Abstract
Purpose
We retrospectively evaluated the efficacy and safety of stereotactic body radiotherapy (SBRT) combined with trans-arterial chemoembolization (TACE) as initial therapy in Barcelona Clinic Liver Cancer (BCLC) system stage B–C hepatocellular carcinoma (HCC).
Patients and methods
Seventy-two patients received a single dose of TACE followed by SBRT 4 weeks later. All patients had tumor sizes ≥5 cm, at least 700 ml of disease-free liver, Child–Pugh (CP) score ≤ B7 and tumor nodules ≤5. SBRT dose, ranging from 6 × 5–8 Gy or 5–10 × 4 Gy, was individualized according to normal tissue constraints. No subsequent scheduled treatment was delivered unless disease progression was observed. Local control (LC), overall survival (OS), progression-free survival (PFS), response rate (RR), and toxicity were evaluated.
Results
The patients’ characteristics were: median age 60 years (range 28–87 years); CP score A/B (n = 68/4); BCLC stage B/C (n = 51/21); solitary/multifocal (n = 37/35); portal vein invasion (n = 18). The median tumor size and GTV were 11.2 cm (range 5.0–23.6 cm) and 751 cm3 (range 41–4009 cm3), respectively. The median equivalent dose in 2 Gy per fraction (EQD2, α/β = 10) was 37.3 Gy2 (range, 28–72 Gy2). The median follow-up time was 16.8 months (range, 3–96 months). The objective RR was 68% and the 1‑year LC rate was 93.6% (95% CI, 87.6–100%). The median OS was 19.8 months (95% CI, 11.6–30.6 months). SBRT-related grade 3 or higher adverse gastrointestinal events and treatment-related death occurred in three (2.8%) and one patient (1.4%) respectively. No patient developed classical radiation-induced liver injury.
Conclusion
Our experience suggests that combined TACE and SBRT can be a safe and effective initial therapy for BCLC stage B–C HCC with appropriate patient selection. Further prospective trials are warranted.
Zusammenfassung
Zielsetzung
Wir bewerteten retrospektiv Wirksamkeit und Sicherheit der stereotaktischen Körperstamm-Strahlentherapie (SBRT) in Kombination mit transarterieller Chemoembolisation (TACE) als Erstlinientherapie für Leberzellkarzinome (HCC) im Stadium B–C nach dem Barcelona-Klinik-Leberkrebs(BCLC)-System.
Patienten und Methoden
Es bekamen 72 Patienten eine einzige TACE-Anwendung gefolgt von einer SBRT 4 Wochen später. Alle Patienten hatten ≥5 cm, mindestens 700 ml tumorfreie Leber, einen Child-Pugh-Score (CP) ≤ B7 und Läsionen ≤ 5. Die SBRT-Dosen im Bereich von 6 × 5–8 Gy oder 5–10 × 4 Gy wurden bezüglich notwendiger Einschränkungen im Normalgewebe individualisiert. Eine weitere Behandlung wurde nur bei entsprechender Progression der Erkrankung durchgeführt. Der primäre Endpunkt war die lokale Kontrolle (LK). Sekundäre Endpunkte umfassten Gesamtüberleben (GS), progressionsfreies Überleben (PFÜ), Ansprechrate (AR) und Toxizität.
Ergebnisse
Patientenmerkmale waren: mittleres Alter 60 Jahre (Spanne 28–87 Jahre); CP-Score A/B (n = 68/4); BCLC-Stadium B/C (n = 51/21); solitär/multifokal (n = 37/35); Pfortaderinvasion (n = 18). Mittlere Tumorgröße und GTV betrugen 11,2 cm (Spanne 5,0–23,6 cm) bzw. 751 cm3 (Spanne 41–4009 cm3). Die mittlere Äquivalentdosis in 2 Gy pro Fraktion (EQD2, α/β = 10) betrug 37,3 Gy2 (Spanne 28–72 Gy2). Die mittlere Nachbeobachtungszeit war 16,8 Monate (Spanne 3–96 Monate). Die Ziel-AR betrug 68 % und die 1‑Jahres-LK 93,6 % (95 %-Konfidenzintervall [KI] 87,6–100 %). Das mediane GS lag bei 19,8 Monaten (95 %-KI 11,6–30,6 Monate). SBRT-induzierte Nebenwirkungen vom Grad 3 oder höher traten bei 3 Patienten (2,8 %) bzw. behandlungsbedingte Todesfälle bei 1 Patienten (1,4 %) auf. Kein Patient entwickelte eine klassische strahleninduzierte Leberschädigung.
Schlussfolgerungen
Unsere Erfahrung zeigt, dass bei entsprechender Patientenauswahl die Kombination von TACE und SBRT eine sichere und effektive Erstlinientherapie für das HCC im BCLC-Stadium B–C sein kann. Prospektive Studien sind gerechtfertigt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and ranks as the second cancer-related cause of death globally, with most of the disease burden in Asia and Africa [1]. One of the most widely adopted staging systems for HCC is the Barcelona Clinic Liver Cancer (BCLC) system, which was developed mainly based on hepatitis C virus (HCV)-related HCC [2]. This system has the advantage of linking the disease staging to treatment recommendations and therefore its therapeutic flowchart plays a pivotal role in patient management and designing clinical trials in Western countries [3].
However, in Asian countries, a majority of HCC is associated with the endemic hepatitis B virus (HBV) infection [4], as opposed to their Western counterparts where HCV infection and alcoholic cirrhosis are two major attributing factors [5]. The natural history and prognosis of HBV- and HCV-related HCC are different [6]; patients with HBV-related HCC often show sizable tumors [7]. It is therefore recognized that the therapeutic recommendation of the BCLC system might not be applicable in Asian countries [8]. For example, among patients with intermediate-stage HCC, trans-arterial chemoembolization (TACE) is the recommended upfront therapy [9, 10]. However, its treatment effect seems to be poor in large tumors, with reported 2‑year survival of HCC patients receiving TACE of 42% versus 0% for tumor sizes of 5–7 cm and ≥8 cm, respectively [11]. The outcome is even worse in advanced stage HCC, for which sorafenib is the standard therapy. Sorafenib rarely induces tumor shrinkage, with response rate of 2–3%, and the survival benefit seems to be limited [12, 13].
All these factors highlight the unmet need of optimizing the loco-regional therapy effect in the management of HBV-related HCC. For example, more aggressive surgical approaches that are widely adopted in Asian countries were associated with better clinical outcome [14], but many advanced stage patients are surgically or medically inoperable. For non-surgical candidates, stereotactic body radiotherapy (SBRT) has emerged as a promising local therapy associated with impressive local control [15,16,17]. At our institution, SBRT was initially utilized in patients who failed or were intolerant to TACE with promising results, which, in turn, prompted us to combine TACE and SBRT as standard upfront loco-regional therapy in those patients who are not amendable to curative surgery.
To date, in patients with HBV-related large HCC, there are a limited number of reports to evaluate the combination of TACE and SBRT as initial therapy. We therefore retrospectively analyze our clinical outcome of combined SBRT and TACE in BCLC stage B or C HCC.
Patients and methods
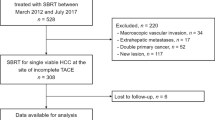
Patients
The diagnosis of HCC was established either by biopsy or by the American Association for the Study of Liver Diseases (AASLD) criteria with characteristic enhancement on two imaging modalities in the presence of cirrhosis. From 2008 to 2015, 72 consecutive BCLC B–C patients who were treated with combined TACE and SBRT according to our institutional protocol were included in this IRB-approved retrospective analysis. Patients were offered treatment under the combined TACE/SBRT protocol if they were unsuitable for resection, liver transplantation, or local ablation therapies, and had a minimum of 700 mL of uninvolved liver, an Eastern Cooperative Oncology Group (ECOG) performance score ≤2, a Child–Pugh (CP) liver score of A to B7, an adequate organ function defined as absolute neutrophil counts (ANC) ≥1.5 × 109/l, creatinine ≥1.5 × ULN, alanine transaminase (ALT) or aspartate transaminase (AST) <2.5 × upper limit of normal (ULN), international normalised ratio (INR) <1.7, and no ascites or encephalopathy clinically. Extra-hepatic diseases were allowed, provided the greatest burden of disease was hepatic. Patients with main portal vein thrombosis (PVT), diffusely infiltrative disease, or more than five tumor nodules were not offered the combined therapy. There was no limit regarding tumor size.
Treatment
TACE was performed by supra-selective cannulation of the supplying tumor artery. The emulsion was prepared by mixing lipiodol with cisplatin in a 1:1 ratio. Various amounts of the emulsion were injected slowly under fluoroscopic monitoring according to the size of the tumor and the arterial blood flow. The maximum dosage of cisplatin and lipiodol injected was 40 mg and 20 ml for each treatment session, respectively. The interval between TACE and simulation computed tomography (CT) was one week, and that between TACE and the start of SBRT was four weeks. The first patients (n = 18) from 2008–2010 were treated using respiratory gating and the subsequent patients (n = 55) from 2011–2015 were treated by a four-dimensional cone beam computed tomography (4DCBCT) guided-approach. The gross tumor volume (GTV) was defined on the plain non-contrast CT when the lipiodol enhancing tumor was visualized. Otherwise, it was defined on the contrast CT, which was usually best visualized at the arterial phase (as hyperintensity) or at the delayed portovenous phase, and included the lipiodol-stained area.
For the first cohort of patients treated with gating, treatment planning was based on 4DCT simulation. The clinical target volume (CTV) was defined as GTV plus a margin of 0 to 5 mm. The internal target volume (ITV) was defined as the composite CTV from the 40 to 60% of respiratory phases. The planning target volume (PTV) margin from ITV ranged from 3 to 5 mm. Radiation was delivered mostly by coplanar 1–2 dynamic conformal arcs, or in a few cases, 5–7 static conformal fields on a 6 MV linac. The treatment setup was performed with the ExacTrac stereotactic body setup system (BrainLab Ltd, Feldkirchen, Germany) together with a stereotactic frame and pre-treatment CT verifications, as described in Wong et al. [18]. For treatments of more than one lesion, multiple isocenters were applied to individual lesions.
For the second cohort of patients treated with the 4DCBCT-guided approach, treatment planning was primarily based on the mid-ventilation concept [19] Volumetric modulated arc radiotherapy (VMAT) was planned on a dual-energy 6 MV and 10 MV linac for all patients, with lesions grouped into a single or dual isocenter for multiple lesions. Technical details of the extraction of the mid-ventilation CT images from the 4DCT and subsequently the formulation of the mid-ventilation PTV at our institution were described in [20]. Pre-treatment 4DCBCT was acquired per treatment isocenter for every fraction. The tumor localization was based on the lipiodol retention whenever it was visible or the diaphragm as a tumor surrogate [21].
The prescribed SBRT dose, ranging from 5.0–6.5 Gy × 6 fractions or 4.0 Gy × 6–10 fractions, was individualized according to the normal tissue constraints. Dose constraints included the normal liver receiving a biological effective dose with α/β ratio of 3 Gy (BED3Gy) of 30 Gy3 < 40% and mean dose <28 Gy3. Doses to 0.1 cc of duodenum, stomach, and small bowel were limited to 4 Gy per fraction for 8 fractions or to 5 Gy per fraction for 6 fractions. We allowed minor dose constraint violations in patients without HBV and HCV carrier and no evidence of cirrhosis.
Evaluation
Patients were assessed every 3 months for the first 2 years and then every 4 months thereafter for treatment response and resectability of lesions. Physical examination and blood work were performed at every follow-up. A tri-phasic liver CT was obtained at 3 months after SBRT and then every 3 months in the first year and every 6 months thereafter. At our institution, the tumor response was routinely measured using Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1.
Local control (LC), progression-free survival (PFS), overall survival (OS), alpha-fetoprotein (AFP) response, and toxicity were evaluated. LC was defined as the absence of progressive disease within the PTV. Patients with liver resection or transplant during follow-up were censored for LC. A new lesion developing outside the PTV was regarded as intra-hepatic out-of-field failure. PFS was defined as the period from the date of starting TACE to the time of disease progression or the time at which the patient passed away, whichever occurred first. OS was calculated from the start of TACE until the date of final follow-up or death. An AFP response was defined as a drop of at least 20% from baseline.
Toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Toxicities were defined as adverse events that occurred >3 months after SBRT. All newly developed toxicities or toxicities that had progressed to 1 grade higher compared to baseline before treatment were considered as adverse events from SBRT. A grade 5 hepatic failure caused by SBRT was defined as death from hepatic failure after the development of acute grade 3 liver toxicities in the first 6 months without intra-hepatic progression.
Statistics
Wilcoxon signed-rank test was used to compare the AFP level at different time points. LC, PFS, and OS were calculated by Kaplan–Meier curves. The log-rank test was used to compare outcomes among survival curves for prognostic factors. Any factors that were significant in univariable analyses were subjected to multivariable analyses using the Cox proportional hazards regression model. A statistical level of p < 0.05 was considered significant. R version 3.2.5 (Vienna, Austria) was used for statistical analysis.
Results
Patients and treatment
From 2008 to 2015, 72 patients were treated under the combined TACE/SBRT protocol as presented above. Baseline patient and treatment characteristics are presented in Table 1. 51 patients (61%) had BCLC stage B disease, while remaining 21 patients (39%) had BCLC stage C disease. Among the patients with BCLC stage C disease, 13 (62%) had branch portal vein (PV)/inferior vena cava (IVC) invasion or minor thrombosis, 2 (10%) had a lymph node metastasis, 1 (5%) had distant metastases, 3 (14%) had both PV invasion and lymph node involvement, and 2 (9%) had both PV invasion and distant metastases. The median tumor size was 11.5 cm (range: 5.0–23.6 cm). 59 patients (82%) are hepatitis B carriers. No patients had received previous treatment. No patient dropped out after TACE, and all except one patient (98.6%) completed the planned SBRT treatment. The median prescription dose in an equivalent dose of 2 Gy per fraction (EQD2, α/β = 10) was 37.3 Gy10 (range, 23.8–72.0 Gy10). The median interval between TACE and SBRT was 22 days (range, 10–66 days).
Response and local control
The median follow-up was 16.8 months (range, 3–96 months). At 1 year and 2 years, there were 41 and 19 patients available for the analysis of local control, respectively. The corresponding LC at 1 year and 2 years was 93.6% (95% confidence interval CI = 87.6–100%) and 83.9% (95% CI = 72.7–96.9%), respectively (Fig. 1). Among the 66 patients who had at least one CT assessment, the best response after full treatment was a partial response (Supplementary material Fig. S1) occurring in 45 patients (68%) and otherwise a stable disease was found in 21 patients (32%). The median time to local recurrence was not reached. The size of the lesion was the only significant factor associated with local control (<15 cm vs. ≥15 cm, hazard ratio HR = 3.18, 95% CI = 1.74–5.81).
Among those patients with AFP elevation, an AFP response was observed in 82.6% of the patients. The median AFP was significantly different from baseline at 3 months, 6 months, 9 months, and 12 months after full treatment (Fig. 2).
Overall survival and time to progression
At the time of analysis, 3 patients (4.2%) were lost during follow-up while 43 patients (59.7%) had died. Death was related to cancer in 39 patients (90.7%) while 1 patient (2.3%) died of treatment-related liver failure, 2 patients (4.7%) died due to post-operative complications in subsequent treatments, and the remaining patient (2.3%) died because of unrelated causes. The median OS of the entire cohort was 19.9 months (95% CI, 11.6–30.6 months). For BCLC stage B and C, the median OS was 25.7 months (95% CI, 16.9–38.7 months) and 8.9 months (95% CI, 5.9–33.1 months), respectively (Fig. 3), with statistical significance show (p = 0.09). Among the patients with BCLC stage C disease (n = 21), the median OS of patients without extra-hepatic disease (n = 13) was significantly better than among those with extra-hepatic metastases (n = 8; 11.5 months versus 6.0 months, p < 0.04). Significant factors associated with OS for the whole patient group were the lesion size, the presence of extra-hepatic disease, and whether the patient received post-TACE/SBRT therapies (surgery, sorafenib, repeated TACE) or not (Table 2).
The median time to progression was 7.2 months (95% CI, 5.3–10.1 months). The PFS was 9.1 months (95% CI, 7.2–19.8 months) and 4 months (95% CI, 3.6–6.3 months) for BCLC B and C patients, respectively. For the first site of progression after initial treatment, 32 patients (56.1%) had new isolated out-of-field lesions in the liver, 10 (17.5%) had distant metastases, 3 (5.3%) had isolated in-field recurrences, while 12 (21.1%) had progression in multiple sites (intra- and extra-hepatic progression in 10 patients and intra-hepatic in-field and out-of-field progression in 2 patients). At the time of progression, 21 patients (36.8%) were treated with further local therapies (TACE or radiofrequency ablation) and 24 patients (17.5%) received sorafenib.
On the other hand, 12 (16.7%) patients with treatment response were subjected to curative surgical resections. Among them, the median size of the initial tumor was 12.5 cm (range 8.7–19.2 cm) compared to 7.7 cm (range 3.6–11 cm) post-treatment. Median reduction of longest tumor diameter was 38.5% (range: 23–59.3%). All patients had margin-negative resection (R0) performed and one had pathological complete response (PCR). Among 12 patients, 83.3% (n = 10) were still alive at the time of analysis and median survival was not yet reached. We found a trend suggesting that the tumor size (>15 cm) is a poor predictive factor for subsequent resection after TACE and SBRT treatment (HR = 0.34, 95% CI = 0.06–1.27, p = 0.11).
Toxicity
The toxicities of the combined treatment are summarized in Table 3. A total of 25 patients (34.7%) reported grade 3 or above toxicities after TACE, most commonly transient elevation of transaminase (n = 11, 15.2%) and bilirubin (n = 9, 12.5%), followed by pain (n = 3, 4.2%). For SBRT toxicities, grade 3 or above adverse events occurred in 12 (16.9%) patients, while gastrointestinal (GI) toxicity was reported in 2 patients (2.8%). No radiation-induced liver disease (RILD) was reported. Grade 3 or above elevation of transaminase was found in 3 individuals (4.2%). A decline in CP class without intra-hepatic progression was found in 10.6, 12.8, and 0% at 3, 6, and 12 months after treatment, respectively. Treatment-related death occurred in one patient (1.4%). The patient had central tumor of 12.5 cm in size compressed on the biliary tree. He developed cholangitis and hepatic failure at around 3 months after completion of SBRT.
Discussion
This study is one of the few reports to evaluate combined TACE with SBRT as initial treatment in advanced stage HCC patients. Compared to previous reports [22,23,24,25], most patients in this study had advanced disease and heavy tumor load. The median tumor size was 11.2 cm, and 25% of tumors had vascular involvement. Combined TACE plus SBRT demonstrated promising anti-tumor activity in this population, with a 1-year local control rate of 93.6% and an objective response rate (ORR) of 68%.
Patients receiving TACE often required repeated sessions, although the benefit of scheduled repeated TACE over on-demand TACE remains controversial [26]. In the work by Terzi et al. [27], only 40% of patients required a second session of trans-arterial treatment after initial TACE. According to our institutional protocol, no scheduled treatment was given unless disease progression occurred, to spare the patients from treatment-associated toxicity. In this series, TACE was given once prior to SBRT in 90% of the patients, and additional sessions of TACE were given in 26% of the patients. Among 55 patients with BCLC B disease, such a treatment approach resulted in a tumor response rate of 73.9%, which compared favorably with the 17–62% of TACE alone [28]. The response to TACE is usually worse in large tumors [29], but in the present series, among 44 evaluable patients with a tumor diameter >10 cm, an objective response was seen in 26 individuals (59%). For the survival outcome, the median OS was 25.7 months (95% CI, 16.9–38.7 months), which appears to be better than the historical results of 6–19.4 months by TACE alone in similar stage [6, 30]. The PFS of 9.1 months (95% CI, 7.2–19.8 months) also compared favorably to the historical results of 3–9 months by TACE alone [6].
In a previous meta-analysis, TACE plus radiotherapy was associated with better survival than TACE alone in unresectable HCC patients [31]. Furthermore, TACE plus SBRT was associated with significantly better OS than SBRT alone in the retrospective series of 127 HCC patients with size >5 cm [25]. Our findings add to the growing body of evidence that the combined approach provides therapeutic advantages over either TACE or SBRT alone.
Radioembolization using yttrium-90 (Y90)-tagged glass (TheraSpheres, MDS Nordion, Ottawa, Canada) or resin (SIR-Spheres, Sirtex Medical, Lane Cove, Australia) microspheres represent another option in this patient population [32, 33]. In the study by Salem et al. including 291 patients with a median tumor size of 7 cm, Y90 therapy was associated with response rate of 42% and time-to-progression of 7.9 months, which is at least comparable to that of TACE [33]. In the other recent phase II study including relatively small tumors (range from 2.3 to 3.7 cm for largest tumor size), Salem et al. demonstrated significantly better time-to-progression with Y90 radioemobilization than TACE (>26 months vs. 6.8 months) [34]. Whether radioembolization will result in similar local control to the combined treatment of TACE with SBRT, in particular in large tumors as in the present study, remains unclear and warrants further investigations.
For patients with BCLC stage C disease, the benefit of local therapy remains controversial [35] and few data are available on TACE plus SBRT in this population. In this study among 21 patients, the tumor response of 55% was significantly better than that of the sorafenib series, albeit with a similar OS of 8.9 months and PFS of 4 months [12, 13]. The improved local control did not translate into better survival in this population with a high competing risk of distant progression; out-field failure represented the predominant mode of progression and cause of death. Further analyses revealed that patients without extra-hepatic disease had better OS than those with distant metastases (11.5 months versus 6 months, p < 0.04), signaling that the combined local therapy may improve outcome if the disease remains within liver parenchyma; however, the results need to be interpreted in caution given the small sample size.
To date, there is no effective down-staging treatment for unresectable HCC patients. In this study, 12 (16.7%) individuals were able to receive curative resections after significant downsizing of tumor by TACE plus SBRT; all patients had margin-negative resection performed, and one individual with an initial tumor of 10 cm had achieved PCR. These findings provide preliminary evidence to support combined TACE and SBRT as down-staging treatment of unresectable HCC. We hypothesized that the favorable tumor response of the combined approach may render more patients suitable for curative resections. However, our analysis was retrospective and came from a single institution; also, the resectability criteria and surgical technique vary among different institutions. Therefore, our findings should not be generalized beyond this study population.
There is no size limit for a lesion in our protocol. Our analysis demonstrated that tumor size >15 cm (n = 20) is a significant poor prognostic factor, with a median OS of 7.2 months compared to 28.6 months for tumor size ≤15 cm (p < 0.001, HR 2.88). Despite the combined therapy can effectively induce tumor shrinkage, patients with advanced disease were often found to develop out-field progression shortly and resulting in limited survival. Based on this observation, the role of combined TACE plus SBRT seems to be limited in this population with short life expectancy. In the ongoing RTOG 1112 trial, patients with HCC > 15 cm were considered to be ineligible for the study [36]. Patients with large HCC often complain of tumor-related symptoms, for example pain, anorexia, and fatigue. Radiotherapy using conventional techniques has been proven to be an effective and efficient way to improve patients’ quality of life and control symptoms [37], and it may still be considered an appropriate local therapeutic approach for patients with huge tumor size.
There were no abnormal safety signals of combined TACE and SBRT demonstrated in the present series. The toxicity profile of merging two local therapies was similar to those of either treatment alone [6, 15, 16]. Treatment was well tolerated, with only 1 patient failing to complete the planned treatment. We observed a low rate of ≥ grade 3 GI toxicity (2.8%) and no cases of radiation-induced liver disease (RILD). CP score progression occurred in around 10% of individuals at 3 months and 6 months, which was similar to that observed in SBRT series [16]. Overall, the chemo-embolization prior to SBRT did not result in unexpected acute or late toxicities.
In a previous meta-analysis, the addition of radiotherapy to TACE increased the incidence of liver enzyme elevation, raised bilirubin, and in particular gastro-duodenal ulcer (odd ratio OR: 12.8) [31]. However, most studies included in the review utilized conformal radiation. In this study, only 1 (1.4%) patient developed ≥3 gastric ulcer; the observed low incidence was consistent with other series using a stereotactic technique, which allows the delivery of highly conformal radiation to maximally spare the adjacent organs at risk, like stomach, duodenum, and uninvolved liver. Most treated HCC lesions were large in size, with median diameter of 11.2 cm and GTV from 41 to 4009 cc. Consequently, the uninvolved liver volume (liver – GTV volume) was relatively small. We have made several efforts to mitigate the liver toxicity. First, we prescribed a modest dose of radiation (median EQD2: 37.3 Gy2); the dose was lower compared to other similar studies with less advanced tumor [22,23,24,25]. Secondly, a moderate dose of cisplatin, 40 mg, was utilized in TACE. Thirdly, an interval of median 3 weeks (median: 22 days, range: 10–66 days) was given after TACE to allow hepatic injuries to recover before SBRT. Finally, every hepatitis B carrier had preemptive anti-viral therapy prescribed.
Despite the fact that most patients were monitored and their data were collected in a prospective manner according to institutional protocol, we cannot entirely eliminate the intrinsic bias of a retrospective design. The single-arm design and short follow-up time also posed a limitation to the robustness of the findings. Furthermore, it was a mono-institutional series: our SBRT protocol adapts the radiation dose according to the dose constraints of organs at risk and allows patients to receive 5–10 fractions of radiation, which is not a common practice around the world. It is also worth noting that our response assessment followed the RECIST v1.1 criteria, which show poor concordance with the newer modified RECIST criteria that take into account tumor viability. Oldrini et al. showed higher rate of CRs was observed using mRECIST v1.1 criteria compared to RECIST v1.1 (57% vs. 20% at 3 months and 91.4% versus 41.6% at 12 months) [38]. Although mRECIST and other criteria such as the EASL (European Association for the Study of Liver Diseases) may be accurate for ablative, embolic, or system therapies, their application to SBRT remains unclear [39, 40]. Additionally, most of our patients were hepatitis B carriers. Further studies and parameters are needed to evaluate such a treatment in a Western population, where hepatitis C and alcoholism are the most frequent etiologies of HCC [41].
In conclusion, our experience suggested that with appropriate patient selection, judicious prescription of treatment, and advancement of radiotherapy technique, combined TACE and SBRT could be a safe and effective initial therapy in patients with unresectable BCLC B–C HCC. However, based on our data, such an approach may not be suitable for patients with large tumors (>15 cm) or extra-hepatic disease. Further trials are warranted to evaluate such treatment prospectively, preferably in the randomized setting to compare with the current standard of care, TACE and sorafenib.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. Ca Cancer J Clin 61(2):69–90. https://doi.org/10.3322/caac.20107
Llovet JM, Brú C, Bruix J (1999) Prognosis of Hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis 19(03):329–338. https://doi.org/10.1055/s-2007-1007122
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: An update. Hepatology 53(3):1020–1022. https://doi.org/10.1002/hep.24199
El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 132(7):2557–2576. https://doi.org/10.1053/j.gastro.2007.04.061
Fattovich G, Stroffolini T, Zagni I, Donato F (2004) Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 127(5, Supplement 1):S35–S50. https://doi.org/10.1053/j.gastro.2004.09.014
Yau T, Tang VYF, Yao T‑J, Fan S‑T, Lo C‑M, Poon RTP (2014) Development of Hongkong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 146(7):1691–1700. https://doi.org/10.1053/j.gastro.2014.02.032
Ng J, Wu J (2012) Hepatitis B‑ and hepatitis C‑related hepatocellular carcinomas in the United States: Similarities and differences. Hepat Mon 12(10 HCC):e7635. https://doi.org/10.5812/hepatmon.7635
Omata M, Lesmana LA, Tateishi R, Chen P‑J, Lin S‑M, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RTP, Shiina S, Cheng AL, Jia J‑D, Obi S, Han KH, Jafri W, Chow P, Lim SG, Chawla YK, Budihusodo U, Gani RA, Lesmana CR, Putranto TA, Liaw YF, Sarin SK (2010) Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 4(2):439–474. https://doi.org/10.1007/s12072-010-9165-7
Lo C‑M, Ngan H, Tso W‑K, Liu C‑L, Lam C‑M, Poon RT-P, Fan S‑T, Wong J (2002) Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35(5):1164–1171. https://doi.org/10.1053/jhep.2002.33156
Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J (2002) Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 359(9319):1734–1739. https://doi.org/10.1016/s0140-6736(02)08649-x
Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT (2005) Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int 25(6):1189–1196. https://doi.org/10.1111/j.1478-3231.2005.01170.x
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J‑F, de Oliveira AC, Santoro A, Raoul J‑L, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz J‑F, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390. https://doi.org/10.1056/NEJMoa0708857
Cheng A‑L, Kang Y‑K, Chen Z, Tsao C‑J, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang T‑S, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34. https://doi.org/10.1016/s1470-2045(08)70285-7
Chan SL, Mo FKF, Johnson PJ, Liem GS, Chan TC, Poon MC, Ma BBY, Leung TWT, Lai PBS, Chan ATC, Mok TSK, Yeo W (2011) Prospective validation of the Chinese University Prognostic Index and comparison with other staging systems for hepatocellular carcinoma in an Asian population. J Gastroenterol Hepatol 26(2):340–347. https://doi.org/10.1111/j.1440-1746.2010.06329.x
Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, Sherman M, Dawson LA (2008) Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 26(4):657–664. https://doi.org/10.1200/jco.2007.14.3529
Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RKS, Dinniwell RE, Kassam Z, Ringash J, Cummings B, Sykes J, Sherman M, Knox JJ, Dawson LA (2013) Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 31(13):1631–1639. https://doi.org/10.1200/jco.2012.44.1659
Sterzing F, Brunner TB, Ernst I, Baus WW, Greve B, Herfarth K, Guckenberger M (2014) Stereotactic body radiotherapy for liver tumors: Principles and practical guidelines of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol 190(10):872–881. https://doi.org/10.1007/s00066-014-0714-1
Wong VYW, Tung SY, Ng AWY, Li FAS, Leung JOY (2010) Real-time monitoring and control on deep inspiration breath-hold for lung cancer radiotherapy—Combination of ABC and external marker tracking. Med Phys 37(9):4673–4683. https://doi.org/10.1118/1.3476463
Wolthaus JWH, Sonke J‑J, van Herk M, Belderbos JSA, Rossi MMG, Lebesque JV, Damen EMF (2008) Comparison of different strategies to use four-dimensional computed tomography in treatment planning for lung cancer patients. Int J Radiat Oncol Biol Phys 70(4):1229–1238. https://doi.org/10.1016/j.ijrobp.2007.11.042
Chan M, Chiang CL, Lee V, Cheung S, Leung R, Wong M, Lee F, Blanck O (2017) Target localization of 3D versus 4D cone beam computed tomography in lipiodol-guided stereotactic radiotherapy of hepatocellular carcinomas. PLoS ONE 12(4):e174929. https://doi.org/10.1371/journal.pone.0174929
Chan MKH, Lee V, Chiang CL, Lee FAS, Law G, Sin NY, Siu KL, Wong FCS, Tung SY, Luk H, Blanck O (2016) Lipiodol versus diaphragm in 4D-CBCT-guided stereotactic radiotherapy of hepatocellular carcinomas. Strahlenther Onkol 192(2):92–101. https://doi.org/10.1007/s00066-015-0929-9
Takeda A, Sanuki N, Tsurugai Y, Iwabuchi S, Matsunaga K, Ebinuma H, Imajo K, Aoki Y, Saito H, Kunieda E (2016) Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer 122(13):2041–2049. https://doi.org/10.1002/cncr.30008
Honda Y, Kimura T, Aikata H, Nakahara T, Naeshiro N, Miyaki D, Nagaoki Y, Kawaoka T, Takaki S, Hiramatsu A, Waki K, Ishikawa M, Kakizawa H, Kenjo M, Awai K, Nagata Y, Chayama K (2014) Pilot study of stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. Hepatogastroenterology 61(129):31–36
Jacob R, Turley F, Redden DT, Saddekni S, Aal AKA, Keene K, Yang E, Zarzour J, Bolus D, Smith JK, Gray S, White J, Eckhoff DE, DuBay DA (2015) Adjuvant stereotactic body radiotherapy following transarterial chemoembolization in patients with non-resectable hepatocellular carcinoma tumours of ≥3 cm. HPB (Oxford) 17(2):140–149. https://doi.org/10.1111/hpb.12331
Su T‑S, Lu H‑Z, Cheng T, Zhou Y, Huang Y, Gao Y‑C, Tang M‑Y, Jiang H‑Y, Lian Z‑P, Hou E‑C, Liang P (2016) Long-term survival analysis in combined transarterial embolization and stereotactic body radiation therapy versus stereotactic body radiation monotherapy for unresectable hepatocellular carcinoma 〉5 cm. BMC Cancer 16:834. https://doi.org/10.1186/s12885-016-2894-9
Facciorusso A, Licinio R, Muscatiello N, Di Leo A, Barone M (2015) Transarterial chemoembolization: Evidences from the literature and applications in hepatocellular carcinoma patients. World J Hepatol 7(16):2009–2019. https://doi.org/10.4254/wjh.v7.i16.2009
Terzi E, Golfieri R, Piscaglia F, Galassi M, Dazzi A, Leoni S, Giampalma E, Renzulli M, Bolondi L (2012) Response rate and clinical outcome of HCC after first and repeated cTACE performed on demand. J Hepatol 57(6):1258–1267. https://doi.org/10.1016/j.jhep.2012.07.025
Schwarz RE, Abou-Alfa GK, Geschwind JF, Krishnan S, Salem R, Venook AP (2010) Nonoperative therapies for combined modality treatment of hepatocellular cancer: Expert consensus statement. HPB (Oxford) 12(5):313–320. https://doi.org/10.1111/j.1477-2574.2010.00183.x
Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind J‑FH (2016) Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 64(1):106–116. https://doi.org/10.1002/hep.28453
Huo Y, Eslick GD (2015) Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: A systematic review and meta-analysis. Jama Oncol 1(6):756–765. https://doi.org/10.1001/jamaoncol.2015.2189
Hong TS (2013) Radiotherapy for hepatocellular carcinoma with tumor vascular thrombus: Ready for prime time? J Clin Oncol 31(13):1619–1620. https://doi.org/10.1200/jco.2012.48.2703
Chow PKH, Gandhi M, Tan S‑B, Khin MW, Khasbazar A, Ong J, Choo SP, Cheow PC, Chotipanich C, Lim K, Lesmana LA, Manuaba TW, Yoong BK, Raj A, Law CS, Cua IHY, Lobo RR, Teh CSC, Kim YH, Jong YW, Han H‑S, Bae S‑H, Yoon H‑K, Lee R‑C, Hung C‑F, Peng C‑Y, Liang P‑C, Bartlett A, Kok KYY, Thng C‑H, Low AS-C, Goh ASW, Tay KH, Lo RHG, Goh BKP, Ng DCE, Lekurwale G, Liew WM, Gebski V, Mak KSW, Soo KC, Asia-Pacific Hepatocellular Carcinoma Trials Group (2018) SIRveNIB: Selective internal radiation therapy versus Sorafenib in asia-pacific patients with Hepatocellular carcinoma. J Clin Oncol 36(19):1913–1921. https://doi.org/10.1200/jco.2017.76.0892
Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, Mulcahy MF, Baker T, Abecassis M, Miller F, Yaghmai V, Sato K, Desai K, Thornburg B, Benson AB, Rademaker A, Ganger D, Kulik L, Lewandowski RJ (2016) Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 151(6):1155–1163. https://doi.org/10.1053/j.gastro.2016.08.029
Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, Sato KT, Wang E, Gupta R, Benson AB, Newman SB, Omary RA, Abecassis M, Kulik L (2010) Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: A comprehensive report of long-term outcomes. Gastroenterology 138(1):52–64. https://doi.org/10.1053/j.gastro.2009.09.006
Radiation Therapy Oncology Group (2012) RTOG 1112 protocol - RANDOMIZED PHASE III STUDY OF SORAFENIB VERSUS STEREOTACTIC BODY RADIATION THERAPY FOLLOWED BY SORAFENIB IN HEPATOCELLULAR CARCINOMA. https://www.rtog.org/Portals/0/RTOG%20Broadcasts/Attachments/1112_master_w_update_5.7.13.pdf
Soliman H, Ringash J, Jiang H, Singh K, Kim J, Dinniwell R, Brade A, Wong R, Brierley J, Cummings B, Zimmermann C, Dawson LA (2013) Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol 31(31):3980–3986. https://doi.org/10.1200/jco.2013.49.9202
Guha C, Kavanagh BD (2011) Hepatic radiation toxicity: Avoidance and amelioration. Semin Radiat Oncol 21(4):256–263. https://doi.org/10.1016/j.semradonc.2011.05.003
Oldrini G, Huertas A, Renard-Oldrini S, Taste-George H, Vogin G, Laurent V, Salleron J, Henrot P (2017) Tumor response assessment by MRI following stereotactic body radiation therapy for hepatocellular carcinoma. PLoS ONE 12(4):e176118. https://doi.org/10.1371/journal.pone.0176118
Vincenzi B, Di Maio M, Silletta M, D’Onofrio L, Spoto C, Piccirillo MC, Daniele G, Comito F, Maci E, Bronte G, Russo A, Santini D, Perrone F, Tonini G (2015) Prognostic relevance of objective response according to EASL criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco-regional therapies: A literature-based meta-analysis. PLoS ONE 10(7):e133488. https://doi.org/10.1371/journal.pone.0133488
Schaub SK, Hartvigson PE, Lock MI, Høyer M, Brunner TB, Cardenes HR, Dawson LA, Kim EY, Mayr NA, Lo SS, Apisarnthanarax S (2018) Stereotactic body radiation therapy for hepatocellular carcinoma: Current trends and controversies. Technol Cancer Res Treat 17:1533033818790217. https://doi.org/10.1177/1533033818790217
Gkika E, Bettinger D, Krafft L, Schultheiss M, Neeff HP, Maruschke L, Schulenburg M, Adebahr S, Kirste S, Nestle U, Thimme R, Grosu A‑L, Brunner TB (2018) The role of albumin–bilirubin grade and inflammation-based index in patients with hepatocellular carcinoma treated with stereotactic body radiotherapy. Strahlenther Onkol. https://doi.org/10.1007/s00066-017-1256-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C.L. Chiang, M.K.H. Chan, C.S.Y. Yeung, C.H.M. Ho, F.A.S. Lee, V.W.Y. Lee, F.C.S. Wong, and O. Blanck declare that they have no competing interests.
Caption Electronic Supplementary Material
66_2018_1391_MOESM1_ESM.tif
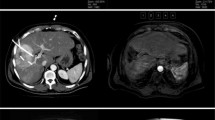
Supplementary material Fig. S1: Comparison of CT images before and after TACE and SBRT. a Baseline axial images, b Baseline coronal images, c Axial images 6 months post treatment, d Coronal images 6 months post treatment
Rights and permissions
About this article
Cite this article
Chiang, C.L., Chan, M.K.H., Yeung, C.S.Y. et al. Combined stereotactic body radiotherapy and trans-arterial chemoembolization as initial treatment in BCLC stage B–C hepatocellular carcinoma. Strahlenther Onkol 195, 254–264 (2019). https://doi.org/10.1007/s00066-018-1391-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1391-2
Keywords
- Stereotactic body radiotherapy
- Transarterial chemoembolization
- Initial therapy
- BCLC stage B-C
- Hepatocellular carcinoma