Abstract
This study reports on the water uptake (WU) and wetting properties of different modified wood materials; furfurylated and N-methylol melamine (NMM) modified Scots pine, and heat-treated (Vacu3 method) Scots pine and beech. All modifications caused a substantial reduction in WU in the longitudinal, tangential and radial directions both after short (24 h) and long contact times (168, 336 h) with a saturated sponge. The water uptake coefficient (w t ) was reduced by approximately 71–89 % in furfurylated wood, with the higher weight percent gain (WPG) providing a slightly greater reduction. The reduction in WU was not found to depend on the NMM solid content. The NMM treatment had the maximum effect on the reduction of tangential w t by 80–84 % and was much smaller in the longitudinal direction (31–68 %). The treatment temperature of 195 °C gave lower WU values than treatment at 210 °C, and the only exception was the radial direction of Scots pine. The longitudinal w t of heat-treated beech represented the highest reduction by 81–89 %, while radial w t was less affected in both species. Sessile drop apparent contact angles for water and diidomethane and corresponding surface energies on planed tangential and radial wood surfaces revealed an increased hydrophobicity and reduced polarity of modified wood. Furfurylated and NMM modified tangential surfaces had a higher increase of apparent contact angles than the radial surfaces but this was not observed in the case of heat treatment. Heat-treated wood showed reduced wetting of surfaces only with water. Apparent contact angles did neither differ with treatment temperature nor with the NMM resin load. The disperse component of surface energy was slightly increased by 20 % maximum in modified wood, while the polar components showed a dramatic decrease by −30 to −90 % with no major differences among treatments and intensities, and between surfaces. The results provide a better understanding of the hygroscopic behaviour of modified wood, which might be useful to predict its adhesion with various polymers such as glues, coatings and paints.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Modification treatments have been studied and applied widely with the aim to improve various technological properties of wood and wood-based products such as dimensional stability, weathering, and durability (Hill 2006). Bulk treatments (e.g. with resins, waxes) by blocking pathways for the water flow and due to the hydrophobic character of the chemicals as well as chemical (e.g. acetylation, furfurylation) or thermal modification by changing the chemical character of wood cell walls due to decreasing of e.g. hydroxyl groups result in reduced water uptake and equilibrium moisture content of wood (Militz 2002; Gsöls et al. 2003; Pétrissans et al. 2003; Mai and Militz 2004; Weigenand et al. 2007; Zhang et al. 2007; Xiao et al. 2010). Although the purpose of the treatments has been achieved, leading thereafter to improved dimensional stability and durability of wood, issues with regard to processing of modified wood such as coating, painting, and gluing might arise due to the more hydrophobic wood surfaces.
The study of wood surface characteristics is of vital interest to understand the effect of various modifications on the adhesion between wood and various polymers such as coatings and glues. According to the wetting or adsorption theory, for example, the less polar and in some cases less porous modified wood surfaces may lead to reduced adhesion due to poorer adhesive wetting of the wood (Hunt et al. 2007).
The wettability of wood can be defined by parameters which describe the molecular polar or non-polar interactions between liquids and solids such as contact angles, surface free energy and work of adhesion (Mantanis and Young 1997; de Meijer et al. 2000; Wålinder and Bryne 2006). Contact angle analysis is a useful tool for estimations of solid–liquid interfacial forces and is performed by using the Wilhelmy, the rising height, and the sessile drop methods (Pétrissans et al. 2003; Bryne and Wålinder 2010). A commonly used technique for investigations on wood is the sessile drop method, which is based on the observation of the profile of a drop deposited on a wood surface (Neumann and Spelt 1996). Limitations associated with the technique include bulk sorption, roughness and porosity of wood, and probe liquid contamination as a result of the wood extractives. The term “apparent contact angle” is preferred because it is a more accurate expression for the measurement of contact angle due to the mentioned finiteness (Bryne and Wålinder 2010).
It is also well-known that swelling and shrinkage of wood has a major effect on the performance of synthetic polymers applied to its surface. Modification techniques reduce the hydrosensitivity of wood substrate and considerably improve its dimensional stability, thus resulting in less wood-polymer debonding (Stamm 1964; Hill 2006). Besides the standard methods used for determining water uptake and water vapour sorption, e.g. submersion in water or moisture sorption tests (Donath et al. 2006; Xie et al. 2011; Ghosh et al. 2013; Pries et al. 2013), the hygroscopic behaviour of modified wood is also evaluated on the basis of water uptake of samples in contact with a saturated sponge (Ghosh et al. 2009; Scholz et al. 2009; Johansson and Kifetew 2010; Xiao et al. 2010). Due to the porous character of wood, the water flows through capillary force in the wood, and also diffuses into the cell walls, and consequently the method used provides estimations of its water uptake behaviour in exterior use as well as of the localisation of chemicals in different morphological regions of modified woody tissues, which may block the fluid flow path (Rowell and Banks 1985).
The objective of this work was to study the wetting phenomena and water uptake of different modified wood materials. As several wood modification technologies are being commercially exploited today and modified wood is increasingly used in combination with other materials such as adhesives, varnishes, paints and coatings, such information could be used to predict the performance of modified wood-polymer systems.
2 Materials and methods
2.1 Wood material and modification methods
The modified wood materials studied were furfurylated Scots pine (Pinus sylvestris L.), melamine treated Scots pine, and heat treated Scots pine and beech (Fagus sylvatica L.). For the modifications, Scots pine (sapwood) and beech boards were used with dimensions 1400 × 100 × 30 mm3 (L × W × T).
Furfurylation was done by Kebony ASA (Norway) within an industrial process with two types of furfuryl alcohol, which are industrially known as Kebony FA 40 and FA 70, with a 65 and 75 % WPG, respectively (Mai 2010).
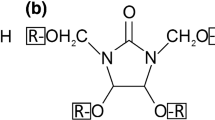
For the melamine treatment, the N-methylol melamine (NMM) resin Madurit MW840/75WA (Ineos Melamines GmbH, Frankfurt, Germany) was supplied as an aqueous stock solution with a solid content of approx. 75 %. Solutions of 10, 20 and 30 % NMM solid content were prepared and impregnation of wood boards was done in a stainless steel vessel using a full cell process, which included an initial vacuum phase of 100 mbar for 1 h and a pressure phase of 12 bar for 2 h. Curing of the NMM resin took place at 120 °C for 1 week in a drying chamber. Heat-treatment was carried with the industrial scale vacuum-press dewatering method (Vacu3) at 195 and 210 °C by Timura Holzmanufaktur GmbH (Germany). In this method, treatment temperature devolves the wood using heating plates enabling a very efficient heat transfer to the lumber, while due to the high vacuum used by-products are condensed at the dryer’s wall and taken out of the system (Niemz 2007). Prior to the testing, all modified wood materials were conditioned at 20 °C and 65 % RH in a climate chamber.
2.2 Water uptake
Water uptake (WU) was measured along the longitudinal direction on samples with dimensions of 20 × 20 × 200 mm3 (R × T × L) and along the tangential and radial directions on samples measuring 40 × 40 × 40 mm3 (R × T × L) according to DIN 52617 (1987). Ten replicates per treatment and direction were used. The samples were sealed with a water resistant coating along all faces except for two (transverse, radial or tangential) to test each time the longitudinal, tangential and radial water uptake. The samples were then placed in a water vessel in contact with a saturated sponge to absorb water through the open faces (Fig. 1). The water level was kept 2 mm above the sponge surface, such that a non-sealed surface of the samples was only partially immersed in water to assess the intensity of water absorption. WU was measured after specific time intervals of 1, 2, 3, 4, 8, 12, 24, 48, 72, 96, 120, 168 and 336 h. In accordance with DIN 52617 (1987), the water uptake coefficient was calculated for every direction by using the equation, \( w_{t } = \Delta W_{t} t^{ - 0.5} \), where w t is the water uptake coefficient (kg m−2 h−0.5), ∆W t is the difference in mass of water uptake per unit area of sample surface (kg m−2) between start and time t; and t is the measuring time (h).
2.3 Contact angle and surface energy
The apparent sessile drop contact angles were measured with the G 10 device (Krüss GmbH, Hamburg, Germany) and DSA 1 software on planed tangential and radial surfaces of wood samples. The probe liquids included water and diiodomethane (dosing volume of 10 μl), and the apparent contact angle value was taken 5 s after droplet deposition. Preliminary tests showed that this period of time was necessary in order to obtain droplet stabilization in both unmodified and modified wood. Furthermore, it has been reported that contact angles measured about 5 s after depositing the water drop on the wood surface minimise the risk of solvent contamination with wood extractives (Wålinder and Johansson 2001). For each sample, 20 representative measurements were performed at different spots to minimise the effects of structural and chemical variations of the wood samples.
There are various methods described in the literature for the calculation of surface energy of solids from contact angle data, mainly using the Young equation as a basis (Żenkiewicz 2007). In the individual methods, various assumptions have been made in finding mathematical relationships to describe quantitatively the phenomena of interfacial interactions in the systems of various liquids and polymeric materials. There appears to be no consensus on the use of the different approaches for surface characterization (Gindl et al. 2001), and thus the surface energy values depend on the method of measurement and analytical computation. In this work, the geometric mean approach developed by Owens–Wendt (1969) was used, which is a commonly used method for calculating the surface energy of wood. The mean values of contact angle measurements were used to calculate the corresponding surface energy by following the Owens–Wendt approach on the basis of Young’s equation, γ S = γ SL + γ L cos θ, where γ S is the surface energy of the solid in mN m−1, γ SL is the interfacial energy between solid and liquid, γ L is the surface tension of the liquid, and θ is the apparent contact angle. In this approach, the total surface energy γ T is considered as a sum of a disperse or non-polar component γ D and a polar component γ P as: γ T = γ D + γ P. A minimum of two measuring liquids is required to determine the dispersed and polar components of surface tension, usually water and diiodomethane. However, the more liquids that are used the more reliable the results become. The surface tension components γ D and γ P for the probe liquids are respectively, 21.8 and 51 mN m−1 for water, and 50.8 and 0 mN m−1 for diiodomethane (van Oss et al. 1990).
3 Results and discussion
3.1 Water uptake
The faster water transport through the axially oriented tracheids of Scots pine and vessel elements of beech caused the highest water uptake (WU) in the longitudinal direction compared to the lateral ones for all modified and unmodified samples (Fig. 2; Table 1). The slightly higher radial WU as compared to the tangential one can be attributed to the presence of rays with large lumens and thin cell walls and open pit structures running along the radial direction, which facilitate the flow of water inside the wood structure (Weigenand et al. 2007; Xiao et al. 2010).
WU was reduced substantially in all directions both after short (24 h) and long submersion times (168, 336 h) by the modifications (Fig. 2; Table 1). For furfurylated wood, the treatment with higher uptake of furfuryl alcohol (FA 70) caused a slightly greater reduction in water uptake in all directions. This reduction effect for higher levels of furfurylation was also shown by Treu et al. (2009) for beech wood. The water uptake coefficient (w t ) was reduced by approximately 84–89 % after 24 h; the values were even high (71–82 %) after 168 and 336 h (Table 1).
As previously reported (Sint 2010), the reduction in water uptake did not follow a certain pattern based on the concentration of the NMM treatment solution. While no differences were noted in the tangential direction among the NMM concentrations, 20 % NMM performed better than the others in the longitudinal direction but it was inferior to 10 and 30 % NMM in the radial direction (Fig. 2). The NMM treatment had the maximum effect on the reduction of tangential w t in all concentrations (80–84 %) already after 24 h (Table 1). These values were comparable to those of radial w t with the exception of 20 % NMM (41–65 %). In contrast, NMM treatment reduced the longitudinal w t after 24 h by only 31–68 %. In the case of 20 and 30 % NMM, the values even declined from 59–68 % to 40–52 % after 336 h. The lower water uptake of NMM treated wood can be explained by the penetration of NMM resin in different morphological regions of wood tissues and cell walls, and by resulting cell wall bulking (Mahnert et al. 2013; Sint et al. 2013; Kielmann et al. 2014). Thus, the available sites in the cell wall to absorb water molecules are reduced. However, the reduction in the velocity of liquid water uptake in the melamine treated wood (Fig. 2) should be attributed mainly to the occlusion of the major penetration pathways for water, e.g. lumens of axial elements (tracheids, vessel elements) and ray cells, by the resin rather than its incorporation in the cell walls.
Heat treatment substantially reduced the WU of wood (Fig. 2), as a result of thermal degradation of wood polymers, mainly destruction and deacetylation of hemicelluloses beginning below 180 °C, and increase of cellulose crystallinity (Militz 2002). Other phenomena such as structural modifications and changes in the chemical structure of lignin, especially when the treatment temperature is above 200 °C, might also play an important role (Alén et al. 2002; Sivonen et al. 2002). Changes in the lignin fraction are mostly registered as depolymerisation of lignin macromolecules, diminishment in the methoxyl content, cleavage of the polysaccharide lignin complexes, and condensation reactions leading to crosslinks within the lignin and possibly between lignin and other wood components (Hill 2006). Changes in the spatial distribution of hydrophobic extractives within the bulk and on the surface of wood after heat treatment (Hakkou et al. 2005; Nuopponen et al. 2003) should also be mentioned. A deviation from the general pattern of reduced WU referred to the higher longitudinal uptake of treated Scots pine than the untreated one at longer submersion times (Fig. 2; Table 1). After the long submersion time of 336 h the increase of longitudinal w t amounted to 7–20 % while previously the short submersion period (24 h) caused a decrease by 23–42 % (Table 1). The phenomenon has previously been observed for Scots pine sapwood at temperatures of 170, 190, and 210 °C compared to untreated material. Water absorption did not decrease until the heat treatment temperature was 230 °C (Metsä-Kortelainen et al. 2006). With the exception of radial uptake of Scots pine, in all other cases the lower treatment temperature of 195 °C gave better results than the treatment at 210 °C. It should be noted that wood defects occurring during heat treatment such as cracking and broken cell walls might also have influenced the results. For example, a collapse of vessels (Boonstra et al. 2006) could explain the substantial reduction in the longitudinal uptake of heat-treated beech (Fig. 2; Table 1). As can be seen in Table 1, this represented the highest reduction in w t (81–89 %) by the heat treatment and was followed by the tangential w t of Scots pine (74–78 %). Radial w t was less affected in both species, and also differences between short and long submersion times were generally small (Table 1).
3.2 Contact angle
Furfurylation generally resulted in a decreased level of wetting force, i.e., an increased apparent contact angle for both probe liquids (Table 2). The highest increase in contact angles was for tangential surfaces. An exception was seen for the radial surface of furfurylated F40 wood which had slightly lower contact angles for water than the unmodified surface. This was also reported by Bryne and Wålinder (2010) and was attributed to the presence of hygroscopic buffering salt agents used in the furfurylation process. The presence of small amounts of unreacted furfuryl alcohol and other by-products of the process might also be responsible for the lower contact angle values (Englund et al. 2009). It should also be noted that the process additives might potentially cause probe liquid contamination and therefore might affect the surface energy properties of furfurylated wood.
A significant increase of hydrophobicity was caused by the NMM treatment and it was independent from the NMM resin load (Table 2). As also observed for furfurylation, the NMM modified tangential surfaces had a higher increase of contact angles than the radial surfaces for both probe liquids. However, the radial surfaces had higher contact angles and thus a lower wetting force.
Heat treatment also affected negatively the wetting of wood surfaces but only for water (Table 2). The migration of extractives at the surface after the heat treatment and the risks associated with the contamination of this probe liquid (Bryne and Wålinder 2010) might be the potential reasons for this result. Specifically, the exudation of mobile hydrophobic substances from the interior to the wood surface causes a minimization of system energy by exposing a more non-polar hydrophobic phase towards the air (Englund et al. 2009). For both species, no major differences were found in contact angles with the treatment temperature (195 and 210 °C) and between the radial and tangential surfaces. However, the contact angles on the tangential surfaces of beech did not change much after heat treatment. In contrast, contact angles for diiodomethane mostly remained unchanged or even decreased in heat-treated wood (e.g. tangential surfaces of beech for both treatment temperatures). A positive effect was only noted for the radial surface of Scots pine treated at 195 °C.
Most literature studies showed a significant increase in wood hydrophobicity after heat treatment. After lab scale heat treatment in the temperature range between 160 and 260 °C, the wood of four European species (pine, spruce, beech and poplar) had a pronounced hydrophobic character with contact angle reaching an average value of 90° (Hakkou et al. 2005). The observations confirmed a previous study using wood of these species treated industrially at 240 °C under inert atmosphere (Pétrissans et al. 2003). Heat treatment of North American white ash and soft maple under gas atmosphere without the presence of oxygen up to 215 °C decreased wood wettability with no differences between the contact angles on tangential and radial surfaces (Kocaefe et al. 2008). The lignin rich surfaces of thermally treated spruce wood were not found to be hydrophobic based on their contact angle data (Bryne and Wålinder 2010).
3.3 Surface energy
All the modifications had a clear impact on the surface energy characteristics leading to a non-polar character of radial and tangential surfaces (Table 3). This is due to an increase in hydrophobicity of wood after modification as a result of chemical changes, which reduce its surface energy (Hill 2006). Thus, the total surface energy was mostly determined by the disperse component of the surface energy. The disperse component of surface energy was slightly increased in modified wood. This effect was more evident for the tangential surfaces (increase up to 15–16 % in heat-treated Scots pine) than the radial surfaces (increase up to 6–7 % in 20 % NMM treated wood, and heat-treated Scots pine and beech at 210 °C). The differences in tangential and radial surface energies may be attributed to differences in wood extractive composition and the diversities in anatomical features in these directions (Nussbaum 1999). The polar components, on the other hand, showed a dramatic decrease ranging from −30 to −90 % with no major differences among treatments and intensities, and between surfaces (radial, tangential). An exception to the above mentioned patterns was observed for disperse and polar components of the radial surface of the F40 modified wood and for the polar component of the tangential surface of the heat-treated beech at 195 °C. The very low surface polarity of modified wood is expected to prevent the formation of attractive polar forces, impair wetting, and accordingly affect negatively the adhesion of glues and paints.
4 Conclusion
The changes in the hygroscopic behaviour and wetting properties of wood caused by various modifications have significant consequences, in particular for the interaction and adhesion of polymers (e.g. glues, paints, coatings). The present analysis of water uptake (WU), apparent contact angle data and surface energy characteristics of furfurylated, NMM modified and heat-treated wood lead to the following conclusions:
-
All modifications resulted in a considerable reduction of WU in the three main directions of wood even after a short contact time (24 h) with a saturated sponge. For furfurylated wood, the higher WPG of furfuryl alcohol had a slightly better effect. The reduction in WU was not found to depend on the concentration of the NMM treatment solution. The NMM treatment had the maximum effect on the tangential WU and the lowest on the longitudinal one. With the exception of radial uptake of Scots pine, heat treatment at 195 °C gave better results than the treatment at 210 °C. The lowest WU caused by the heat treatment was noted longitudinally for beech.
-
Hydrophobicity of wood, as expressed by apparent contact angle data, was increased by the modifications. Some exceptions were observed, mainly for heat-treated wood. The load of furfuryl alcohol and NMM had no effect on contact angles, and so it was for the treating temperatures in the case of heat-treated wood. For both furfurylation and NMM treatment the tangential surfaces showed the highest increase of contact angles, while the temperature had no effect on contact angles between the heat-treated surfaces (radial, tangential).
-
Modifications provided radial and tangential surfaces with a non-polar character due to the vast decrease of the polar components of the surface energy and the slight increase of the disperse ones. No major differences in surface polarity were found for different treatments, intensities, and wood surfaces.
References
Alén R, Kotilainen R, Zaman A (2002) Thermochemical behavior of Norway spruce (Picea abies) at 180–225 °C. Wood Sci Technol 36:163–171
Boonstra MJ, Rijsdijk JF, Sander C, Kegel E, Tjeerdsma B, Militz H, van Acker J, Stevens M (2006) Microstructural and physical aspects of heat treated wood. Part 2. Hardwoods. Maderas Cienc Tecnol 8:209–217
Bryne LE, Wålinder MEP (2010) Ageing of modified wood. Part 1: wetting properties of acetylated, furfurylated, and thermally modified wood. Holzforschung 64(3):295–304
de Meijer M, Haemers S, Cobben W, Militz H (2000) Surface energy determinations of wood: comparison of methods and wood species. Langmuir 16(24):935–939
DIN 52617 (1987) Determination of the water absorption coefficient of construction materials. DIN German Institute for Standardization, Berlin
Donath S, Militz H, Mai C (2006) Creating water-repellent effects on wood by treatment with silanes. Holzforschung 60:40–46
Englund F, Bryne LE, Ernstsson M, Lausmaa J, Wålinder M (2009) Some aspects on the determination of surface chemical composition and wettability of modified wood. In: Proceedings of the 4th European conference on wood modification, Stockholm, pp 553–560
Ghosh S, Militz H, Mai C (2009) The efficacy of commercial silicones against blue stain and mould fungi in wood. Eur J Wood Prod 67:159–167
Ghosh S, Militz H, Mai C (2013) Modification of Pinus sylvestris L. wood with quat- and amino-silicones of different chain lengths. Holzforschung 67(4):421–427
Gindl M, Sinn G, Gindl W, Reiterer A, Tschegg S (2001) A comparison of different methods to calculate the surface free energy of wood using contact angle measurements. Colloids Surf A 181:279–287
Gsöls I, Raetzsch M, Ladner C (2003) Interactions between wood and melamine resins—effect on dimensional stability properties and fungal attack. In: Van Acker J, Hill C (eds) Proceedings first european conference on wood modification. Ghent, Belgium, pp 221–225
Hakkou M, Pétrissans M, El Bakali I, Gérardin P, Zoulalian A (2005) Wettability changes and mass loss during heat treatment of wood. Holzforschung 59:35–37
Hill CAS (2006) Wood modification: chemical, thermal and other processes. Wiley, Chichester
Hunt CG, Brandon R, Ibach RE, Frihart CR (2007) What does bonding to modified wood tell us about adhesion? In: Proceedings 5th COST E34 international workshop: bonding of modified wood. Bled, Slovenia, pp 47–56
Johansson J, Kifetew G (2010) CT-scanning and modelling of the capillary water uptake in aspen, oak and pine. Eur J Wood Prod 68(1):77–85
Kielmann BC, Adamopoulos S, Militz H, Koch G, Mai C (2014) Modification of three hardwoods with an N-methylol melamine compound and a metal-complex dye. Wood Sci Technol 48:123–136
Kocaefe D, Poncsak S, Doré G, Younsi R (2008) Effect of heat treatment on the wettability of white ash and soft maple by water. Holz Roh Werkst 66:355–361
Mahnert K-C, Adamopoulos S, Koch G, Militz H (2013) Topochemistry of heat-treated and N-methylol melamine-modified wood of koto (Pterygota macrocarpa K. Schum.) and limba (Terminalia superba Engl. et. Diels). Holzforschung 67(2):137–146
Mai C (2010) Processes of chemical wood modification (in German). Holztechnologie 51:22–26
Mai C, Militz H (2004) Modification of wood with silicon compounds. Treatment systems based on organic silicon compounds—a review. Wood Sci Technol 37(6):453–461
Mantanis GI, Young RA (1997) Wetting of wood. Wood Sci Technol 31:339–353
Metsä-Kortelainen S, Antikainen T, Viitaniemi P (2006) The water absorption of sapwood and heartwood of Scots pine and Norway spruce heat-treated at 170 °C, 190 °C, 210 °C and 230 °C. Holz Roh Werkst 64:192–197
Militz H (2002) Thermal treatment of wood: European processes and their background. International Research Group on Wood Preservation, IRG/WP 02-40241
Neumann AW, Spelt JK (1996) Applied surface thermodynamics. Marcel Dekker Inc, New York
Niemz P (2007) Thermisch vergütetes Holz in der Schweiz. (Thermally treated wood in Switzerland) (in German). Holz-Zentralblatt 133(40):1102
Nuopponen M, Vuorinen T, Jämsä S, Viitaniemi P (2003) The effect of heat treatment on the behaviour of extractives in softwood studied by FTIR spectroscopic methods. Wood Sci Technol 37:109–115
Nussbaum RM (1999) Natural surface inactivation of Scots pine and Norway spruce evaluated by contact angle measurements. Holz Roh Werkst 57:419–424
Owens DK, Wendt RC (1969) Estimation of the surface free energy of polymers. J Appl Polym Sci 13:1741–1747
Pétrissans M, Gérardin P, El bakali I, Serraj M (2003) Wettability of heat-treated wood. Holzforschung 57(3):301–307
Pries M, Wagner R, Kaesler K-H, Militz H, Mai C (2013) Acetylation of wood in combination with polysiloxanes to improve water-related and mechanical properties of wood. Wood Sci Technol 47:685–699
Rowell RM, Banks WB (1985) Water repellency and dimensional stability of wood. Gen Tech Rep FPL-50. Madison, WI
Scholz G, Krause A, Militz H (2009) Capillary water uptake and mechanical properties of wax soaked Scots pine. In: Proceedings of the 4th European conference on wood modification, Stockholm, pp 209–212
Sint KM (2010) Promoting utilization potential of B. ceiba Linn and B. insigne wall through enhancement of wood quality and technological properties by modification with melamine resin. Sierke Verlag, Thesis Univ. Göttingen
Sint KM, Adamopoulos S, Koch G, Hapla F, Militz H (2013) Impregnation of Bombax ceiba and Bombax insigne wood with a N-methylol melamine compound. Wood Sci Technol 47:43–58
Sivonen H, Maunu SL, Sundholm F, Jamsa S, Viitaniemi P (2002) Magnetic resonance studies of thermally modified wood. Holzforschung 56:648–654
Stamm AJ (1964) Wood and cellulose science. Ronald Press Company, New York
Treu A, Pilgård A, Puttmann S, Krause A, Westin M (2009). Material properties of furfurylated wood for window production. International Research Group on Wood protection, IRG/WP 09-40480
van Oss CJ, Giese RF Jr, Good RJ (1990) Reevaluation of the surface tension components and parameters of polyacetylene from contact angles of liquids. Langmuir 6(11):1711–1713
Wålinder MEP, Bryne LE (2006) Wood adhesion mechanisms: prediction of wood-thermoplastic-water interactions. In: CR Frihart (ed) Wood adhesives 2005. Forest Products Society. Proceedings No 7230, Madison WI, US, pp 385–392
Wålinder MEP, Johansson I (2001) Measurement of wood wettability by the Willhelmy method. Part 1. Contamination of probe liquids by extractives. Holzforschung 55:21–32
Weigenand O, Militz H, Tingaut P, Sèbe G, De Jeso B, Mai C (2007) Penetration of amino-silicone micro- and macro-emulsions into Scots pine sapwood and the effect on water-related properties. Holzforschung 61(1):51–59
Xiao Z, Xie Y, Militz H, Mai C (2010) Effect of glutaraldehyde on water related properties of solid wood. Holzforschung 64(4):483–488
Xie Y, Hill CAS, Xiao Z, Militz H, Mai C (2011) Dynamic water vapour sorption properties of wood treated with glutaraldehyde. Wood Sci Technol 45(1):49–61
Żenkiewicz M (2007) Methods for the calculation of surface free energy of solids. J Achiev Mater Manuf Eng 24:137–145
Zhang Y, Jin J, Wang S (2007) Effects of resin and wax on the water uptake behavior of wood strands. Wood Fiber Sci 39(2):271–278
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bastani, A., Adamopoulos, S. & Militz, H. Water uptake and wetting behaviour of furfurylated, N-methylol melamine modified and heat-treated wood. Eur. J. Wood Prod. 73, 627–634 (2015). https://doi.org/10.1007/s00107-015-0919-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-015-0919-8