Abstract
We examined functional outcomes and quality of life of whole brain radiotherapy (WBRT) with integrated fractionated stereotactic radiotherapy boost (FSRT) for brain metastases treatment. Eighty seven people with 1–3 brain metastases (54/87 lung primary, 42/87 single brain metastases) were enrolled on this Phase II trial of WBRT (30 Gy/10) + simultaneous FSRT, (60 Gy/10). Median overall follow-up and survival was 5.4 months, 6 month actuarial intra-lesional control was 78 %; only 1 patient exhibited grade 4 toxicity (worsened seizures); most treatment related toxicity was grade 1 or 2; 2/87 patients demonstrated asymptomatic radiation necrosis on follow-up imaging. Mean (Min–Max) baseline KPS, Mini Mental Status Exam (MMSE) and FACT-BR quality of life were 83 (70–100), 28 (21–30) and 143 (98–153). Lower baseline MMSE (but not KPS or FACT-Br) was associated with worse survival after adjusting for age, number of metastases, primary and extra-cranial disease status. Crude rates of deterioration (>10 points decrease from baseline for KPS and FACT-Br, MMSE fall to <27) ranged from 26 to 38 % for KPS, 32–59 % for FACT-Br and 0–16 % for MMSE depending on the time-point assessed with higher rates generally noted at earlier time points (≤6 months post-treatment). Using a linear mixed models analysis, significant declines from baseline were noted for KPS and FACT-Br (largest effects at 6 weeks to 3 months) with no significant change in MMSE. The effects on function and quality of life of this integrated treatment of WBRT + simultaneous FSRT were similar to other published series combining WBRT + radiosurgery.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Radiosurgery (SRS) alone provides high rates of local control and high rates of intracranial control when combined with whole brain radiotherapy (WBRT) for brain metastases (BM) [1–5] but utilization of SRS may be low where access to dedicated SRS platforms is limited [6]. Linear accelerators with integrated on-board image guidance (IGRT) and intensity modulated radiotherapy(IMRT) provide an attractive alternative to traditional SRS that may be more widely available. We conducted a Phase II trial for patients with 1-3 BM based on a successful Phase I trial of synchronous WBRT + FSRT using such an integrated platform [7, 8]. Here we report clinical outcomes assessments (COAs) acquired as secondary endpoints on this trial: Karnofsky Performance Status (KPS), Mini-mental Status Exam (MMSE) and Functional Assessment of Cancer Therapy-Brain (FACT-Br).
Methods and materials
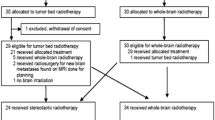
Full details regarding the trial are published. Eligibility criteria were designed to match those of RTOG 9508 and included maximum of 3 BM (none ≥ 3 cm), extra-cranial cancer controlled or under treatment and KPS ≥ 70. Prior surgery was allowed as long as there was at least one intra-cranial lesion in situ; boost to the surgical cavity was not allowed. The co-primary endpoints for the trial were overall survival and lesional and intracranial control as measured by serial MRI; assessment of COAs were planned secondary endpoints. Patients received 30 Gy/10 fractions WBRT with synchronous FSRT of 60 Gy/10 fractions. Patient status, toxicity as assessed by NCI CTC V3 and COAs were collected at baseline and 6 weeks and 3, 6, 9 and 12 months post treatment [8].
Statistics
For all tests P values less than 0.05 were considered statistically significant and data were analyzed using SAS 9.3. (Cary, NC, USA) Cox proportional hazards regression was used to explore relationships between baseline COAs and survival. The association between baseline COAs and age and steroid use were evaluated using Pearson correlation coefficients. Association between baseline COAs and number of metastases and the extracranial disease status was examined using unpaired t-tests. Clinically relevant changes in COA over time were defined as (1) a decline in KPS of 10 points from baseline (2) a decline in FACT-Br of 10 points from baseline (3) MMSE less than 27. To account for a ceiling effect (due to eligibility criteria) we also analyzed (4) fall in KPS to less than 70. Changes in COA scores over time were analyzed using a linear mixed model (LMM) approach with a flexible covariance structure and Dunnett’s test was used to compare COA means at each time point against baseline [9]. Effect sizes at each time point in the LMM were categorized as <0.5 small, >0.5 to <0.8 moderate and ≥0.8 large. At 3 and 6 months COAs for those with and without intra-cranial progression were compared using unpaired t-tests.
Results
Patient demographics are available in Table 1. Median overall survival (and median follow-up) for all patients (n = 87) was 5.4 months [10]. Six-month actuarial intra-lesional control was 78 %. Only 1 patient exhibited grade 4 toxicity (worsened seizures); most treatment related toxicity was grade 1 or 2. There were 2/87 patients who demonstrated suspected, asymptomatic radiation necrosis on follow-up imaging as reported by the neuro-radiologist. Mean (Median, Min–Max) baseline KPS, MMSE and FACT-BR were 83 (80, 70–100), 28 (29, 21–30) and 143 (146, 98–153). Baseline MMSE was found to be associated (HR (95 % CI) = 0.81 (0.72, 0.91), p < 0.001) with overall survival (Table 2). This association remained significant when adjustment was made for age, presence of extra-cranial disease, and number of metastases (HR (95 % CI) = 0.82 (0.73, 0.93), p = 0.003).While there was no significant association between baseline COAs with number of metastases and presence of extra-cranial disease there were small negative associations between increasing age and lower baseline KPS (r = −0.29, p = 0.006) and lower baseline MMSE (r = −0.24, p = 0.027). Significant negative association between steroid use and KPS and FACT-Br was noted at baseline (r = −0.238, p = 0.039, r = −0.381, p = 0.001) but not at later time points.
As expected, the absolute number of patients assessable over time decreased due to disease progression (39/87 alive at 6 months, 19/87 at 12 months). Overall compliance with serial assessment of COAs was 80–100 %.Mean baseline and follow-up KPS, MMSE and FACT-Br with standard deviations are illustrated in Fig. 1a–c. KPS and FACT-Br scores tended to fall at 6 weeks post treatment with a gradual recovery (but still below baseline) over time. Crude rates of clinicallyrelevant deterioration (Table 3) were highest for FACT-Br (32–52 %), lowest for MMSE (3–12 %) and intermediate for KPS (16–26 %) with the largest changes seen at earlier times (≤3 months). Using the linear mixed models (Table 4) statistically significant differences from baseline were seen for KPS and FACT-Br with moderate effect sizes at 6 weeks and 3 months for KPS and at 6 weeks for FACT-Br. KPS and FACT-Br scores were slightly lower among patients with intra-cranial progression at 3- and 6 months but not statistically significant compared to patients without progression.
Discussion
The primary hypothesis regarding local control compared to historical results (RTOG 9508) of this Phase II trial was satisfied (intracranial control rate of 78 % at 6 months) [10]. Secondary COA endpoints are explored here and compared to the published Phase III literature examining WBRT + SRS [2–5]. While the limitations of the COAs assessed in our study are well recognized [11] these tools have been extensively utilized in other prospective studies of BM patients [3, 5, 11–13] and carry advantages of familiarity, ease of use and relatively low patient and family burden for completion.
KPS is a provider assessed measure of patient function and assigns a score from 0 to 100 with patients rated 90–100 being minimally symptomatic or asymptomatic; patients rated 70–80 being symptomatic but able to carry on most normal activities and patients rated <70 being increasingly compromised in their function. KPS is commonly used as a stratification criterion for clinical trials and is incorporated in BM prognostic indices [11, 12, 14]. RTOG 9508 reported 43/76 patients (56 %) in the WBRT + SRS arm with a worse KPS at 6 months [2]. Aoyama noted KPS of <70 in approximately 2/3 of 44 patients followed to 12 months post treatment [3]. Kocher reported a median time of 10 months for a fall in function to WHO Performance Status >2 (KPS <60) [15]. In comparison we noted 20–30 % with a clinically relevant KPS decline and the largest changes noted at 6 weeks and 3 months post treatment. The reduced discriminatory ability of KPS at the high end of the scale has been noted [11, 12] and our entrance eligibility of KPS > 70 may have biased our results towards adverse KPS changes (ceiling effect).
The MMSE was originally developed as a screening tool for dementia/delirium and uses a series of questions testing orientation, memory, ability to follow commands as well as spatial and written ability. A maximum MMSE score is 30 and scores of <27 are associated with clinically relevant cognitive compromise. Despite its known lack of sensitivity to subtle neurocognitive changes, MMSE has been commonly used in BM trials [11, 12]. In the RTOG 9508 MMSE decline was noted in 21/50 patients (42 %) at 6 months [2]. In the JPRSOG study, among patients receiving whole WBRT + SRS actuarial rates of neurocognitive preservation (freedom from a 3 point decline or a MMSE < 27) were approximately 80 % at 6 and 12 months [3, 16]. In comparison, the NCCTG randomized trial of SRS ± WBRT [17] noted cognitive progression (as measured by standardized neuro-cognitive tests) among the majority (88 %) of patients receiving WBRT. In our trial crude rates of decline in MMSE to <27 were noted in 5–12 % of patients at time points over 12 months but the changes in MMSE were not statistically significant (perhaps reflecting the low sensitivity of this scale). Similar to others [18] we found lower MMSE to be an independent predictor of worse OS on MVA. Correlations between lower MMSE and larger volume of BM and edema have been noted, suggesting MMSE may be a surrogate measure of volume of intra-cranial disease [16].
The Functional Assessment of Cancer Therapy (FACT) integrates the effects of treatment and disease to provide an overall measure of general health related quality of life (HRQoL) [11]. A brain subscale addresses issues such as self-assessed cognitive function (e.g. “I can remember new things”), physical functioning (e.g. “I get frustrated that I cannot do things that I used to do”) and symptoms (e.g., “I get headaches”) and the combination FACT-Br has been validated among patients with primary and metastatic brain tumors [19, 20]. While specific neurocognitive domains are not assessable by FACT-Br, a strong association between neurocognitive function and FACT-Br scores has been noted [21]. Chang et al. [5] noted no difference in FACT-Br scores at 4 months in patients treated with SRS alone versus WBRT + SRS but did not report on changes compared to baseline. The EORTC reported declines in HRQoL (EORTC QLQ-BN20 and EORTC QLC-C30) among patients receiving WBRT with SRS or surgery, with the greatest differences noted at 2 and 9 months [15]. In comparison we noted crude rates of clinically significant deterioration in FACT-Br in about half of patients with the largest effect size noted at 6 weeks.
WBRT with lesion directed therapy maximizes the probability of intra-cranial control.The technique examined here allows efficient delivery of WBRT with lesional FSRT boost and is deployable using standard IMRT and IGRT. Exploratory analysis of secondary COA endpoints suggested effects on function and quality of life were comparable to that reported in the literature for WBRT + SRS delivered on other platforms; were consistent with known toxicity profiles associated with WBRT [22] and were consistent with modeled comparisons to sequential WBRT + SRS [23]. Our COA assessments in this trial are limited by the instruments used, overall moderate size patient cohort and patient attrition on trial. Thus characterization of COAs at later time points and incorporating effects of factors like histologic subtype were limited.
Since the initiation of this trial, clinical practice has shifted away from the routine WBRT in favor of lesion only treatment with SRS/FSRT because of potential neurotoxicity [17, 24]. High rates of lesional control suggest the FSRT boost used in this trial (60 Gy/10 fractions) could be used for lesion treatment only with WBRT held for salvage of subsequent intracranial failures, in line with current recommendations [24].Alternatively, early results suggest preservation of neurocognitive function and HRQoL may be possible with WBRT with hippocampal sparing. Incorporating hippocampal sparing into our technique is another possible strategy to reduce the impact on COA noted [13, 25].
Conclusions
In this Phase II trial WBRT + simultaneous FSRT boost was associated with modest negative impacts on functional and quality of life outcomes comparable to those reported for sequential WBRT + SRS strategies.
References
Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, Sperduto PW, Vogelbaum MA, Radawski JD, Wang JZ, Gillin MT, Mohideen N, Hahn CA, Chang EL (2012) Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2:210–225
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29:134–141
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044
Hodgson DC, Charpentier AM, Cigsar C, Atenafu EG, Ng A, Bahl G, Zadeh G, San Miguel J, Menard C (2013) A multi-institutional study of factors influencing the use of stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 85:335–340
Rodrigues G, Yartsev S, Yaremko B, Perera F, Dar AR, Hammond A, Lock M, Yu E, Ash R, Caudrelier JM, Khuntia D, Bailey L, Bauman G (2011) Phase I trial of simultaneous in-field boost with helical tomotherapy for patients with one to three brain metastases. Int J Radiat Oncol Biol Phys 80:1128–1133
Rodrigues G, Yartsev S, Tay KY, Pond GR, Lagerwaard F, Bauman G (2012) A phase II multi-institutional study assessing simultaneous in-field boost helical tomotherapy for 1-3 brain metastases. Radiat Oncol 7:42
Helen B, Robin P (2006) Applied mixed models in medicine, 2nd edn. Wiley, Chicester
Rodrigues G, Yartsev V, Roberge D, MacRae R, Roa W, Panet-Raymond V, Masucci L, Yaremko B, D’Souza D, Palma D (2015) A phase 2 multi-institutional clinical trial assessing fractionated simultaneous in-field boost radiation therapy for brain oligometastases. Int J Radiat Oncol Biol Phys 93:S39
Lin NU, Wefel JS, Lee EQ, Schiff D, van den Bent MJ, Soffietti R, Suh JH, Vogelbaum MA, Mehta MP, Dancey J, Linskey ME, Camidge DR, Aoyama H, Brown PD, Chang SM, Kalkanis SN, Barani IJ, Baumert BG, Gaspar LE, Hodi FS, Macdonald DR, Wen PY (2013) Response Assessment in Neuro-Oncology, Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes, a report from the RANO group. Lancet Oncol 14:e407–416
Dirven L, Armstrong TS, Taphoorn MJ (2015) Health-related quality of life and other clinical outcome assessments in brain tumor patients: challenges in the design, conduct and interpretation of clinical trials, Neuro-Oncol Pract 2
Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, Konski AA, Bauman GS, Shah S, Shi W, Wendland M, Kachnic L, Mehta MP (2014) Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 32:3810–3816
Rodrigues G, Bauman G, Palma D, Louie AV, Mocanu J, Senan S, Lagerwaard F (2013) Systematic review of brain metastases prognostic indices. Pract Radiat Oncol 3:101–106
Soffietti R, Kocher M, Abacioglu UM, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Mueller RP, Tridello G, Collette L, Bottomley A (2013) A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 31:65–72
Aoyama H, Tago M, Kato N, Toyoda T, Kenjyo M, Hirota S, Shioura H, Inomata T, Kunieda E, Hayakawa K, Nakagawa K, Kobashi G, Shirato H (2007) Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys 68:1388–1395
Brown PD, Asher AL, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG, Richard RD, Burri S, Menard C, Chung C, S. VW, Pollock PE, Galanis E, Buckner JC, J. KA (2015) NCCTG N0574 (Alliance): A phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases. American Society of Clinical Oncology, J Clin Oncol, Chicago, pp. abstr LBA4
Murray KJ, Scott C, Zachariah B, Michalski JM, Demas W, Vora NL, Whitton A, Movsas B (2000) Importance of the mini-mental status examination in the treatment of patients with brain metastases: a report from the Radiation Therapy Oncology Group protocol 91-04. Int J Radiat Oncol Biol Phys 48:59–64
Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA (1995) The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer 75:1151–1161
Thavarajah N, Bedard G, Zhang L, Cella D, Beaumont JL, Tsao M, Barnes E, Danjoux C, Sahgal A, Soliman H, Chow E (2014) Psychometric validation of the functional assessment of cancer therapy–brain (FACT-Br) for assessing quality of life in patients with brain metastases. Support Care Cancer 22:1017–1028
Li J, Bentzen SM, Li J, Renschler M, Mehta MP (2008) Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys 71:64–70
Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD (2005) Radiosurgery with or without whole-brain radiotherapy for brain metastases: the patients’ perspective regarding complications. Am J Clin Oncol 28:173–179
Bauman G, Yartsev S, Fisher B, Kron T, Laperriere N, Heydarian M, VanDyk J (2007) Simultaneous infield boost with helical tomotherapy for patients with 1–3 brain metastases. Am J Clin Oncol 30:38–44
ASTRO, ASTRO releases second list of five radiation oncology treatments to question, as part of national Choosing Wisely® campaign (2015) http://www.choosingwisely.org/astro-releases-second-list. Accessed April 2016
Gutierrez AN, Westerly DC, Tome WA, Jaradat HA, Mackie TR, Bentzen SM, Khuntia D, Mehta MP (2007) Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: a planning study. Int J Radiat Oncol Biol Phys 69:589–597
Acknowledgments
Thank you to Anne O’Connell, Frances Whiston and Laura Bailey for clinical trials management support for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Bauman, G., Yartsev, S., Roberge, D. et al. Assessment of function and quality of life in a phase II multi-institutional clinical trial of fractionated simultaneous in-field boost radiotherapy for patients with 1–3 metastases. J Neurooncol 128, 431–436 (2016). https://doi.org/10.1007/s11060-016-2128-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2128-7