increase in CrI of the treated samples. Many previous studies have reported that biomass pretreatment could increase the CrI by removing amorphous substances in the biomass, improving enzymatic hydrolysis [50,51,52,53,54,55]. The reduction of CrI in samples treated with more than 10% NaOH is due to the destruction of crystalline structure of cellulose along with removal of lignin and hemicelluloses. The increases of crystallinity of treated samples obtained below 10% NaOH are attributed to the degradation of amorphous hemicellulose and amorphous regions of cellulose [55]. However, the severe decomposition of crystalline cellulose in biomass may occur at 20% NaOH treatment, which causes the decrease of crystallinity. It can also be seen that cellulose amount is also decreased in 20% treated samples (Table 1). It had been observed that there was a slight decrease of total CrI of corn stover after hydrotropic pretreatment, which was associated with the destruction of cellulose [56]. Newman mentioned that the crystalinity index of wood essentially depends on its cellulose content [57]. The increased CrI of NaOH–treated wood suggests that more cellulose became exposed to bind with CR. Therefore we got highest CR adsorption on 10% NaOH treated samples.
Enzymatic Saccharification
Figure 6 shows the reducing sugar yield from raw pine, water treated, 1%, 1.5%, 2% and 2.5% NaOH–treated samples. After 72 h, the raw pine wood exhibited maximum 15.3% reducing sugars yield. However, reducing sugar yield was increased significantly with increasing NaOH concentration and the yield was observed 68% and 76% for 7.5% and 10% NaOH treated samples, respectively. Interestingly, further increment of NaOH concentration caused lower reducing sugar yield amounting 72.7% and 70.8% for 12.5% and 20% treated samples, respectively. This is due to the presence of lower amount of cellulose in higher NaOH treated samples (Table 1). Kuo and Lee reported that cellulose degradation during N-methylmorpholine-N-oxide pretreatment not only reduces the hydrolysis yield of regenerated cellulose, the degraded products may also inhibit the cellulase activity and results in a slower hydrolysis rate [58]. Therefore, 10% NaOH can be considered as a useful alkali concentration in this treatment regime.
Reducing sugar yields from raw, water treated, pine wood treated with different NaOH concentration. All treatments were carried out at 130 °C for 30 min. Each error bar shows the standard deviation of triplicate tests for a sample. Different letters next to the bars indicate significant difference (p < 0.05) from each other
Conclusions
In this study, the changes of pine wood structure were evaluated based on CR adsorption. SEM and FTIR results showed that the 10% NaOH treatment removed amorphous lignin substantially. In addition, the total cellulose content decreased when treatment carried out with more than 10% NaOH, therefore highest amount of cellulose in 10% NaOH treated sample adsorbed more CR. The crystallinity was increased with increasing NaOH concentration. However, there was only insignificant increase in lignin and hemicellulose removal by increasing NaOH concentration from 10 to 12.5%. Moreover, after 72 h reducing sugar yield was the highest for 10% NaOH treated sample. Therefore, it is likely to conclude that 10% NaOH is an effective alkali concentration for pine wood treatment in the mentioned conditions. We cannot say this is an optimization of NaOH treatment for pine wood as we did not consider other treatment temperature and time except 130 °C and 30 min, respectively. Besides, 10% NaOH treatment may not be economical. But this CR approach is supposed to be helpful for the researchers to select the optimum condition for biomass pretreatment.
References
Mohammed, I.Y., Abakr, Y.A., Kazi, F.K., Yusuf, S.: Effects of pretreatments of napier grass with deionized water, sulfuric acid and sodium hydroxide on pyrolysis oil characteristics. Waste Biomass Valoriz. 8, 755–773 (2017)
Wistara, N.J., Pelawi, R., Fatriasari, W.: The effect of lignin content and freeness of pulp on the bioethanol productivity of Jabon wood. Waste Biomass Valoriz. 7, 1141–1146 (2016)
Salehian, P., Karimi, K.: Alkali pretreatment for improvement of biogas and ethanol production from different waste parts of pine tree. Ind. Eng. Chem. Res. 52, 972–978 (2013)
Arenas-Cardenas, P., Lopez-Lopez, A., Moeller-Chavez, G.E., Leon-Becerril, E.: Current pretreatments of lignocellulosic residues in the production of bioethanol. Waste Biomass Valoriz. 8, 161–181 (2017)
Munoz, C., Baeza, J., Freer, J., Mendonca, R.T.: Bioethanol production from tension and opposite wood of Eucalyptus globulus using organosolv pretreatment and simultaneous saccharification and fermentation. J. Ind. Microbiol. Biotechnol. 38, 1861–1866 (2011)
Mantanis, G.I., Young, R.A., Rowell, R.M.: Swelling of compressed cellulose fiber webs in organic liquids. Cellulose 2, 1–22 (1995)
Mantanis, G.I., Young, R.A., Rowell, R.M.: Swelling of wood. Part IV. A statistical model for prediction of maximum swelling of wood in organic liquids. Wood Fiber Sci. 27, 22–24 (1995)
Bilal, M., Asgher, M., Iqbal, H.M.N., Ramzan, M.: Enhanced bioethanol production from old newspapers waste through alkali and enzymatic delignification. Waste Biomass Valoriz. 8, 2271–2281 (2017)
Geng, X., Henderson, W.A.: Pretreatment of corn stover by combining ionic liquid dissolution with alkali extraction. Biotechnol. Bioeng. 109, 84–91 (2012)
Matthews, J.F., Skopec, C.E., Mason, P.E., Zuccato, P., Torget, R.W., Sugiyama, J., Himmel, M.E., Brady, J.W.: Computer simulation studies of microcrystalline cellulose Iβ. Carbohydr. Res. 341, 138–152 (2006)
Wang, Y., Zhao, Y., Deng, Y.: Effect of enzymatic treatment on cotton fiber dissolution in NaOH/urea solution at cold temperature. Carbohydr. Polym. 72, 178–184 (2008)
Haque, M.A., Kim, M.K., Barman, D.N., Kim, M.K., Yun, H.D.: A potential cellulose microfibril swelling enzyme isolated from Bacillus sp. AY8 enhances cellulose hydrolysis. Process Biochem. 50, 807–815 (2015)
Jager, G., Girfoglio, M., Dollo, F., Rinaldi, R., Bongard, H., Commandeur, U., Fischer, R.: Spiess, A.C., Buchs, J.: How recombinant swollenin from Kluyveromyces lactis affects cellulosic substrates and accelerates their hydrolysis. Biotechnol. Biofuels 4, 33 (2011)
Ciovica, S., Vlaic, M., Stanciu, C., Chiuaru, R., Asandei, N.: Some aspects concerning viscose fibers dyeing uniformity. I, staple viscose dyeing uniformity. Cellul. Chem. Technol. 24, 251–261 (1990)
Ougiya, H., Hioki, N., Watanabe, K., Morinaga, Y., Yoshinaga, F., Samejima, M.: Relationship between the physical properties and surface area of cellulose derived from adsorbates of various molecular sizes. Biosci. Biotechnol. Biochem. 62, 1880–1884 (1998)
Lee, S.H., Teramoto, Y., Endo, T.: Enzymatic saccharification of woody biomass micro/nanofibrillated by continuous extrusion process I—effect of additives with cellulose affinity. Bioresour. Technol. 100, 275–279 (2009)
Park, S.R., Cho, S.J., Kim, M.K., Ryu, S.K., Lim, W.J., An, C.L., Hong, S.Y., Kim, J.H., Kim, H., Yun, H.D.: Activity enhancement of Cel5Z from Pectobacterium chrysanthemi PY35 by removing C-terminal region. Biochem. Biophys. Res. Commun. 291, 425–430 (2002)
Cho, K.M., Hong, S.Y., Lee, S.M., Kim, Y.H., Kahng, G.G., Kim, H., Yun, H.D.: A cel44C-man26A gene of endophytic Paenibacillus polymyxa GS01 has multi-glycosyl hydrolases in two catalytic domains. Appl. Microbiol. Biotechnol. 73, 618–630 (2006)
Haft, R.F., Gardner, J., Keating, D.: Quantitative colorimetric measurement of cellulose degradation under microbial culture conditions. Appl. Microbiol. Biotechnol. 94, 223–229 (2012)
Yamaki, S.B., Barros, D.S., Garcia, C.M., Socoloski, P., Oliveira, O.N., Atvars, T.D.Z.: Spectroscopic studies of the intermolecular interactions of congo red and tinopal CBS with modified cellulose fibers. Langmuir 21, 5414–5420 (2005)
Samiey, B., Dargahi, M.R.: Kinetics and thermodynamics of adsorption of congo red on cellulose. Cent. Eur. J. Chem. 8, 906–912 (2010)
Venkataraman, K.:Synthetic dyes. The Chemistry of Synthetic Dyes. 1 (1952). https://doi.org/10.1021/ed029p426.3
Schuurmann, G., Funar-Timofei, S.: Multilinear regression and comparative molecular field analysis (CoMFA) of azo dye – fiber affinities. 2. Inclusion of solution-phase molecular orbital descriptors. J. Chem. Inf. Comput. Sci. 43, 1502–1512 (2003)
Segal, L., Creely, J.J., Martin, A.E., Conrad, C.M.: An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res.J. 29, 786–794 (1959)
TAPPI, Acid-Insoluble Lignin in Wood and Pulp, T222 om-11. TAPPI Standard Methods. TAPPI, Sirsa (2011)
TAPPI, Alpha-, beta- and gamma-cellulose in pulp, T203 cm-99. TAPPI standard methods. TAPPI, Sirsa (1999)
Pentosans in wood and pulp. T 223cm-01 (2001)
Lin, L., Yan, R., Liu, Y., Jiang, W.: In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: cellulose, hemicellulose and lignin. Bioresour. Technol. 101, 8217–8223 (2010)
Blasi, C.D., Signorelli, G., Di Russo, C., Rea, G.: Product distribution from pyrolysis of wood and agricultural residues. Ind. Eng. Chem. Res. 2216–2224 (1999)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Haque, M.A., Barman, D.N., Kang, T.H., Kim, M.K., Kim, J., Kim, H., Yun, H.D.: Effect of dilute alkali pretreatment on structural features and enhanced enzymatic hydrolysis of Miscanthus sinensis at boiling temperature with low residence time. Biosyst. Eng. 114, 294–305 (2013)
Mazeau, K., Wyszomirski, M.: Modelling of Congo red adsorption on the hydrophobic surface of cellulose using molecular dynamics. Cellulose 19, 1495–1506 (2012)
Wang, H., Srinivasan, R., Yu, F., Steele, P., Li, Q., Mitchell, B.: Effect of acid, alkali, and steam explosion pretreatments on characteristics of bio-oil produced from pinewood. Energy Fuels 25, 3758–3764 (2011)
You, Z., Wei, T., Cheng, J.J.: Improving anaerobic codigestion of corn stover using sodium hydroxide pretreatment. Energy Fuels 28, 549–554 (2014)
Chilari, D., Dimos, K., Georgoula, G., Paschos, T., Mamma, D., Louloudi, A., Papayannakos, N., Kekos, D.: Bioethanol production from alkali-treated cotton stalks at high solids loading applying non-isothermal simultaneous saccharification and fermentation. Waste Biomass Valoriz. 8, 1919–1929 (2017)
Jeoh, T., Ishizawa, C.I., Davis, M.F., Himmel, M.E., Adney, W.S., Johnson, D.K.: Cellulase digestibility of pretreated biomass is limited by cellulose accessibility. Biotechnol. Bioeng. 98, 112–122 (2007)
Kabel, M.A., Bos, G., Zeevalking, J., Voragen, A.G.J., Schols, H.A.: Effect of pretreatment severity on xylan solubility and enzymatic breakdown of the remaining cellulose from wheat straw. Bioresour. Technol. 98, 2034–2042 (2007)
Bhatia, L., Johri, S.: FTIR Analysis and optimization of simultaneous saccharification and fermentation parameters for sustainable production of ethanol from peels of Ananas cosmosus by Mucor indicus MTCC 4349. Waste Biomass Valoriz. 7, 427–438 (2016)
Soundarrajan, C., Vennison, J.S., Saraswathi, K., Emmanuel, C.E.S.: Does chip size of the lignocellulosic bagasse influence lignin degradation with NaOH treatment? Biosci. Biotechnol. Res. Asia 8, 765–769 (2011)
Liu, L., Sun, J., Li, M., Wang, S., Pei, H., Zhang, J.: Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl3 pretreatment. Bioresour. Technol. 100, 5853–5858 (2009)
Lu, J., Zhou, P.: Optimization of microwave-assisted FeCl3 pretreatment conditions of rice straw and utilization of Trichoderma viride and Bacillus pumilus for production of reducing sugars. Bioresour. Technol. 102, 6966–6971 (2011)
Sun, X.F., Xu, F., Sun, R.C., Wang, Y.X., Fowler, P., Baird, M.S.: Characteristics of degraded lignins obtained from steam exploded wheat straw. Polym. Degrad. Stab. 86, 245–256 (2004)
Xiao, B., Sun, X.F., Sun, R.: Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym. Degrad. Stab. 74, 307–319 (2001)
Kacurakova, M., Wilson, R.H.: Developments in mid-infrared FT-IR spectroscopy of selected carbohydrates. Carbohydr. Polym. 44, 291–303 (2001)
Sun, X.F., Xu, F., Sun, R.C., Fowler, P., Baird, M.S.: Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr. Res. 340, 97–106 (2005)
Barman, D.N., Haque, M.A., Kang, T.H., Kim, G.H., Kim, T.Y., Kim, M.K., Yun, H.D.: Effect of mild alkali pretreatment on structural changes of reed (Phragmites communis Trinius) straw. Environ. Technol. 35, 232–241 (2014)
Gupta, B.S., Jelle, B.P., Gao, T.: Wood facade materials ageing analysis by FTIR spectroscopy. Proc. Inst. Civ. Eng. Constr. Mater. 168, 219–231 (2015)
Haque, M.A., Akhtar, M., Halilu, A., Yun, H.D.: Validation and extended application of cellulose microfibril swelling enzyme assay method to alkali induced swelling of cellulose. J. Chem. Eng. Bioanal. Chem. 2, 62–69 (2017)
Li, C., Knierim, B., Manisseri, C., Arora, R., Scheller, H.V., Auer, M., Vogel, K.P., Simmons, B.A., Singh, S.: Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour. Technol. 101, 4900–4906 (2010)
Kim, S., Holtzapple, M.T.: Effect of structural features on enzyme digestibility of corn stover. Bioresour. Technol. 97, 583–591 (2006)
Bak, J.S., Ko, J.K., Han, Y.H., Lee, B.C., Choi, I.G., Kim, K.H.: Improved enzymatic hydrolysis yield of rice straw using electron beam irradiation pretreatment. Bioresour. Technol. 100, 1285–1290 (2009)
Barman, D.N., Haque, M.A., Kang, T.H., Kim, M.K., Kim, J., Kim, H., Yun, H.D.: Alkali pretreatment of wheat straw (Triticum aestivum) at boiling temperature for producing a bioethanol precursor. Biosci. Biotechnol. Biochem. 76, 2201–2207 (2012)
Sheikh, M.M.I., Kim, C.H., Park, H.J., Kim, S.H., Kim, G.C., Lee, J.Y., Sim, S.W., Kim, J.W.: Effect of torrefaction for the pretreatment of rice straw for ethanol production. J. Sci. Food Agric. 93, 3198–3204 (2013)
Garmakhany, A.D., Kashaninejad, M., Aalami, M., Maghsoudlou, Y., Khomieri, M., Tabil, L.G.: Enhanced biomass delignification and enzymatic saccharification of canola straw by steam-explosion pretreatment. J. Sci. Food Agric. 94, 1607–1613 (2014)
Zeng, Y., Yang, X., Yu, H., Zhang, X., Ma, F.: Comparative studies on thermochemical characterization of corn stover pretreated by white-rot and brown-rot fungi. J. Agric. Food Chem. 59, 9965–9971 (2011)
Mou, H., Li, B., Fardim, P.: Pretreatment of corn stover with the modified hydrotropic method to enhance enzymatic hydrolysis. Energy Fuels 28, 4288–4293 (2014)
Newman, R.H.: Homogeneity in cellulose crystallinity between samples of Pinus radiata wood. Holzforschung 58, 91–96 (2004)
Kuo, C.H., Lee, C.K.: Enhanced enzymatic hydrolysis of sugarcane bagasse by N-methylmorpholine-N-oxide pretreatment. Bioresour. Technol. 100, 866–871 (2009)
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (03-2010-0250), Republic of Korea. Dhirendra Nath Barman was supported by a scholarship from the BK21 Plus Program, Ministry of Education & Human Resources Development, ROK.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barman, D.N., Haque, M.A., Hossain, M.M. et al. Deconstruction of Pine Wood (Pinus sylvestris) Recalcitrant Structure Using Alkali Treatment for Enhancing Enzymatic Saccharification Evaluated by Congo Red. Waste Biomass Valor 11, 1755–1764 (2020). https://doi.org/10.1007/s12649-018-00547-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-00547-z