Abstract
Purpose
Published results of quality of life (QoL) studies mostly concern whole brain radiotherapy for limited or multiple brain metastases. This prospective multicentre study was designed to compare the QoL of patients with limited (1–3) brain metastases treated with either whole brain (WBRT) or stereotactic radiotherapy (SRT).
Methods
From 01/2007–03/2011, 90 limited brain metastases patients who were previously untreated (n = 77) or had undergone primary surgery (n = 13) were recruited at 14 centres in Germany and Austria. QoL was measured with the EORTC-QLQ-C15-PAL and BN20 brain modules before the start of radiotherapy and after 3 months.
Results
Fifty-two patients (58%) received WBRT and 38 (42%) received SRT. At 3 months, 67 patients (74%) were still living, and 92.6% of the 3‑month survivors completed the second set of questionnaires. Analysis of the QLQ-C15-PAL and BN20 scales revealed significant deterioration in patients treated with WBRT and SRT in physical function (p < 0.001 and p = 0.007), fatigue (p < 0.001 and p = 0.036), nausea (p = 0.003 and p = 0.002), appetite loss (p < 0.001 and p = 0.025), drowsiness (p < 0.001 and p = 0.011), hair loss (p = 0.019 and p = 0.023) and itchy skin (p = 0.030 and p = 0.018). Motor dysfunction (p < 0.001), communication deficits (p = 0.002) and leg weakness (p < 0.001) declined significantly only in patients treated with WBRT. Comparing the two radiotherapy techniques over time, the results showed significant differences in symptom scores for future uncertainty, fatigue and appetite loss.
Conclusions
QoL data as an outcome of the paper should be considered in decision making on the irradiation technique in patients with small number of brain metastases. Larger studies are required to verify the results according to subgroups.
Zusammenfassung
Hintergrund
Bisher publizierte Ergebnisse von Studien zur Lebensqualität (LQ) berücksichtigten überwiegend die Ganzhirnbestrahlung (GHRT) für limitierte und multiple Hirnmetastasen. In dieser prospektiven, multizentrischen Studie wurde die LQ von Patienten mit limitierten (1–3) Hirnmetastasen, die entweder mit GHRT oder stereotaktischer Bestrahlung (SRT) behandelt wurden, verglichen.
Patienten und Methoden
Von 01/2007–03/2011 wurden 90 Patienten mit bisher unbehandelten (n = 77) oder primär chirurgisch versorgten (n = 13) limitierten Hirnmetastasen an 14 Zentren in Deutschland und Österreich rekrutiert. Die LQ wurde mit dem EORTC-QLQ-C15-PAL und dem Hirn-Modul BN20 vor Beginn der Strahlentherapie und 3 Monate danach gemessen.
Ergebnisse
52 Patienten (58 %) erhielten eine GHRT und 38 (42 %) eine SRT. Nach 3 Monaten lebten noch 67 (74 %) Patienten. 92,6 % der 3‑Monats-Überlebenden vervollständigten das zweite Fragebogenset. Die Auswertung bezüglich der QLQ-C15-PAL- und BN20-Skalen zeigten eine signifikante Verschlechterung sowohl bei Patienten mit GHRT als auch mit SRT in der physikalischen Funktion (p < 0,001 und p = 0,007), Fatigue (p < 0,001 und p = 0,036), Übelkeit (p = 0,003 und p = 0,002), Appetitverlust (p < 0,001 und p = 0,025), Schwindel (p < 0,001 und p = 0,011), Haarverlust (p = 0,019 und p = 0,023) und Juckreiz (p = 0,030 und p = 0,018). Die motorische Dysfunktion (p < 0,001), Kommunikationsdefizite (p = 0,002) und Beinschwäche (p < 0,001) verschlechterten sich signifikant nur bei Patienten mit GHRT. Beim Vergleich beider Bestrahlungstechniken im zeitlichen Verlauf, zeigten die Ergebnisse signifikante Unterschiede in den Symptomskalen für Zukunftsangst, Fatigue und Appetitverlust.
Schlussfolgerung
Die Daten zur LQ in dieser Untersuchung können helfen, über die Bestrahlungstechnik bei Patienten mit limitierten Hirnmetastasen zu entscheiden.
Größere Studien sind nötig um die Ergebnisse für einzelne Subgruppen zu verifizieren.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Between 10 and 40% of cancer patients develop brain metastases (BM) [27], and the incidence is increasing [1]. The aim of antineoplastic treatment for such patients is to provide disease control with a good quality of life (QoL). However, in patients with brain metastases, a palliative setting always prevails, suggesting that therapies with a low burden for the patient are preferable. Over the past several years, there has been a shift from treating these patients with whole brain radiotherapy (WBRT) towards applying hypofractionated stereotactic radiotherapy (hfSRT) [19, 35], stereotactic radiosurgery (SRS) [42], novel cytotoxic agents and other targeted therapies [1].
Studies comparing treatment combinations showed that WBRT with SRS resulted in better intracranial and local control but not in better overall survival (OS) than SRS alone [3, 6, 12, 32]. A matched pair analysis showed that treatment outcomes were not significantly different after WBRT and SRS compared with surgery with additional WBRT and local boost radiotherapy. Rades et al. concluded that WBRT and SRS are less invasive than surgery and may be preferable for patients with one or two brain metastases [31]. Churilla et al. showed a similar local control of BM between SRS and surgical resection [10]. A meta-analysis concluded that patients with limited BM have no OS benefit with WBRT plus SRS boost compared with SRS alone [43]. Therefore, SRS alone should be considered a routine treatment option due to favourable neurocognitive outcomes, less risk of late side effects, and no adverse effects on a patient’s performance status [43].
The Radiation Therapy Oncology Group (RTOG) 9508 analysed patients with 1, 2 or 3 BM treated with WBRT and SRS versus WBRT alone. An update of these RTOG analysis with predominantly lung cancer patients shows no survival advantage in the group overall with WBRT and SRS treatment. However, in patients with high Graded Prognostic Assessment (GPA) scores (3.5–4), there is a survival advantage regardless of whether they have 1, 2, or 3 BM. This benefit did not extend to patients with lower GPA scores [37].
Concerns over WBRT regarding limited treatment response, cognitive deficits, neurological deficits and reduced QoL [2, 29] motivate researchers to adopt more focused radiotherapy options, such as SRS or hfSRT [2, 23], in cases of limited BM. To the best of our knowledge, no publication to date has focused on comparing the QoL between patients treated with WBRT and patients treated with stereotactic radiotherapy (SRT). Information on health-related QoL could help physicians, patients and family members make appropriate decisions between the various treatment options. Overall, the number of published articles regarding QoL in patients with BM is limited [40], and most studies report QoL outcomes in patients who have received WBRT [2, 14, 20, 24]. The discussion pertaining to whether patients with limited BM benefit more from WBRT or local radiotherapy with regard to maintained or improved QoL led us to address this question prospectively within a time frame of three months after radiotherapy.
Patients and methods
Recruitment
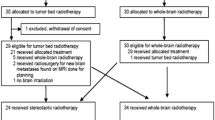
Patients with limited (1–3) BM of solid tumours were recruited at 14 radiation oncology centres from February 2007 to March 2011. Patients with BM of any solid primary tumour scheduled for radiotherapy of the whole brain or parts of it were eligible. Patients were excluded if they had received previous radiotherapy of the cranium or if chemotherapy was planned during the time of irradiation. Furthermore, patients were excluded from this study if their physical or cognitive function was not sufficient to complete the questionnaire. Informed consent was obtained from all individual participants included in the study.
General eligibility criteria included age ≥18 years, Karnofsky Performance Status (KPS) ≥50, sufficient compliance, satisfactory German language skills and no major psychological impairment. The decision regarding which radiotherapy technique was used, i.e. either WBRT or SRT, was determined by the treating physician or according to the policy of the respective radiotherapy centre. WBRT was planned as a two-field opposite technique without hippocampal sparing. If a boost was indicated, it was applied after a three-dimensional (3D)-planning procedure. The dose of hfSRT was determined based on the localization and size of the metastasis, the irradiated whole brain volume and the different institutional protocols. Postoperatively, 10 × 4 Gy was used. In hfSRT, the tumour lesion in the fused axial T1-weighted contrast enhanced Magnetic Resonance images (MRI) or the tumour bed in the postoperative MRI was defined as Gross Tumour Volume (GTV), and a 4-mm safety margin was chosen for the Planning Tumour Volume (PTV).
SRS was applied only in definitive situations and in cases of lesions up to 3 cm or greater than 3 cm with 20 or 18 Gy (95% enclosing isodose), respectively. Doses were reduced near the brain stem.
QoL was assessed before radiotherapy using the European Organization for Research and Treatment of Cancer (EORTC) questionnaires, QLQ-C15-PAL and QLQ-BN20 (see below). Important patient and tumour characteristics were also collected, such as KPS score and Barthel Index [25], the number of brain metastases, Recursive Partitioning Analysis (RPA) and GPA class [15, 36], primary tumour characteristics, and extracranial disease. The same set of questionnaires was mailed to the patients three months after the first radiotherapy session. Patients who did not respond were contacted repeatedly thereafter to obtain the completed questionnaire and information to determine survival status. Ethics approval was obtained from the ethics committee at the University of Wuerzburg, Germany and from the Medical School Hannover, Germany.
Quality-of-life questionnaires
The QLQ-C15-PAL is a validated shortened version of the QLQ-C30 [16]. We selected this version because it was more appropriate for the patients’ palliative setting. The QLQ-C15-PAL contains 15 items for the following nine domains: physical function, emotional function, global QoL, pain, fatigue, appetite, dyspnoea, constipation and sleep. Each item is scored from 1 to 4 (“not at all”, 1; “a little”, 2; “quite a bit”, 3; and “very much”, 4). As an exception, the global QoL is scored from 1 (“very poor”) to 7 (“excellent”).
The validated BN20 questionnaire contains 20 items grouped into four domains (future uncertainty [four items], visual disorder [three items], motor dysfunction [three items] and communication deficit [three items]) and seven single items (headaches, seizures, drowsiness, hair loss, itchy skin, weakness of legs, and bladder control) [41]. The BN20 questionnaire is a brain-specific module that is often used in addition to non-site/disease-specific QoL questionnaires.
High scores represent good functioning/good QoL for functional scales and global QoL, whereas high scores represent more symptoms/lower functioning in the symptom scales of the QLQ-C15-PAL and in all scales of the BN20 [41]. The scores for each symptom or function on the BN20 and QLQ-C15-PAL questionnaires were transformed to numbers from 0–100, where 0 represents “not at all” and 100 represents “very much” [41].
To determine the clinical relevance of changes in health-related quality-of-life (HRQOL) scores, the method of Osoba et al. was applied. On a scale from 0 to 100, a difference of 10 points was classified as the minimum clinically meaningful change in the mean value of a HRQOL parameter [28]. Mean changes ≥10 and <20 points were rated as moderate changes, and mean changes ≥20 points were considered large changes in QoL. Important clinical changes can be documented as either improvement or worsening of the specific symptom.
Statistical analysis
Student’s t‑test for independent samples was used to assess differences in QoL between the groups (WBRT vs. SRT) at baseline. Paired t‑tests were used to compare the patients’ mean scores between the points in time. The significance level was set to 0.05.
Clinically relevant changes were defined as follows: changes had to be statistically significant (p < 0.05), and mean score changes had to be ≥10 points for moderate changes and ≥20 points for large clinical changes [28].
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS; version 22).
Results
Patient and treatment characteristics
From January 2007 to March 2011, 90 patients with limited (1–3) BM were recruited at 14 centres in Germany and Austria. The dominant radiotherapy strategy (n = 52, 58.2%) was WBRT (3 patients with boost) compared to SRT alone (n = 38, 41.8%). Patient and treatment characteristics, including the pretreatment KPS score and Barthel index, are presented in Table 1.
Survival data and intracranial control
Survival status was known in all 90 patients. Twenty-three patients (25.6%) died within 3 months after the beginning of radiation therapy (16 patients treated with WBRT and 7 patients treated with SRT). The median OS was 9.3 months in all patients, with longer survival in the SRT group (10.9 months) compared to the WBRT group (7.2 months; p = 0.055). Of the 67 3‑month survivors, 3 of 36 patients (8%) who received WBRT had intracranial progression and 6 of 31 patients (19%) who received SRT showed progression within the brain. Intracranial status was unknown for 23 patients (18 with WBRT (48.6%) and 5 with SRT (16%)).
Higher GPA scores correlated with better survival. Whereas 56% of patients with a GPA score of 0–1.5 (worse performance) died after 3 months, only 37% of patients with GPA score of 2–4 died.
Steroid use in 3‑month survivors was significantly lower at the second time point (61.4% vs. 31.6%, p = 0.006).
Baseline QoL scores in both groups, test for differences by unpaired test
All 90 patients completed the baseline EORTC QLQ-C15-PAL and BN20 questionnaires. As shown in Table 2, there were significant differences in baseline physical function, hair loss and nausea between the WBRT and SRT groups before the start of radiotherapy, with better physical functioning and less nausea in the SRT group and less hair loss in WBRT patients.
We calculated QoL due to all symptoms and scales and observed no difference according to GPA classification.
Evaluation of the QoL scores: changes after 3 months within and between groups
In all, 63 patients completed the questionnaires at both time points. The response rate of 3‑month survivors was 63 of 67 (94%), including 32 of 37 patients treated with WBRT (87%) and all 31 surviving patients treated with SRT. To prevent potential bias, only questionnaires that were completed at both time points (pretreatment and 3 months after therapy) were included and evaluated. A paired test within groups, baseline and after 3 months, regarding the QLQ-C15-PAL scales revealed significant deterioration in patients treated with WBRT and SRT in physical function (p = 0.000 and p = 0.007), fatigue (p = 0.000 and p = 0.036), nausea (p = 0.003 and p = 0.002) and appetite loss (p = 0.000 and p = 0.025). In the organ-specific BN20 module, significant deterioration was noted in patients treated with WBRT and SRT in drowsiness (p = 0.000 and p = 0.011), hair loss (p = 0.019 and p = 0.023) and itchy skin (p = 0.030 and p = 0.018). Motor dysfunction (p = 0.000), communication deficits (p = 0.002) and weakness of legs (p = 0.000) declined significantly only in patients treated with WBRT. Although future uncertainty increased significantly in patients treated with WBRT (p = 0.046), it decreased significantly in patients treated with SRT (p = 0.001). Scores for headache and visual disorder remained unchanged in both groups (Fig. 1).
Self-assessed quality of life (QoL) on preselected QLQ scales of patients with brain metastases before and 3 months after start of radiotherapy. a The EORTC QLQ C15-PAL: global QoL, physical (phys.) function, and emotional (emot.) function (a higher score is better). b The EORTC QLQ C15-PAL and BN20: symptom scales (a higher score is worse). *Significant change after 3 months within groups (p < 0.05). #Significant change after 3 months between groups (p < 0.05). WBRT Whole brain radiotherapy, SRT stereotactic radiotherapy, RT radiotherapy
When comparing QoL between WBRT and SRT patients over time, significant differences after three months were discovered in fatigue (p = 0.017) and appetite loss (p = 0.001), with less deterioration and decreased future uncertainty (p = 0.000, Fig. 1) in SRT patients.
Number of patients experiencing clinically relevant changes in QoL scores in both groups
Significant results according to the clinical relevance of changes in QoL scores are shown in Table 3. In symptom scales fatigue, hair loss and appetite loss more than 50% of patients described a ≥20 point decrease. Whereas 79% of patients with WBRT suffered from appetite loss (≥20 points decrease), only 37% of patients with SRT declared this high level.
Discussion
Studies on how the QoL of patients with BM is affected by radiotherapy have been previously reported [8, 38, 44]. Nevertheless, this is one of the first comparisons of QoL effects between patients undergoing SRT or WBRT for limited (1–3) BM using a brain-specific tool.
In general, SRT delivers a high dose of focal irradiation to the tumour while minimizing irradiation to healthy brain tissue. Therefore, better cognitive function, QoL, and local control results are expected after this treatment [19, 21].
At our study, most symptoms and domains showed significant declines after three months in both the WBRT and SRT groups. Decreases in QoL after WBRT [13, 30, 39, 44] or SRT have been confirmed in other studies.
Bauman et al. examined QoL in patients with 1–3 brain metastases treated with WBRT and integrated fractionated stereotactic radiotherapy boost. The rates of deterioration (>10-point decrease from baseline on the FACT-Br questionnaire) ranged from 32–59% on the FACT-Br questionnaire depending on the timepoint assessed, with the greatest effects from 6 weeks to 3 months [5].
Habets et al. measured QoL in 97 patients with BM before SRT and 1, 3, and 6 months after SRT [17]. Patients showed worsened physical functioning and fatigue at 6 months. Miller et al. used the EuroQol (EQ)-5D and the Patient Health Questionnaire (PHQ)-9 [26] for patients undergoing SRS and reported that all subscores of the EQ-5D instrument worsened significantly at the last follow-up. Patients with more than three BM experienced more rapid QoL deterioration than those with a single metastasis.
Soffietti et al. showed that adjuvant WBRT after surgery or SRS for a limited number of BM from solid tumours may negatively impact some aspects of QoL. Overall, patients in the observation-only arm reported better QoL scores than patients who received WBRT. The differences were statistically significant and clinically relevant mostly during the early follow-up period (for global health status at 9 months, physical functioning at 8 weeks, cognitive functioning at 12 months, and fatigue at 8 weeks) [34]. Slotman et al. investigated QoL on the basis of the BN-20 questionnaire and the QLQ-C30 in patients with extensive disease small-cell lung cancer (ED-SCLC) [33]. Unlike our study, they randomly assigned patients to either the observation or prophylactic cranial irradiation (PCI) group. Almost every other patient in the PCI arm experienced worsening fatigue compared to baseline for up to three months (49% worsening vs. 51% no worsening). These results are consistent with our study in which fatigue increased ≥20 points (57% WBRT, 44% SRT). Slotman et al. also showed clinically relevant aggravation of emotional function after radiotherapy in 21.4% of patients with PCI. In our cohort, these values were 26% (WBRT) and 23% (SRT).
Lester-Coll et al. [22] compared SRS alone with SRS and WBRT to evaluate the theoretical benefits of intracranial tumour control with adjuvant WBRT against its possible side effects using quality-adjusted life expectancy (QALE). In a cohort of patients with 1 to 3 BM, treatment with SRS yielded 6.2 quality-adjusted life months (QALMs). The addition of initial WBRT reduced QALE by 1.2 QALMs. They concluded that SRS alone results in improved quality of life in patients with 1 to 3 BM compared to SRS and immediate WBRT [22]. Therefore, immediate treatment with WBRT after SRS can be reserved for patients who would have a poor performance status regardless of treatment. In our study, QoL after “superior” SRT for BM did not elicit significantly higher QoL scores in most QoL parameters as calculated in the study of Lester-Coll et al. [22], possibly due to the progression of the intra- and extracranial tumour status or further tumour therapies, especially chemotherapy [22].
Cole et al. [11] examined self-reported cognitive abilities in a group of patients (n = 50) with BM. Patients treated with SRT reported better attention and memory function than patients treated with WBRT, although they also showed deterioration over time (6 weeks, 3 months and 6 months after RT). In addition, patients treated with WBRT showed significantly decreased motivation.
Brown et al. enrolled 194 patients and randomly assigned them to SRS (98 patients) or WBRT (96 patients) postoperatively. Cognitive-deterioration-free survival was longer in patients assigned to SRS than in patients assigned to WBRT, and cognitive deterioration at 6 months was less frequent in patients who received SRS than in those who received WBRT [7].
The study by Chang et al. [9] was terminated because the patients who received SRT plus WBRT were significantly more likely to show a decline in learning and memory function 4 months after RT than patients assigned to receive SRS alone. Chang et al. included some additional QoL values for the FACT-Br questionnaire. The baseline mean was 59.8 for SRS plus WBRT and 64.6 for SRS. The 4‑month mean was 58 for SRS plus WBRT and 65.6 for SRS alone. The FACT-BR mean difference between the groups at 4 months compared with baseline was 2.8 (95% confidence interval [CI] −26 to 21; p = 0.76). The wide CI indicates that the results are inconclusive and should not be interpreted as indicating no difference between the two groups [9].
Aoyama et al. [4] detected steady deterioration in performance in the Mini-Mental Status Examination over time, but they did not detect a significant group difference between patients treated with WBRT plus SRS or SRS alone.
Another main finding in our study was the difference in the score for “future uncertainty” over time according to treatment modality. After 3 months, this score decreased in patients undergoing SRT and increased in patients undergoing WBRT. “Future uncertainty” was scored highest in both groups among the symptom scales at baseline. This result is consistent with Caissie et al. [8] and Steinmann et al. [39], who also reported future uncertainty to be the most prominent baseline symptom in patients with BM after evaluation with the QLQ-BN20. Future uncertainty is a symptom that includes the following aspects: future childcare, finances, spousal support, and the arrangement of care when the disease progresses. Therefore, Hickmann et al. stated that providing non-medical support for the aforementioned topics (e.g., handing out information, suggesting support groups, establishing connections with respective authorities, setting up appointments with social services, and assisting in dealing with insurance companies) could improve QoL and decrease distress [18]. In patients treated with SRT who received this support during their treatment, future uncertainty was lower. In patients treated with WBRT, higher fatigue scores and depressive mood were observed.
The study has several limitations. First, we have to consider that the reliability of patients’ declarations could be biased since follow-up QoL questionnaires were not completed during a control visit but were returned by mail. Therefore, we could not assess a patient’s general condition ourselves. Additionally, completion of the questionnaires in the hospital was not observed.
Second, despite the multicentre study design, 67 survivors is a relatively small sample, reflecting the difficulty of studies in this field. However, the return rate of the questionnaires was very high (94%) compared to that in other studies [34].
Another significant limitation is the lack of prior randomization. The decision regarding which radiotherapy technique was applied, i.e. either WBRT or SRT, was determined by the treating physician or according to the policy of the respective radiotherapy centre. Patient characteristics and patient wishes were considered at all times. There may be a potential bias because patients receiving WBRT had different baseline characteristics from patients undergoing SRT. Patients undergoing WBRT had lower KPS scores, a higher RPA class, more corticosteroid use, more than one metastasis, and received postoperative radiotherapy.
Therefore, further studies with randomization are necessary.
Conclusion
Overall, the QoL of patients with 1–3 brain metastases was negatively affected by WBRT and SRT with respect to physical function, fatigue, appetite loss, nausea, drowsiness, hair loss, and itchy skin. Motor dysfunction, communication deficits and leg weakness decreased significantly only in patients treated with WBRT. Interestingly, future uncertainty decreased in patients with local treatment but increased in patients treated with WBRT. When comparing the effects of the two radiotherapy techniques on QoL over time, we showed significant differences in scores for fatigue and appetite loss. Therefore, the outcome of the paper should be considered in decision making on the irradiation technique in patients with small number of brain metastases.
References
Ahluwalia MS, Vogelbaum MV, Chao ST et al (2014) Brain metastasis and treatment. F1000Prime Rep 6:114
Ammirati M, Cobbs CS, Linskey ME et al (2010) The role of retreatment in the management of recurrent/progressive brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96:85–96
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S et al (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491
Aoyama H, Tago M, Kato N et al (2007) Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys 68:1388–1395
Bauman G, Yartsev S, Roberge D et al (2016) Assessment of function and quality of life in a phase II multi-institutional clinical trial of fractionated simultaneous in-field boost radiotherapy for patients with 1–3 metastases. J Neurooncol 128:431–436
Brown PD, Jaeckle K, Ballman KV et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316:401–409
Brown PD, Ballman KV, Cerhan JH et al (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1049–1060
Caissie A, Nguyen J, Chen E et al (2012) Quality of Life in Patients with Brain Metastases Using the EORTC QLQ-BN20+2 and QLQ-C15-PAL. Int J Radiat Oncol Biol Phys 83:1238–1245
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10(11):1037–1044
Churilla TM, Chowdhury IH, Handorf E et al (2018) Comparison of local control of brain metastases with Stereotactic Radiosurgery vs surgical resection: a secondary analysis of a randomized clinical trial. JAMA Oncol 5(2):243–247
Cole AM, Scherwath A, Ernst G et al (2013) Self-reported cognitive outcomes in patients with brain metastases before and after radiation therapy. Int J Radiat Oncol Biol Phys 87:705–712
El Gantery MM, Abd El Baky HM, El Hossieny HA et al (2014) Management of brain metastases with stereotactic radiosurgery alone versus whole brain irradiation alone versus both. Radiat Oncol 9:116-717X-9-116
Feyer P, Jahn F, Jordan K (2014) Radiation induced nausea and vomiting. Eur J Pharmacol 722:165–171
Gaspar LE, Mehta MP, Patchell RA et al (2010) The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96:17–32
Gaspar LE, Scott C, Murray K et al (2000) Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys 47:1001–1006
Groenvold M, Petersen MA, Aaronson NK et al (2006) The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer 42:55–64
Habets EJJ, Dirven L, Wiggenraad RG et al (2016) Neurocognitive functioning and health-related quality of life in patients treated with stereotactic radiotherapy for brain metastases: a prospective study. Neuro-oncology 18:435–444
Hickmann A, Hechtner M, Nadji-Ohl M et al (2017) Evaluating patients for psychosocial distress and supportive care needs based on health-related quality of life in primary brain tumors: a prospective multicenter analysis of patients with gliomas in an outpatient setting. J Neurooncol 131:135–151
Ishihara T, Yamada K, Harada A et al (2016) Hypofractionated stereotactic radiotherapy for brain metastases from lung cancer. Strahlenther Onkol 192:386–393
Kalkanis SN, Kondziolka D, Gaspar LE et al (2010) The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96:33–43
Kocher M, Wittig A, Piroth MD et al (2014) Stereotactic radiosurgery for treatment of brain metastases. Strahlenther Onkol 190:521–532
Lester-Coll N, Dosoretz AP, Yu JB (2014) Decision analysis of Stereotactic radiation surgery versus Stereotactic radiation surgery and whole-brain radiation therapy for 1 to 3 brain metastases. Int J Radiat Oncol Biol Phys 89:563–568
Linskey ME, Andrews DW, Asher AL et al (2010) The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96:45–68
Mahmood U, Kwok Y, Regine WF et al (2010) Whole-brain irradiation for patients with brain metastases: still the standard of care. Lancet Oncol 11:221–222 (author reply 223)
Mahoney FI, Barthel DW (1965) Functional evaluation: the Barthel index. Md State Med J 14:61–65
Miller J, Kotecha R, Barnett G et al (2017) Quality of life following Stereotactic Radiosurgery for single and multiple brain metastases. Neurosurgery 81:147–155
Olson JJ, Paleologos NA, Gaspar LE et al (2010) The role of emerging and investigational therapies for metastatic brain tumors: a systematic review and evidence-based clinical practice guideline of selected topics. J Neurooncol 96:115–142
Osoba D, Rodrigues G, Myles J et al (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16:139–144
Patchell RA, Tibbs PA, Walsh JW et al (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500
Pulenzas N, Khan L, Tsao M et al (2014) Fatigue scores in patients with brain metastases receiving whole brain radiotherapy. Support Care Cancer 22:1757–1763
Rades D, Kueter JD, Pluemer A et al (2009) A matched-pair analysis comparing whole-brain radiotherapy plus stereotactic radiosurgery versus surgery plus whole-brain radiotherapy and a boost to the metastatic site for one or two brain metastases. Int J Radiat Oncol Biol Phys 73:1077–1081
Rades D, Kueter J, Hornung D et al (2008) Comparison of stereotactic radiosurgery (SRS) alone and whole brain radiotherapy (WBRT) plus a stereotactic boost (WBRT + SRS) for one to three brain metastases. Strahlenther Onkol 184:655–662
Slotman BJ, Mauer ME, Bottomley A et al (2009) Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol 27:78–84
Soffietti R, Kocher M, Abacioglu UM et al (2013) A European Organisation for research and treatment of cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or Radiosurgery: quality-of-life results. J Clin Oncol 31:65–72
Specht HM, Kessel KA, Oechsner M et al (2016) HFSRT of the resection cavity in patients with brain metastases. Strahlenther Onkol 192:368–376
Sperduto CM, Watanabe Y, Mullan J et al (2008) A validation study of a new prognostic index for patients with brain metastases: the Graded Prognostic Assessment. J Neurosurg 109(Suppl):87–89
Sperduto PW, Shanley R, Luo X et al (2014) Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1–3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int J Radiat Oncol Biol Phys 90:526–531
Steinmann D, Schäfer C, van Oorschot B, Wypior HJ, Bruns F, Bölling T, Sehlen S, Hagg J, Bayerl A, Geinitz H, Hipp M, Vordermark D (2009) Effects of radiotherapy for brain metastases on quality of life (QoL). Prospective pilot study of the DEGRO QoL working party. Strahlenther Onkol 185:190–197
Steinmann D, Paelecke-Habermann Y, Geintiz H et al (2012) Prospective evaluation of quality of life effects in patients undergoing palliative radiotherapy for brain metastases. BMC Cancer 12:283
Steinmann D, Vordermark D, Geinitz H et al (2013) Proxy assessment of patients before and after radiotherapy for brain metastases. Results of a prospective study using the DEGRO brain module. Strahlenther Onkol 189:47–53
Taphoorn MJ, Claassens L, Aaronson NK et al (2010) An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer 46:1033–1040
Treuer H, Hoevels M, Luyken K et al (2015) Intracranial stereotactic radiosurgery with an adapted linear accelerator vs. robotic radiosurgery. Strahlenther Onkol 191:470–476
Tsao M, Xu W, Sahgal A (2012) A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer 118:2486–2493
Wong E, Zhang L, Rowbottom L et al (2016) Symptoms and quality of life in patients with brain metastases receiving whole-brain radiation therapy. Support Care Cancer 24:4747–4759
Acknowledgements
We thank A. Bayerl, U. Eichenseder-Seiss, J. Hagg, M. Hipp, F. Zehentmayr, S. Sehlen, T. Bölling, B. van Oorschot, E. Bosch, I. Kleff, F. Bruns and J. Gerstein for their good ideas in initiation and analysis of this study and helping to recruit patients.
Funding
The study was supported by a foundation of the Equal Opportunities Office of the Medical School Hannover.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
D. Steinmann, D. Vordermark, W. Gerstenberg, R. Aschoff, N. Gharbi, A. Müller, C. Schäfer, M. Theodorou, H.-J. Wypior and H. Geinitz declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Steinmann, D., Vordermark, D., Gerstenberg, W. et al. Quality of life in patients with limited (1–3) brain metastases undergoing stereotactic or whole brain radiotherapy. Strahlenther Onkol 196, 48–57 (2020). https://doi.org/10.1007/s00066-019-01506-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-019-01506-w