Abstract
The selection of appropriate conditions for increasing the extraction efficiency is important. The response surface method (RSM) is a statistical and mathematical technique which it can be used to examine the dependence between the responses with variables in a process and also determine the optimal conditions. In this study, the experimental design of General full factorial (GFF) was used by Minitab 17 software. The variables were time (30, 60 and 90 min), solvent (water, methanol and n-hexane) and method (maceration and ultrasound-assisted extraction). The main effects and also interaction terms were investigated on amounts of total phenolic content (TPC), total flavonoid content (TFC) and the antioxidant activity (according to DPPH, FRAP, IC50 and BCB) of extract of Arenaria hispanica L.. A multiple regression, first degree model was used to express the responses. An analysis of variance (ANOVA) with 95% confidence level was then carried out for each response (TPC, TFC, FRAP, IC50 and BCB) in order to test the model significance and suitability. In optimum conditions, the maceration method was optimum method for TPC, FRAP and BCB, while for TFC was ultrasound-assisted extraction. Water was optimal solvent for TPC, FRAP and BCB, but methanol was obtained as the best solvent for TFC and IC50. Also, the extraction time of TPC was 60 min and for TFC, IC50, FRAP and BCB obtained 30 min. The mounts of TPC, TFC, IC50, FRAP and BCB in the optimum conditions obtained 173.93 ± 0.50 (mg GAE/g extract), 184.27 ± 4.04 (mg QC/g extract), 0.69 ± 0.03 (mg/mL), 734.80 ± 2.02 (mmol Fe2+/g exctract) and 21.23 ± 0.22; respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The application of natural and low-risk antioxidants is an unavoidable necessity. Natural antioxidants increase the strength of antioxidants of plasma. Phenol and total flavonoid are derived from secondary metabolites of plants have the potential to eliminate free radicals (Katalinic et al. 2006; Theriault et al. 2006; Aeschbach et al. 1994).
The antioxidant activity of phenol compounds in plants is mainly due to their oxidation–reduction properties and chemical structure, which plays an important role in neutralizing free radicals.
In recent years it has been proven that free radicals, in addition to undesired organoleptic effects in food products, eliminating vitamins, destroying of essential fatty acids and creating toxic compounds can lead to side effects such as inflammatory diseases, diabetes and decreased immune system(Robards et al. 1988; Benzie 1996; Estevez and Cava 2006; Tamaino et al. 2005; Antolovich et al. 2002).
Therefore, the use of antioxidants is necessary to reduce the rate of oxidation in food materials.There is a great deal of evidence of toxicity and undesirable effects of synthetic antioxidants such as butylatedhydroxytoluene (BHT), butylatedhydroxyanisole (BHA) and tert-butylhydroquinone (TBHQ) (Frankel 1991). Due to this fact, attention is focused on the use of natural antioxidants that extracted from medicinal plants.
Solvent type, physicochemical properties of the solvent, extraction time, agitation speed, the solvent to sample ratio and temperature are important parameters on extraction efficiency. Previous studies have shown that solvent type than other parameters in determination of phenol and flavonoid compounds in different extraction methods is more effective (Cheok et al. 2012).
Also, the standard extraction methods such as soxhlet, microwave-assisted (MA), ultrasound-assisted (UA), supercritical fluid (SF) and accelerated solvent (AS) have a great role in optimizing the quantity and quality of effective compounds of plants. On the other hand, the selection of appropriate conditions for increasing the extraction efficiency is important. The response surface method (RSM) is a statistical and mathematical technique which it can be used to examine the dependence between the responses with variables (dependent and interaction terms) in a process and also the determination the optimal conditions (Chen and Chen 2009).
The caryophyllaceae family have 2000 species with 88 genus. Arenaria hispanica L. from this family is a native of Asia and Europe. It is also found in North America. A. hispanica has flowers with white petals; leaves are simple and have five petals. Due to the lack of studies on Arenaria hispanica L. and the beneficial effects of it, this plant has been investigated.
In this study was used the experimental design of General full factorial (GFF). The variables were time (30, 60 and 90 min), solvent (water, methanol and n-hexane) and method (maceration and ultrasound-assisted extraction). The main effects and also interaction terms were investigated on amounts of total phenolic content (TPC), total flavonoid content (TFC) and the antioxidant activity (according to IC50, FRAP, and BCB) of extract of Arenaria hispanica L. Under optimum conditions, based on the model, the experimental and predicated values for TPC, TFC, IC50, FRAP, and BCB of A. hispanica extract were studied.
Materials and methods
Acetic acid (CH3COOH), butylated hyroxytoluene (C15H24O), methanol (CH3OH), 2,4,6–tripyridyl–s–triazine (C18H12N6), sodium sulphate (Na2SO4), n–hexane (C6H14), iron(II) ammonium sulfate ((NH4)2 Fe(SO4)2.6H2O), acetate potassium (CH3COOK), iron (III) chloride hexahydrate (FeCl3.6H2O), n- hexane, gallic acid (C7H6O5), linoleic acid (C18H32O2), tween 80 (C64H124O26), aluminium chloride (AlCl3) and quercetin (C15H10O7) (Darmstadt, Germany) were purchased all from Merck with high purity. β-carotene was prepared from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of plant
The plant material was collected in April of 2017 from the Baba Amman Mountains of North Khorasan province in Iran. The plant was identified and confirmed by Natural products and Medicinal plants Research center of North Khorasan university of Medical Sciences with voucher number NP23/2-1. The geographical map of the sampling area of Arenaria hispanica L. is shown in Fig. 1.
The aerial organs were dried at 25 °C in the shade. After 4 days, the dried aerial parts were finely grinded. The dried samples were kept in the cold and dry place.
Extraction procedure
Extraction was performed by maceration (M) and ultrasound-assisted (UA) techniques. 10 g of powdered plant were mixed with 100 mL of solvent (H2O, CH3OH and n-hexane). The mixture became homogenous. After than 30, 60 and 90 min, the contents were filtered. In UAE, the homogenous contents were transferred to the ultrasonic water bath (Bandelin Sonorex, Germany, 480 W, 20 kHz) and the extraction action were made at different extraction times (30, 60 and 90 min). In both techniques, the obtained extracts were concentrated using rotary evaporator (RV 05 BASIC 1-B 115 V IKA 8017901). Then, the concentrated extracts were dried and stored at 4 °C.
Result
The total phenolic content (TPC) of A. hispanica extract was determined using the Folin-Ciocalteu method (Singleton and Rossi 1965). For this purpose, the amounts of 1 g of the obtained extracts was dissolved in 1 mL of solvent and shaken after increasing 1.5 mL of sodium carbonate (20% w/w) and 500 μL of Folin-Ciocalteu reagent. Then, the mixture was kept in the dark for 2 h. The absorbance of solutions was measured at 750 nm. The standard calibration curve was plotted using gallic acid solution (0.03–0.22 mg/mL). The results were expressed as mg GAE/g extract.
The total flavonoid content (TFC) of crude extract was determined by the aluminum chloride colorimetric method (Woisky and Salation 1998). To 0.01 g of prepared extract was added 1.5 mL of methanol, 0.1 mL of AlCl3 (10% v/v in ethanol), 0.1 mL of acetate potassium with concentration 1 mol/L and 2.8 mL of H2O. After 30 min at ambient temperature, the absorbance at 415 nm was measured. Quercetin solutions within the range of 10–100 mg/L were used for the plot of calibration curve. The antioxidant capacity was expressed in mg QC/g extract.
3.9 mL of DPPH solution (6 × 10−6 mmol/L) was mixed with 100 μL of the prepared extract. This mixture was shaken and incubated for 30 min at room temperature; the absorbance of solutions was measured using uv–visible spectrophotometer (UVO-2960-shimadzu) at 517 nm. Inhibition percent (I %) was calculated by following equation (Saha et al. 2004):
Ablank and Asample are the absorbance of the blank and sample, respectively. IC50 was calculated from the plot of inhibition percentage against extract concentration using Graph pad prism 7software.
3 mL of FRAP reagent (Benzie and Strain 1999) was mixed with 50 μL of the extract. This mixture was incubated at 37 °C for 20 min and then its absorbance was measured at 593 nm using uv–visible spectrophotometer. Aqueous solutions of FeSO4 in the range of 2 to 10 mg/L were used for the plot of calibration curve. The antioxidant capacity was expressed in mmol Fe2+/g extract.
In this study, 5 mg of β-carotene was dissolved in 10 mL of chloroform. 1 mL of β-carotene solution, 25 μL of linoliec acid and 200 mg of tween 40 were mixed (Tuntachote and Berghofer 2005), then the solvent of this mixture was completely removed by rotary evaporator. Finally, 100 mL of distilled water was added to it and the mixture was emulsified. 2.5 mL of this emulsion was mixed with 350 μL of extract and incubated at 50 °C for 48 h. The absorbance was measured in 490 nm. The antioxidant activity (AA) was calculated using following equation:
A0 and At are the absorbance of the blank and sample solutions at t = 0 min and t = 48 h; respectively.
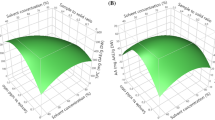
Response surface methodology (RSM) was applied to determine the optimized conditions using the Minitab 17 (Minitab Inc., State College, PA, USA) software. General full factorial design (GFFD) was used to investigate the effect of independent variables (time, solvent and method) and interaction terms on the TPC, TFC, IC50, FRAP and BCB. The independent variables with their corresponding levels (i, j and k) for the experimental design are shown in Table 1.
A multiple regression, first degree model was used to express the responses (Bachcecitapar et al. 2016):
where Yijkn is response in n’th, replicate, β0 is regression coefficients for intercept; βi, βj and βk are linear regression coefficients for main variables (X1, X2 and X3); βij, βik and βjk are regression coefficients for 2-way interactions and finally βijk is regression coefficients for 3-way interactions.
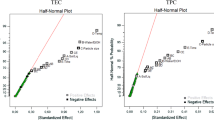
An analysis of variance (ANOVA) with 95% confidence level was carried out for each response (TPC, TFC, IC50, FRAP and BCB) in order to test the model significance and suitability. The significance of independent variables in the form of main effect and interaction terms were analyzed by computing the F-value at probability (p) of 0.001 and 0.05.
The experimental data for TPC, TFC, IC50, FRAP and BCB of A.hisponica extract under different treatment conditions are shown in Table 2.
Discussion
The results of variance analysis (ANOVA) for extraction variables and regression coefficients on responses are presented in Table 3. As are shown in this Table, the regression parameters of the model, the linear and interaction terms have significant effects. In the linear model, time of 60 min (βi (60)), solvent of H2O (βj (H2O)), solvent of CH3OH (βj (CH3OH)) and maceration method (βk (M)) as the independent variables and in 2-way interactions, solvent of H2O with maceration method (βjk (H2O) (M)) and solvent of CH3OH with maceration method (βjk (CH3OH) (M)) were highly significant (p < 0.001) on the TPC. Also, in 3-way interactions, all of interactions were significant on TPC response. Based on Table 3, the effect of solvent and the interaction of solvent with the method has been more effective than other variables on the TPC value and the highest amount of TPC was observed by the maceration method in water solvent.
The results of variance analysis showed that all of variables (independent and interaction terms) were significant (p < 0.001 and p ≤ 0.05) on the amount of TFC, IC50 and FRAP (Table 3).
In BCB, the coefficients for linear and interaction terms were significant (p < 0.001 and p ≤ 0.05) except time of 60 in maceration method (βin (60) (M)) and interaction between methanol solvent with maceration method at 30 min (p ≥ 0.05) that were not significant.
Based on results of Table 2, the highest amount for responses was found in H2O (p′ = 10.2) and CH3OH (p′ = 5.1) solvents and the lowest value was observed in n-hexane (p′ = 0.1) solvent that it can be related to increasing the efficiency of extraction for polar and semi-polar compounds such as alkaloids, flavonoids and terpenesin in polar solvents compared with non-polar ones.
Optimum extraction conditions were obtained by maximizing the response of model for TPC, TFC, FRAP and BCB and minimizing of it for IC50 response. The amount of TFC in the methanol solvent obtained higher than water and n-hexane, which can be related to the better extraction of flavonoid aglycones in CH3OH solvent in addition to flavonoid glycones. Less polar solvents were used for extraction of flavonoid aglycones, while more polar ones were used to extract flavonoid glycosides (Dordoevic et al. 2000). Veljkovic et al. (2007) showed that the petroleum ether extracts of S. officinalis contained unpolar compounds (flavonoid aglycones), the aqueous ethanolic (70%V/V) extracts contained polar and unpolar compounds, while the water extracts did not show visible spots of flavonoid aglycones.
Sound waves create mechanical oscillations in a material. Unlike electromagnetic waves, sound waves propagate in a material and produce cycles of expansion and contraction due to effect of cavitation. In expansion mode, the negative pressure in the solvent creates bubbles. As bubbles break down at the material level, their cell wall is degraded and the process of mass transfer into the solvent is made easier and faster. The intensity of the ultrasonic cavitation effect depends on the surface tension, viscosity and vapor pressure of the medium (Chen et al. 2007). By reducing viscosity and surface tension, the effect of cavitation due to decrease density and increased penetration coefficient is more. The values of viscosity and surface tension for the used solvent were found to be 0.59 cP, 2.55 dyn/cm for methanol and 1cP, 72.8 dyn/cm for water. As be seen, viscosity and surface tension of water is higher than methanol; therefore phenomenon of cavitation occurs more in methanol than water solvent. On the other hands, according to the predicted model and the obtained regression coefficient for methanol solvent (βj(CH3OH) = 68.7) (Table 3), the effect of CH3OH solvent is more than other variables for TFC response. Therefore, an increasing the efficiency of TFC for A.Hispanic extract in methanol solvent with ultrasonic method is expected. Falleh et al. (2012) showed that solvent is more effective than extraction time on the amount of of total phenolic content, total flavonoid content and antioxidant activity of Mesembry anthemumedule L.
The results showed that the highest value for antioxidant activity (DPPH, FRAP and BCB) of the extract of A.Hispanic was observed in the maceration method while the maximum amount of TFC was obtained in the UAE method. The solvent extraction process consists of two stages of immersing the plant tissue in the solvent in order to absorb and swell the tissue and then transfer the material from the tissue into the solvent through diffusion and osmosis processes. Ultrasound facilitates both stages of the extraction process(i.e. tissue swelling as well as the removal of compounds from it by creating porosity in the cell wall and improving the diffusion and mass transfer). Therefore, in this method, the efficiency of the extraction process and the speed of the extraction process increase (Vinatoru 2001). Azvanida showed that the choice of extraction method depends on the type of plant, material content, suitability and economic feasibility of the method (Azwanida 2015).
In optimum conditions, water was optimal solvent for TPC, FRAP and BCB, but methanol was obtained for TFC and IC50. Also, the extraction time for TPC, TFC, IC50, FRAP and BCB was 90, 30, 30, 30 and 90 min; respectively. The mounts of TPC, TFC, IC50, FRAP and BCB in the optimum conditions obtained 173.93 ± 0.50 (mg GAE/g extract), 184.27 ± 4.04 (mg QC/g extract), 0.69 ± 0.03 (mg/mL), 734.80 ± 2.02 (mmol Fe2+/g exctract) and 21.23 ± 0.22; respectively. The R2 values for responses (TPC, TFC, IC50, FRAP and BCB) were close to unity, which indicates the compatibility between the experimental and the real data. Also, the comparison R-sq (adj) with R-sq (pred) for the responses (Table 2) and the residual plots in Fig. 2 confirmed the choice of the model.
Conclusion
The results showed that the antioxidant activity of extract of A. hispanica can be related to the phenolic and flavonoid compounds in this plant. Also, the effect of solvent and solvent with extraction method were more important than extraction time (as main effect) and others interaction terms on TPC, TFC, IC50, FRAP and BCB responses.
Despite the reduction in extraction time and solvent consumption in UAE, in the most cases, maceration was selected as optimum method. The results of this study and previous studies showed that the choice of extraction method depends on different factors, such as plant type and target compounds.
References
Aeschbach R, LoEliger J, Scott BC, Murcia A, Butler J, Halliwell B, Aruoma OI (1994) Antioxidant action of thymol, carvacrol, 6- gingerol, zingerone and hydroxytyrosol. Food Chem Toxicol 32:31–36
Antolovich M, Prenzler PD, Patsalides E, Mcdonald S, Robards K (2002) Methods for testing antioxidant activity. Analyst 127:183–198
Azwanida NN (2015) A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants 4:1–6
Bachcecitapar MK, Karadag O, Aktas S (2016) Estimation of sample size and power for general full factorial designs. J Stat Stat Actuar 9:79–86
Benzie IFF (1996) Lipid peroxidation: a review of causes, consequence, measurement and dietary influences. Int J Food Sci Nutr 47:233–261
Benzie IF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method Enzymol. 299:15–27
Chen KN, Chen MJ (2009) Statistical optimization: response surface methodology. In: Erdogdu F (ed) Optimization in food engineering. CRC Press, Boca Raton, pp 115–116
Chen F, Sun Y, Zhao G, Liao X, Hu X, Wu J, Wang Z (2007) Optimization of ultrasound-assisted extraction of anthocyanins in red raspherries and identification of anthocyanins in extract using high- performnce liquid chromatography- mass spectrometry. Ultrason Sonochem 14:767–778
Cheok CY, Chin NL, Yusof YA, Law CL (2012) Extraction of total phenolic content from Garciniamangostana Linn. hull. I. Effects of solvents and uv–vis spectrophotometer absorbance method. Food Bioproc Tech 5:2933–2933
Dordoevic S, Cakic M, Amr S (2000) The extraction of apigenin and luteolin from the sage (Salvia officinalis L.) from Jordan. FU Phys Chem Technol 1:87–93
Estevez M, Cava R (2006) Effectiveness of rosemary essential oil as inhibitor of lipid and protein oxidation: contradictory effects in different types of frankfurters. Meat Sci 72:348–356
Falleh H, Ksouri R, Lucchessi ME, Abdelly C (2012) Ultrasound-assisted extraction: effect of extraction time and solvent power on the levels of polyphenols and antioxidant activity of Mesembryanthemum edule L. Aizoaceae shoots. Trop. J. Pharm. Res. 11:243–249
Frankel EN (1991) Recent advances in lipidoxidation. J Sci Food Agric 54:495–511
Juntachote T, Berghofer E (2005) Antioxidative, properties and stability of ethanolic extracts of Holy Basil and Galangal. Food Chem 92:193–202
Katalinic V, Milos M, Kulisic T, Jukic M (2006) Screening of 70 medical plant extracts for antioxidant capacity and total phenols. Food Chem 94:550–577
Robards K, Kerr A, Patsalides E (1988) Rancidity and its measurement in edible oils and snack foods: a review. Analyst 113:213–224
Saha K, Lajis NH, Israf DA, Hamazah AS, Khozirah S, Khamis S, Syahida A (2004) Evaluation of antioxidant and nitric oxide inhibitory activities of selected Malaysian medicinal plants. J Ethnopharmacol 92:263–267
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Tamaino A, Cimino F, Zimbalatti V, Venuti V, Salfaro V, Pasquale AD, Saija A (2005) Influence of heating on antioxidant activity and chemical composition of some spice essential oil. Food Chem 89:549–554
Theriault M, Caillet S, Kermash S, Lacroix M (2006) Antioxidant, antiradical and at mutagenic activity of phenolic compounds present in maple products. Food Chem 98:490–501
Velickovic DT, Nikolova M, Ivancheva SV, Stojanoviv JB, Veljkovic VB (2007) Extraction of flavonoids from garden (Salvia officinalis L.) and glutinous (Salvia glutinosa L.) sage by ultrasonic and classical maceration. J Serb Chem Soc 72:73–80
Vinatoru M (2001) An owerview of the ultrasonically assisted of bioactive principles from herbs. Ultrason Sonochem 8:303–313
Woisky RG, Salatino A (1998) Analysis of propolis: some parameters and procedures for chemical quality control. J Apic Res 37:99–105
Acknowledgements
The authors gratefully acknowledgment the support of this research by Bojnourd Branch, Islamic Azad University, Bojnourd-Iran.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
Malihe Samadi Kazemi has no conflict of interest. Somayeh Firouzeh has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kazemi, M.S., Firouzeh, S. Evaluation of the extraction process of Arenaria hispanica L. using response surface methodology on amounts of total phenolic content, total flavonoid content and the antioxidant activity. ADV TRADIT MED (ADTM) 22, 325–332 (2022). https://doi.org/10.1007/s13596-020-00539-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-020-00539-2