Abstract
In this study, for the first time, optimized phenolic compounds and antioxidant properties were derived from the extraction process of Cortex fraxini (CFR) using response surface methodology. Four factors and three levels of Box-Behnken (BBD) were used to study the extraction time (X1), extraction temperature (X2), ethanol concentration (X3), and solvent-material ratio (X4). The analysis of variance (ANOVA) revealed that temperature and solvent-material ratio had a relatively large effect on the response. The optimal combination of phenolic compounds and antioxidant activity was obtained with the optimal conditions of X1 = 1.629 h, X2 = 53.367 ℃, X3 = 60.971% (v/v), and X4 = 45.582 mL/g under ultrasonic assisted extraction (UAE). The experimental parameters were consistent with the predicted values. Caffeic acid, erucic acid, ferulic acid and p-hydroxycinnamic acid and other 19 phenolic acids were characterized by high performance liquid chromatography-mass spectrometry (HPLC–MS). The results of this study indicate that optimization of the extraction process is essential for the accurately determined measurement of phenolic components and antioxidants in CFR for its industrial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cortex fraxini (CFR) is the dried branch bark or dried bark of the bitter toast ash tree, ash tree, sharp-leaved ash tree or persistent ash tree of the family Myrtaceous, which grows on mountain slopes, sparse forests and beside ditches. The trunk, bark and flowers from CFR are all capable of being used as medicinal herbs, but currently the bark is used more extensively. CFR has a long history of use as a traditional Chinese herbal medicine in the treatment of heat relief, drying humidity, eyesight, and dysentery relief. In particular, it possesses multiple bioactivities, including anti-inflammatory (Li et al. 2019), antioxidant (Kaneko et al. 2003), and inhibition of melanin production (Masamoto et al. 2004), and it is frequently used to treat diseases, such as hyperuricemia (Wang et al. 2017), anticoagulant (Liang et al. 2017), atherosclerosis (Wang et al. 2022) and antidiabetic (Prabakaran and Ashokkumar 2012). In addition, CFR was found to possess abundant antioxidant components including coumarins and polyphenolic compounds (Wang et al. 2011; Wu et al. 2007). In a recent study, Wang et al. (2011) showed that the phenolic compounds of CFR significantly inhibit linoleic acid peroxidation more effectively than the same concentration of butylated hydroxytoluene. Meanwhile, Li et al. (2015) reported the polyphenolic compounds of CFR exert neuroprotective effects by inhibiting 6-hydroxydopamine (6-OHDA)-induced reactive oxygen species production in rat pheochromocytoma (PC12) cells. Therefore, the phenolic content of CFR and its antioxidant properties are of interest to study, as it has been little studied so far. Hence, it is a necessity to find a fast, green and efficient extraction method to obtain a large amount of antioxidant flavonoids and polyphenolic compounds from CFR.
In the science of natural product isolation, disruption of plant cell walls is a critical aspect in obtaining natural plant secondary products of nutritional and utilization value. The traditional extraction methods, including maceration, Soxhlet extraction and distillation, have the disadvantages of being time-consuming, high-energy consumption and low extraction yield (Zhou et al. 2017; Savic Gajic et al. 2019), which would not satisfy the requirements of the development of green industry. To overcome the drawbacks of traditional extraction methods, the emphasis was placed on green extraction methods such as microwave (Liu et al. 2015), ultrasonic and supercritical fluid extraction, which are low in energy consumption, short in time, and high in efficiency (Yerena-Prieto et al. 2022). Further, considering both industrial cost and reduction in active substance degradation, the ultrasonic extraction method (UAE) seems to be a more attractive technique for bioactive compounds extraction. UAE is based on the formation of high-frequency ultrasonic waves that are capable of causing cavitation effects through the process of solute expansion and contraction cycles (Abreu-Naranjo et al. 2018). The cell wall of the solid solute is destroyed during the cavitation cycle, while the cavitation effect generates thermal energy to further enhance solvent penetration and mass transfer, improving the efficiency of extraction (Živković et al. 2018; Sharmila et al. 2016). The extraction by using UAE was reported by Yang et al. (2011) for coumarin contents from CFR, which increased the release of active substances from CFR into the solution effectively, reduced the degradation of active substances and improved bioavailability. Similar conclusions were drawn by Fu et al. (2014). Meanwhile, UAE is also commonly used for extraction of flavonoids and polyphenols from different plants, such as apricot (Kasapoğlu et al. 2021), tamarillo fruit (Rohilla and Mahanta 2021), and Cirsium setosum (Wang et al. 2019), which proved that UAE possesses several advantages to be widely accepted in the extraction process. However, there are few reports about effective methods for extracting the flavonoid content and phenolic content of CFR.
In addition, UAE is the process of releasing active substances into the extraction solvent. The content of flavonoids and polyphenolic compounds obtained by UAE is usually affected by various factors, such as extraction time, extraction temperature, solvent-material ratio, frequency, extraction solvent and its concentration, etc. Nevertheless, only the effect of individual parameter changes on the output has been studied without considering the interactions between specific parameters, resulting in the inability to explain the overall effect of the factors on the response (Pandey et al. 2018; Feki et al. 2021; Huang et al. 2021). Thus, there is a requirement to establish multivariate statistical methods to optimize the extraction process parameters. Response surface methodology (RSM) is the most popular multivariate statistical method that can be used to perform, modify, and optimize the independent variables affecting the response in an experiment. RSM is a multifactorial approach to reduce the number of experiments and evaluate multiple parameters and interactions between each other in a single experiment (Wang et al. 2020). At the same time, RSM establishes a mathematical model for prediction based on the statistics of data compatibility with polynomial models (Sekhar et al. 2021). It has been widely used as an optimization method for different extraction experiments of polysaccharides, polyphenols, saponins, alkaloids, etc. (Teng and Choi 2012; Yang et al. 2020; Pandey et al. 2018; Raza et al. 2017). The Box-Behnken design (BBD) is convenient to explore the linear effects, quadratic effects and interaction effects of the extraction process (Feki et al. 2021).
To date, this study is the first attempt to optimize the UAE conditions for total flavonoid content (TFC) and total phenolic content (TPC) in CFR by BBD and to determine the antioxidant activity. RSM was used to investigate the effects and interactions of four factors: extraction time (X1), extraction temperature (X2), the ethanol concentration (X3), and solvent-material ratio (X4). High performance liquid chromatography-mass spectrometry (HPLC–MS) was applied to the qualitative and quantitative analysis of phenolic acids in CFR. This study contributes to further understanding of the industrial applications and pharmacological activity analysis of phenolic compounds in CFR.
2 Materials and methods
2.1 Materials
CFR was purchased from Jiangsu Provincial Hospital of Traditional Chinese Medicine (Nanjing, China). The plant materials were authenticated by Dr. Huang, College of Chemical Engineering, Nanjing Forestry University (Nanjing Forestry University – Herbarium No: 5563). Ferric chloride, ferrous sulfate, sodium carbonate, sodium hydroxide, hydrochloric acid, ethyl acetate and potassium persulfate were purchased from Sinopharm Chemical Reagent Co., Ltd (Ningbo, China). Methanol, acetonitrile, and acetic acid were purchased from ANPEL. Trans-ferulic acid, 4-hydroxybenzoic acid, hydrocinnamic acid, trans-cinnamic acid, vanillic acid, vanillin, gallic acid, phenylalanine, catechin, epicatechin, p-hydroxycinnamic acid, benzoic acid, protocatechualdehyde, 3,4-dihydroxybenzoic acid, caffeic acid, syringic acid, syringaldehyde, 4-hydroxy-3,5-dimethoxycinnamic acid and salicylic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Gallic acid was purchased from Aladdin (Shanghai, China). 2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl (DPPH) was purchased from MACKLIN (Shanghai, China). Potassium ferricyanide and Folin-Ciocalteu's phenol reagent were purchased from local dealers.
2.2 Experimental design
2.2.1 Selection of variables
Different conditions have significant effects on the extraction yield and phytochemical content, such as extraction time, temperature, solvent-material ratio, solvent type, and solvent concentration (Pandey et al. 2018). Generally, acetone, ethyl acetate, methanol, ethanol and deionized water can be selected based on their polarity for the extraction of polyphenolic compounds. Owing to the green security and efficient production of ethanol in the manufacturing process, it was selected as the solvent in this experiment (Celli et al. 2015). According to previous work, bioactive compounds are degraded when ultrasonic power exceeds 100 W (Chen et al. 2018). Therefore, the factors such as extraction temperature, extraction time, ethanol concentration and solvent-material ratio were selected as variables to comprehensively evaluate the extraction yield by analyzing the total flavonoids content (TFC), total phenol content (TPC), antioxidant activity (DPPH) and total reducing power (TRP).
2.2.2 Experimental design
RSM is an extensively used technique in mathematical and statistical research, which allows the design, optimization, and evaluation of extraction processes. BBD model is used for designing, building and interpretation of the experiments. According to the single-factor experiment model, a three-level, four-variable experimental design was adopted to statistically optimize the extraction conditions of polyphenols from CFR.
The BBD model consists of 29 trial runs, including the 24 factors and 5 central points, where the independent variables were extraction time (X1), extraction temperature (X2), ethanol concentration (X3), and solvent-material ratio (X4). The effects of four independent variables on four related responses YTFC, YTPC, YDPPH, YTRP were evaluated by BBD. The response surface to the design experiment is shown in Table 1. The empirical model is obtained through multiple regression analysis to evaluate the data relationship between dependent variables and independent variables. In order to predict the best point, the fitting model adopts the following formula,
where Y is the predictive response variable associated with each three-level combination, and A0 Ai, Aii, and Aij are the regression coefficients of intercept, linear, quadratic term and cross product effect, respectively. Xi and Xj are independent variables. The model evaluates the impact of each independent variable on the response.
2.3 Extraction of CFR
The powdered sample (1 g) of CFR was mixed with diffirent volumes (30~70 mL) of ethanol solution with different concentrations (30~70%) in a 100 mL Erlenmeyer flask and uniformly mixed with a vortex oscillator for 30 s. Subsequently, the mixture was fully extracted at different extraction temperatures (30~70 °C) for different time (0.5~2.5 h) by the UAE method. The variables of extraction temperature, extraction time, solvent-material ratio and ethanol concentration are given in Table 1, respectively. Finally, the mixture was separated by a centrifuge, and the collected supernatants were stored at 4 °C for further use. All experiments were carried out in triplicate to ensure the repeatability of the experiment.
2.4 Total flavonoids content (TFC) analysis
TFC was measured based on the aluminum chloride colorimetric procedure as described by Lučinskaitė et al. (2021). Briefly, a 5 mL volume of each eightfold diluted sample and 0.3 mL sodium nitrite (5%, w/v) were added to the test tube and incubated at room temperature for 6 min. At the end of this period, 0.3 mL aluminum nitrate (10%, w/v) was added to the test tube for additional 6 min at room temperature. Following this, 4 mL sodium hydroxide (4%, w/v) and 0.4 mL deionized water were added and the absorbance spectrum of the mixture was measured at 510 nm after 15 min. TFC was quantified in triplicate.
The TFC was measured by the standard curve of rutin equivalents (mg RE/g; y = 0.2027x − 0.1555, R2 = 0.994),
where x represents the extraction concentration of CFR; V represents the volume of CFR; m represents the mass of CFR.
2.5 Total phenolic content (TPC) analysis
TPC was measured based on the Folin-Ciocalteu procedure with minor modification (Vázquez et al. 2012). Briefly, 0.2 mL of diluted CFR extract solution and 0.4 mL of Folin-Ciocalteu’s reagent were mixed in the dark for 15 min; then 2.5 mL of Na2CO3 solution (20% w/v) was added and mixed evenly, and the resultant mixture was diluted to 10 mL with deionized water and reacted for 1 h in the dark. Finally, the absorbance spectrum of the mixture was measured at 760 nm.
The TPC was measured by the standard curve of gallic acid graph (mg GAE/g; y = 1.0023x + 0.1191, R2 = 0.998),
where x represents the extraction concentration of CFR; V represents the volume of CFR; m represents the mass of CFR.
2.6 Antioxidant activity by DPPH assay
The scavenging activity of polyphenols to DPPH free radicals was measured according to reported methods with some modifications (Jung et al. 2016; Xiao et al. 2021). Briefly, the extracted polyphenolic solution was first diluted to 1.0~5.0 mg/mL by deionized water. Then, 2 mL of DPPH·-ethanolic solution (0.1 mmol/L) was mixed with 0.5 mL of the sample solution. The mixture was incubated at 37 °C for 30 min in the dark prior to absorbance measurement at 517 nm. The scavenging activity to DPPH free radicals was calculated according to the following equation,
where Ai is the absorbance value of the sample with DPPH solution, Aj is the absorbance of the mixture under identical conditions to Ai with distilled water instead of DPPH, A0 replace the sample solution with deionized water under the same conditions as Ai.
2.7 Total reducing power (TRP)
The total reducing power was determined based on a previously reported method with minor modification (Aroso et al. 2017). First, the extracted sample was diluted with deionized water to prepare solutions with different concentrations (1~5 mg/mL). Then, 1 mL of the sample solution was mixed with 2.5 mL phosphate buffer solution (2 mol/L, pH = 6.6) and 2.5 mL of potassium ferricyanide (1% v/w), which was reacted in a water bath at 50 °C for 20 min. 2.5 mL trichloroacetic acid (10% v/w) was added to the cooled mixture and centrifuged. Finally, 5 mL of the supernatant was evenly mixed with 5 mL of deionized water and 1 mL of ferric chloride (0.1% v/w). The absorbance of the mixed solution was measured at 700 nm.
2.8 Optimal condition and verification of the CFR
The optimized variables were kept at maximum value (TFC, TPC, DPPH, TRP) based on the values obtained using RSM. The optimized extraction conditions determine all the responses. The effectiveness of the model will be determined by comparing the experimental value with the predicted value of CV%.
2.9 HPLC–MS analysis of polyphenols composition
Approximately 100 mg of CFR samples were treated with 2 mL of 4 M aqueous NaOH. The mixed solution was hydrolyzed at 40 °C for 2 h in a gas bath with shaking and protection from light. The pH value was adjusted to 2 by adding 4 M aqueous HCl. The mixture was shaken with 2 mL of n-hexane at room temperature for 20 min to remove the n-hexane layer. Ethyl acetate (2 × 2 mL) was used to extract the aqueous layer, and the mixed extracts were concentrated to nearly dry on a rotary evaporator at 35 °C under reduced pressure. Prior to the analysis, the residue was dissolved in 200 μL of 50% methanol/water and transferred to insert-equipped vials.
The polyphenols of CFR composition were characterized according to the reported method with minor modifications (Fu et al. 2021). The sample extracts were analyzed using an UPLC—Orbitrap—MS system (UPLC, Vanquish; MS, QE). The analytical conditions were as follows: UPLC: column, Waters HSS T3 (50*2.1 mm, 1.8 μm); column temperature, 40 ℃; flow rate, 0.3 mL/min; injection volume, 2μL; solvent system, water (0.1% acetic acid): acetonitrile (0.1% acetic acid); gradient program, 90:10 V/V at 0 min, 90:10 V/V at 2.0 min, 40:60 V/V at 6.0 min, 40:60 V/V at 8.0 min, 90:10 V/V at 8.1 min, 90:10 V/V at 12.0 min.
HRMS data were recorded on a Q Exactive hybrid Q–Orbitrap mass spectrometer equipped with a heated ESI source (Thermo Fisher Scientific) utilizing the SIM MS acquisition methods. The ESI source parameters were set as follows: spray voltage, − 2.8 kV; sheath gas pressure, 40 arb; aux gas pressure, 10 arb; sweep gas pressure, 0 arb; capillary temperature, 320 ℃; and aux gas heater temperature, 350 ℃.
2.10 Statistical analysis
All analyses were performed in triplicate, and data were expressed as means ± standard deviation (SD). The Design-Expert software was used for experimental design calculation, data analysis and prediction. The data were analyzed by analysis of variance (ANOVA, Pb 0.05). Comparisons of means between multiple groups were performed using Duncan’s multiple range test. The result from HPLC acquired on the Q-Exactive using Xcalibur 4.1 (Thermo Scientific), and processed using TraceFinder™4.1 Clinical (Thermo Scientific). Quantified data were output into Excel format.
3 Results and discussion
3.1 Fitting the model
The effects of UAE on the phenolic yields and antioxidant properties in CFR were verified by RSM and BBD. The experiments were randomly grouped as shown in Table 2.
ANOVA revealed the idealization of regression equation and significance of the model by high F-value and low p value (p < 0.05). Lack of fit of each model was not significant (p < 0.05), demonstrating the relationship between independent variables and dependent variables in the established model. The values of determination coefficients (R2) for the TFC, TPC, DPPH, TRP were as follows: 0.9754, 0.9507; 0.9126, 0.9286, respectively, the overall R2 value is more than 0.9, close to 1, indicating the significant correlation of the experiment and the predicted value. In addition, the coefficient of variation (CV < 10%) suggests a positive impact on the experimental runs and a high reliability result. The three-dimensional (3D) response surface graphs were generated corresponding to the interaction of each response variable.
3.2 Effect of different variables on the extraction of CFR
3.2.1 Effect of extraction variables on total flavonoid content (TFC)
The model was extremely significant (p < 0.0001) according to the experimental data. Based on the linear effects of ethanol concentration (X3), quadratic effects of temperature (X22), and interaction of temperature \(\times \) solvent-material ratio (X2X4) showed a significant effect (p < 0.05) on the TFC, and solvent-material ratio (X4) and X42 showed an extremely significant effect on the TFC (p < 0.0001) (Table 2). TFC was remarkably major dependent on X4, X3, X2, X32 and extraction time according to the regression coefficient (β). Based on the multiple regression analysis of the experimental data, the prediction model equation using the coefficients shown in Table 2 is
The lack of fitted insignificant value (F = 1.96) indicates that the model fitted the response to the CFR impact variable with good prediction (R2 = 0.9754; Radj2 = 0.9509). The low degree of the coefficient of variation (CV = 5.03%) shows that the experimental data values were of high precision and trustworthiness.
Ultrasound-assisted extraction had a significant effect on the flavonoid content, and the solvent-material ratio had a major role in the flavonoid concentration content (p < 0.0001). The possible reason for this result is based on the mass transfer principle, where a concentration difference is formed between the solid and the solvent, with increasing the solvent-material ratio to enhance the contact area between the solid raw material and the solvent (Živković et al. 2018), and reduce the viscosity of the solution, thereby preventing the solution from reaching saturation in the low concentration state (Hou et al. 2019; Wang and Zhou 2021). Similarly, there was a significant (p < 0.05) positive effect of the ethanol concentration, where the flavonoid compounds in plants had a good affinity with lipophilic substances, and they are stable regardless of their affinity with ethanol/water (Mokrani and Madani 2016), which was also confirmed in the present research.
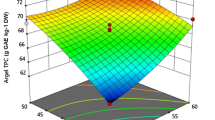
Nonetheless, the interaction between extraction temperature and solvent material (X2X4) illustrated a significant (p < 0.01) effect. The extraction of yield of TFC increased with increasing temperature and solvent material. At lower temperatures and solvent material, the interaction was not observed. However, the interaction of extraction temperature and solvent material increased the TFC as the temperature and the liquid ratio increased as also reported in other research (Sirichan et al. 2022; Zheng et al. 2021). This can be explained by the possibility that the increase in the temperature and the solvent-material ratio contributed to the penetration of the solvent into the plant cells and increased the solubility (Belwal et al. 2016), while the increase in temperature accelerated (Xu et al. 2013) the diffusion rate and led to the increasing extraction yield (Fig. 1).
Interactive effect of extraction variables on total flavonoid content (TFC). Interactive effect of A extraction temperature and extraction time on extraction yields of TFC, B ethanol concentration and extraction time on extraction yields of TFC, C solvent-material ratio and extraction time on extraction yields of TFC, D ethanol concentration and extraction temperature on extraction yields of TFC, E solvent-material ratio and extraction temperature on extraction yields of TFC, F solvent-material ratio and ethanol concentration on extraction yields of TFC
3.2.2 Effect of extraction variables on total phenolic content (TPC)
The model was shown to be highly significant (p < 0.0001) as demonstrated by the experimental data. As shown in Table 2, the linear effects of the solvent-material ratio (X4) illustrated a significant (p < 0.05) effect on TPC, and extraction time (X1), temperature (X2) suggested a highly significant effects(p < 0.01) on TPC, and quadratic effects of temperature (X22) and solvent-material ratio (X42) were extremely significant (p < 0.0001) on the TPC. TPC was greatly dependent on X2, X1X4, X3X4, X4 showing major positive effect according to the regression coefficient (β). Based on the multiple regression analysis of the experimental data, the prediction model equation using the coefficients shown in Table 2 is
The lack of fitted insignificant value (F = 1.01) indicates that the model fitted the response to the CFR impact variable with good prediction (R2 = 0.9507; Radj2 = 0.9013). The low degree of the coefficient of variation (CV = 4.02%) affirms that the experimental data values have a high degree of precision and reliability.
It can be observed that extraction time had a highly significant effect (p < 0.01) on the yield of CFR from the ANOVA. With the increase in extraction time, the cavitation effect and prolonged implosion time in the air bubbles would cause rupture of plant cell walls, and improve the quality transfer of solvent to plant cells (Zemouri-Alioui et al. 2019; Wong et al. 2017). However, excessively long extraction times can lead to degradation of the active substances and reduce the extraction efficiency (Fig. 2), as reported in previous studies (Sharmila et al. 2016; Zhang et al. 2019). Simultaneously, temperature also had a significant effect on the extraction efficiency of total phenols, and the extraction yield could be accelerated by the movement and diffusion of substances (Chen et al. 2018).
Interactive effect of extraction variables on total phenolic content (TPC). Interactive effect of A extraction temperature and extraction time on extraction yields of TPC; B ethanol concentration and extraction time on extraction yields of TPC, C solvent-material ratio and extraction time on extraction yields of TPC, D ethanol concentration and extraction temperature on extraction yields of TPC, E solvent-material ratio and extraction temperature on extraction yields of TPC, F solvent-material ratio and ethanol concentration on extraction yields of TPC
3.2.3 Effect of extraction variables on antioxidant activity and total reducing power (TRP)
The model was verified to be highly significant (p < 0.0001) and reproduced in the experiment by analyzing the experimental data (DPPH, TRP).
For scavenging DPPH free radicals, the linear effects of extraction time (X1), temperature (X2) and the ethanol concentration (X3) showed a significant (p < 0.05) effect on antioxidant activity, and quadratic effects of solvent-material ratio (X42) demonstrated an extremely significant effect (p < 0.0001) on antioxidant activity (Table 2). The optimal positive effect of X3, temperature \(\times \) the ethanol concentration (X2X3), X1 as well as extraction time \(\times \) temperature (X1X2) on antioxidant activity was shown based on the regression coefficient (β). Based on the multiple regression analysis of the experimental data, the prediction model equation using the coefficients shown in Table 2 is
With respect to the TRP, the linear effects of temperature (X2) and solvent-material ratio (X4), the interaction between extraction time \(\times \) the ethanol concentration (X1X3) and the ethanol concentration \(\times \) solvent-material ratio (X3X4) showed a significant (p < 0.05) effect on antioxidant activity, following by quadratic effects of all independent variable revealed a significant effect (p < 0.01) on antioxidant activity (Table 2). The optimal positive effect of X3 on antioxidant activity was shown based on the regression coefficient (β). Based on the multiple regression analysis of the experimental data, the prediction model equation using the coefficients shown in Table 2 is
The lack of fitted insignificant value (FDPPH = 2.05; FTPR = 0.36) indicates that the model fitted the response to the CFR impact variable with good prediction (RDPPH2 = 0.9126, Radj2 = 0.8253; RTRP2 = 0.9286, Radj2 = 0.8571). The experimental data values with high reliability and accuracy are determined from the low degree of the coefficient of variation (CVDPPH = 1.04%; CVTRP = 1.06%).
By contrast, extraction temperature had a positive effect on all reactions. The antioxidant activity was inextricably linked to the TFC and TPC. Phenolic compounds possess phenolic hydroxyl functional groups on the benzene ring, resulting in antioxidant properties that can inhibit or quench free radical reactions based on their hydrogen atom supply capacity, solubility and reducing power of chelating properties (Barreca 2021; Abbas et al. 2017). TRP could be considered as a key parameter of antioxidant performance, by supplying electrons to reactive radicals to terminate free radical chain reactions (Kandasamy and Aradhya 2014).
With increasing temperature, the scavenging ability of radicals gradually increased until the maximum activity was reached. According to the reason pointed out in previous reports (Chotphruethipong et al. 2017; Lin et al. 2020) it is due to the strong correlation between antioxidant activity and TPC, the increase in time and temperature accelerates the solvent flow and extraction speed (Fig. 3), allowing the extraction of TPC with higher content in a short time (Benchikh et al. 2019) (Fig. 4), indirectly improving the scavenging efficiency of DPPH radicals (Yap et al. 2021; Yue et al. 2021).
Interactive effect of extraction variables by DPPH. Interactive effect of A extraction temperature and extraction time on extraction yields of DPPH, B ethanol concentration and extraction time on extraction yields of DPPH, C solvent-material ratio and extraction time on extraction yields of DPPH, D ethanol concentration and extraction temperature on extraction yields of DPPH, E solvent-material ratio and extraction temperature on extraction yields of DPPH, F solvent-material ratio and ethanol concentration on extraction yields of DPPH
Interactive effect of extraction variables by TRP. Interactive effect of A extraction temperature and extraction time on extraction yields of TRP, B ethanol concentration and extraction time on extraction yields of TRP, C solvent-material ratio and extraction time on extraction yields of TRP, D ethanol concentration and extraction temperature on extraction yields of TRP, E solvent-material ratio and extraction temperature on extraction yields of TRP, F solvent-material ratio and ethanol concentration on extraction yields of TRP
3.3 Optimization of extraction conditions and validation
The optimal combination and conditions for the relationship between the independent (extraction time, extraction temperature, ethanol concentration and solvent-material ratio) and dependent variables (TPC, TCF, DPPH and TRP) were determined using response surface and Design-express software. In the optimization of extraction conditions: extraction time is 1.629 h, temperature of 53.364 ℃, ethanol concentration of 60.957% (v/v) and solvent-material ratio of 47.7249.599 mL/g. All experiments were repeated three times under optimal extraction conditions and the assay (Table 3). The TFC and TPC models developed to optimize the extraction of CFR conditions are adapted to this result, so the method is repeatable, precise and reliable.
3.4 Identification and quantification of the chemical composition by HPLC−MS analysis
A total of 19 standards were used in this work which were all detectable in the CFR. In Table 4 and Fig. 5, the type, retention time (RT), mass-to-charge ratio (quan peak) and concentration of each phenolic acid component in CFR are provided. Phenolic acids can be classified into two categories, derived from benzoic and cinnamic acids, respectively (Bicudo et al. 2014). In this study, 19 phenolic acids were determined from the CFR as 12 hydroxybenzoic acid (gallic acid, 3,4-dihydroxybenzoic acid, protocatechualdehyde, catechin, vanillic acid, syringic acid, l-epicatechin, 4-hydroxybenzoic acid, vanillin, syringaldehyde, salicylic acid, benzoic acid) and 7 hydroxycinnamic acid (phe(l-phenylalanine), caffeic acid, p-hydroxycinnamic acid, 4-hydroxy-3,5-dimethoxycinnamic acid, ferulic acid, hydrocinnamic acid, trans-cinnamic acid). Among the phenolic acids identified, the peak 7 at 5.54 min with a negative molecular ion at [MS-H]+ at an m/z of 179.04 showed the fragment identified as caffeic acid (553.31 μg/mg, DW). Caffeic acid as the most abundant phenolic acid in CFR has a highly antioxidant capacity, with previous studies (Dudylina et al., 2019) suggesting that caffeic acid in the bark could be a healthy source of antioxidants. In addition, based on the chromatogram peaks 14 and 15 at 6.67 min, 6.69 min and negative molecular ion at [MS-H]+ at an m/z of 223.06 and 193.05 were identified as 4-Hydroxy-3,5-dimethoxycinnamic acid (62.94 μg/mg, DW) and ferulic acid (80.98 μg/mg, DW), respectively. 4-Hydroxy-3,5-dimethoxycinnamic acid has a high antioxidant capacity equivalent to the caffeic acid (Míguez et al. 2022). Ferulic acid is one of the most common acids extracted from cinnamic acid, and it has been shown that large amounts of ferulic acid can produce a mighty effect on antiproliferative activity (Zduńska et al. 2018). Peaks 10, 3, 4 and 8 were corresponding to p-hydroxycinnamic acid, 3,4-dihydroxybenzoic, protocatechualdehyde and syringic acid, respectively, which were also higher in the CFR, with the content of 26.92, 21.87, 20.35, 10.09 μg/mg. In addition, there are small amounts of phenolic compounds such as gallic acid, phe (l-phenylalanine) catechin, vanillic acid, l-epicatechin, 4-hydroxybenzoic acid, vanillin, syringaldehyde, salicylic acid, benzoic acid, hydrocinnamic acid, and trans-cinnamic acid. This study confirmed that the well-known components which may have potential pharmacological effects in CFR have significant antioxidant effects, and that high concentrations of cinnamic acid derivatives, such as caffeic acid, 4-Hydroxy-3,5-dimethoxycinnamic acid and ferulic acid, conferred stronger antioxidant capacity to CFR.
HPLC–MS chromatograms of CFR phenolic acids: 1-gallic acid; 2-phe (l-phenylalanine); 3–3,4-dihydroxybenzoic acid; 4-protocatechualdehyde; 5-catechin; 6-vanillic acid; 7-caffeic acid; 8-syringic acid; 9-l-epicatechin; 10-p-hydroxycinnamic acid; 11–4-hydroxybenzoic acid; 12-vanillin; 13-syringaldehyde; 14–4-hydroxy-3,5-dimethoxycinnamic acid; 15-ferulic acid; 16-salicylic acid; 17-benzoic acid; 18-hydrocinnamic acid; 19-trans-cinnamic acid
4 Conclusion
In this study, a method to optimize the ultrasound-assisted extraction of phenolic compounds and antioxidant activity from Cortex fraxini was designed. Optimal physicochemical properties were obtained for 4 variables: extraction time, extraction temperature, ethanol concentration, and solvent-material ratio by running 29 randomized experiments with response surface methodology. In the case of optimal total flavonoid and total polyphenol content, the optimal conditions for the four parameters were: extraction time 1.629 h, extraction temperature 53.364 °C, ethanol concentration (v/v) 60.957% and solvent-material 49.599 mL/g. Model validation experimental results agreed with predicted values. The main phenolic compounds in Cortex fraxini are caffeic acid, erucic acid, ferulic acid and p-hydroxycinnamic acid. In any case, the optimized extraction method is advantageous for the extraction and separation of phenolic compounds in Cortex fraxini, which is useful for the pharmaceutical, food and cosmetic fields.
Data availability
Data will be made available on request.
References
Abbas M, Saeed F, Anjum FM, Afzaal M, Tufail T, Bashir MS, Ishtiaq A, Hussain S, Suleria HAR (2017) Natural polyphenols: an overview. Int J Food Prop 20:1689–1699
Abreu-Naranjo R, Arteaga-Crespo Y, Bravo-Sanchez LR, Pérez-Quintana ML, García-Quintana Y (2018) Response surface methodology for optimisation of total polyphenol content and antioxidant activity of extracts from Maytenus macrocarpa bark by means of ultrasound-assisted extraction. Wood Sci Technol 52:1359–1376
Aroso IM, Araújo AR, Fernandes JP, Santos T, Batista MT, Pires RA, Mano JF, Reis RL (2017) Hydroalcoholic extracts from the bark of Quercus suber L. (Cork): optimization of extraction conditions, chemical composition and antioxidant potential. Wood Sci Technol 51:855–872
Barreca D (2021) Mechanisms of plant antioxidants. Action Plants 10:35
Belwal T, Dhyani P, Bhatt ID, Rawal RS, Pande V (2016) Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem 207:115–124
Benchikh Y, Zaoui A, Derbal R, Bachir Bey M, Louaileche H (2019) Optimisation of extraction conditions of phenolic compounds and antioxidant activity of Ruta chalepensis L. using response surface methodology. J Food Meas Charact 13:883–891
Bicudo MOP, Ribani RH, Beta T (2014) Anthocyanins, phenolic acids and antioxidant properties of Juçara fruits (Euterpe edulis M.) along the on-tree ripening process. Plant Food Hum Nutr 69:142–147
Celli GB, Ghanem A, Brooks MS (2015) Optimization of ultrasound-assisted extraction of anthocyanins from haskap berries (Lonicera caerulea L.) using response surface methodology. Ultrason Sonochem 27:449–455
Chen SS, Zeng Z, Hu N, Bai B, Wang HL, Suo YR (2018) Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem 242:1–8
Chotphruethipong L, Benjakul S, Kijroongrojana K (2017) Optimization of extraction of antioxidative phenolic compounds from cashew (Anacardium occidentale L.) leaves using response surface methodology. J Food Biochem 41:e12379
Dudylina AL, Ivanova MV, Shumaev KB, Ruuge EK (2019) Superoxide formation in cardiac mitochondria and effect of phenolic antioxidants. Cell Biochem Biophys 77:99–107
Feki F, Klisurova D, Masmoudi MA, Choura S, Denev P, Trendafilova A, Chamkha M, Sayadi S (2021) Optimization of microwave assisted extraction of simmondsins and polyphenols from Jojoba (Simmondsia chinensis) seed cake using Box-Behnken statistical design. Food Chem 356:129670
Fu S, Zhang J, Li T, Wang S, Ding WJ, Zhao MM, Du YF, Wang Q, Jia J (2014) Multi-responses extraction optimization based on response surface methodology combined with polarity switching HPLC–MS/MS for the simultaneous quantitation of 11 compounds in Cortex Fraxini: application to four species of Cortex Fraxini and its 3 confusable species. J Pharmaceut Biomed 91:210–221
Fu XZ, Wang D, Belwal T, Xu YQ, Li L, Luo ZS (2021) Sonication-synergistic natural deep eutectic solvent as a green and efficient approach for extraction of phenolic compounds from peels of Carya cathayensis Sarg. Food Chem 355:129577
Hou MY, Hu WZ, Wang AS, Xiu ZL, Shi YS, Hao K, Sun X, Cao D, Lu RS, Sun J (2019) Ultrasound-assisted extraction of total flavonoids from Pteris cretica L: process optimization, HPLC analysis, and evaluation of antioxidant activity. Antioxidants 8:425
Huang CB, You CQ, Xiong RH, Wang F (2021) Research progress of natural polysaccharide in the application of biomedical materials. J for Eng 6(3):1–8
Jung JY, Park H, Yang J (2016) Optimization of ethanol extraction of antioxidative phenolic compounds from torrefied oak wood (Quercus serrata) using response surface methodology. Wood Sci Technol 50:1037–1055
Kandasamy S, Aradhya SM (2014) Polyphenolic profile and antioxidant properties of rhizome of commercial banana cultivars grown in India. Food Biosci 8:22–32
Kaneko T, Tahara S, Takabayashi F, Bshizuoka JC, Aredox RRG (2003) Suppression of lipid hydroperoxide-induced oxidative damage to cellular DNA by esculetin. Biol Pharm Bull 26:840–844
Kasapoğlu ED, Kahraman S, Tornuk F (2021) Optimization of ultrasound assisted antioxidant extraction from apricot pomace using response surface methodology. J Food Meas Charact 15:5277–5287
Li JJ, Zhou SY, Zhang H, Lam KH, Lee SM, Yu PH, Chan SW (2015) Cortex fraxini (Qingpi) protects rat pheochromocytoma cells against 6-hydroxydopamine-induced apoptosis. Parkinson’s Disease 2015:11
Li WF, Li WQ, Yu JJ, Liu F, Zhang LL, Xiao X, Zhao JM, Yao Q, Niu XF (2019) Fraxin inhibits lipopolysaccharide-induced inflammatory cytokines and protects against endotoxic shock in mice. Fund Clin Pharmacol 34:91–101
Liang CY, Ju WH, Pei SM, Tang YH, Xiao YD (2017) Pharmacological activities and synthesis of esculetin and its derivatives: a mini-review. Molecules 22:387
Lin DJ, Ma Q, Zhang YW, Peng ZY (2020) Phenolic compounds with antioxidant activity from strawberry leaves: a study on microwave-assisted extraction optimization. Prep Biochem Biotech 50:874–882
Liu ZZ, Gu HY, Yang L (2015) An approach of ionic liquids/lithium salts-based microwave irradiation pretreatment followed by ultrasound-microwave synergistic extraction for two coumarins preparation from Cortex fraxini. J Chromatogr A 1417:8–20
Lučinskaitė I, Laužikė K, Žiauka J, Baliuckas V, Čėsna V, Sirgedaitė-Šėžienė V (2021) Assessment of biologically active compounds, organic acids and antioxidant activity in needle extracts of different Norway spruce (Picea abies (L.) H. Karst) half-sib families. Wood Sci Technol 55:1221–1235
Masamoto Y, Murata Y, Baba K, Shimoishi Y, Tada M, Takahata K, Osaka UOPS, Graduate SONS, Faculty OA, Faculty OP, Okayama U (2004) Inhibitory effects of esculetin on melanin biosynthesis. Biol Pharm Bull 27:422–425
Míguez C, Cancela Á, Álvarez X, Sánchez Á (2022) The reuse of bio-waste from the invasive species Tradescantia fluminensis as a source of phenolic compounds. J Clean Prod 336:130293
Mokrani A, Madani K (2016) Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep Purif Technol 162:68–76
Pandey A, Belwal T, Sekar KC, Bhatt ID, Rawal RS (2018) Optimization of ultrasonic-assisted extraction (UAE) of phenolics and antioxidant compounds from rhizomes of Rheum moorcroftianum using response surface methodology (RSM). Ind Crop Prod 119:218–225
Prabakaran D, Ashokkumar N (2012) Antihyperglycemic effect of esculetin modulated carbohydrate metabolic enzymes activities in streptozotocin induced diabetic rats. J Funct Foods 4:776–783
Raza A, Li F, Xu XQ, Tang J (2017) Optimization of ultrasonic-assisted extraction of antioxidant polysaccharides from the stem of Trapa quadrispinosa using response surface methodology. Int J Biol Macromol 94:335–344
Rohilla S, Mahanta CL (2021) Optimization of extraction conditions for ultrasound-assisted extraction of phenolic compounds from tamarillo fruit (Solanum betaceum) using response surface methodology. J Food Meas Charact 15:1763–1773
Savic Gajic I, Savic I, Boskov I, Žerajić S, Markovic I, Gajic D (2019) Optimization of ultrasound-assisted extraction of phenolic compounds from black locust (Robiniae pseudoacaciae) flowers and comparison with conventional methods. Antioxidants 8:248
Sekhar SC, Karuppasamy K, Kumar MV, Bijulal D, Vedaraman N, Sathyamurthy R (2021) Rain tree (Samanea saman) seed oil: solvent extraction, optimization and characterization. Journal of Bioresources and Bioproducts 6:254–265
Sharmila G, Nikitha VS, Ilaiyarasi S, Dhivya K, Rajasekar V, Kumar NM, Muthukumaran K, Muthukumaran C (2016) Ultrasound assisted extraction of total phenolics from Cassia auriculata leaves and evaluation of its antioxidant activities. Ind Crop Prod 84:13–21
Sirichan T, Kijpatanasilp I, Asadatorn N, Assatarakul K (2022) Optimization of ultrasound extraction of functional compound from making seed by response surface methodology and antimicrobial activity of optimized extract with its application in orange juice. Ultrason Sonochem 83:105916
Teng H, Choi YH (2012) Optimization of extraction of bioactive alkaloids and antioxidants from rhizome coptidis (Coptis chinensis Franch) by using response surface methodology. Food Sci Biotechnol 21:799–806
Vázquez G, Santos J, Freire MS, Antorrena G, González-Álvarez J (2012) Extraction of antioxidants from eucalyptus (Eucalyptus globulus) bark. Wood Sci Technol 46:443–457
Wang Q, Zhu K (2021) Study on the seasonal variation law of the essential oil content and composition of Cupressus lusitanice. J for Eng 6:88–92
Wang K, Zhang Y, Ekunwe SIN, Yi XH, Liu XX, Wang HS, Pan YM (2011) Antioxidant activity and inhibition effect on the growth of human colon carcinoma (HT-29) cells of esculetin from Cortex fraxini. Med Chem Res 20:968–974
Wang Y, Zhao M, Ye H, Shao YZ, Yu YB, Wang M, Zhao CJ (2017) Comparative pharmacokinetic study of the main components of cortex fraxini after oral administration in normal and hyperuricemic rats. Biomed Chromatogr 31:e3934
Wang B, Lv DY, Huang P, Yan F, Liu C, Liu H (2019) Optimization, evaluation and identification of flavonoids in Cirsium setosum (Willd.) MB by using response surface methodology. J Food Meas Charact 13:1175–1184
Wang W, Li XW, Chen K, Yang HY, Jialengbieke B, Hu XD (2020) Extraction optimization, characterization and the antioxidant activities in vitro and in vivo of polysaccharide from Pleurotus ferulae. Int J Biol Macromol 160:380–389
Wang QH, Qin SW, Jiang JG (2022) Improvement effects of esculetin on the formation and development of atherosclerosis. Biomed Pharmacother 150:113001
Wong KH, Li GQ, Li KM, Razmovski-Naumovski V, Chan K (2017) Optimization of Pueraria is flavonoids by response surface methodology using ultrasonic-assisted extraction. Food Chem 231:231–237
Wu CR, Huang MY, Lin YT, Ju HY, Ching H (2007) Antioxidant properties of Cortex fraxini and its simple coumarins. Food Chem 104:1464–1471
Xiao DC, Jin XZ, Song YY, Zhang Y, Li X, Wang F (2021) Enzymatic Acylation of proanthocyanin dimers from Acacia Mearnsii Bark: effect on lipophilic and antioxidant properties. J Bioresour Bioprod 6:359–366
Xu Q, Shen YY, Wang HF, Zhang NP, Xu S, Zhang L (2013) Application of response surface methodology to optimize extraction of flavonoids from fructus sophorae. Food Chem 138:2122–2129
Yang L, Liu Y, Zu YG, Zhao CJ, Zhang L, Chen XQ, Zhang ZH (2011) Optimize the process of ionic liquid-based ultrasonic-assisted extraction of aesculin and aesculetin from Cortex fraxini by response surface methodology. Chem Eng J 175:539–547
Yang BY, Zhang MY, Weng HY, Xu Y, Zeng LH (2020) Optimization of ultrasound assisted extraction (UAE) of kinsenoside compound from Anoectochilus roxburghii (Wall) Lindl by response surface methodology (RSM). Molecules 25:193
Yap JY, Hii CL, Ong SP, Lim KH, Abas F, Pin KY (2021) Quantification of carpaine and antioxidant properties of extracts from Carica papaya plant leaves and stalks. J Bioresourc Bioprod 6:350–358
Yerena-Prieto BJ, Gonzalez-Gonzalez M, Vázquez-Espinosa M, González-de-Peredo AV, García-Alvarado MÁ, Palma M, Rodríguez-Jimenes GDC, Barbero GF (2022) Optimization of an ultrasound-assisted extraction method applied to the extraction of flavonoids from Moringa Leaves (Moringa oleífera Lam). Agronomy 12:261
Yue FX, Lin MS, Qian Y, Lv FC (2021) Recent advances of anti-UV radiation of lignin. J for Eng 6:12–20
Zduńska K, Dana A, Kolodziejczak A, Rotsztejn H (2018) Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol Phys 31:332–336
Zemouri-Alioui S, Bachir Bey M, Kurt BZ, Sonmez F, Louaileche H (2019) Optimization of ultrasound-assisted extraction of total phenolic contents and antioxidant activity using response surface methodology from jujube leaves (Ziziphus jujuba) and evaluation of anticholinesterase inhibitory activity. J Food Meas Charact 13:321–329
Zhang L, Jiang YH, Pang XN, Hua PY, Gao X, Li Q, Li ZC (2019) Simultaneous optimization of ultrasound-assisted extraction for flavonoids and antioxidant activity of Angelica kaiseki using response surface methodology (RSM). Molecules 24:3461
Zheng XX, Zhao YT, Pan B, Wang XZ (2021) Extraction, distribution and composition of tannin in Picea sitchensis. J for Eng 6:108–113
Zhou Y, Zheng J, Gan RY, Zhou T, Xu DP, Li HB (2017) Optimization of ultrasound-assisted extraction of antioxidants from the mung bean coat. Molecules 22:638
Živković J, Šavikin K, Janković T, Ćujić N, Menković N (2018) Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep Purif Technol 194:40–47
Acknowledgements
This work was supported by National Natural Science Foundation of China (Nos. 21774060, 52163002, and 51903235). We also thank Advanced Analysis & Testing Center, Nanjing Forestry University for HPLC-MS characterization.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, D., Wang, J., Li, F. et al. Optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Cortex fraxini using response surface methodology. Eur. J. Wood Prod. 81, 685–697 (2023). https://doi.org/10.1007/s00107-022-01912-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-022-01912-5