Abstract
Purpose
Retrospective evaluation of stereotactic body radiation therapy (SBRT) in patients with hepatocellular carcinoma (HCC).
Methods
We retrospectively analyzed 36 patients (45 lesions) treated between 2011 and 2017. Twenty-seven had previous treatments. Current treatment consisted of SBRT alone (n = 15) or selective transarterial chemoembolization (TACE) followed by SBRT to the same lesions (n = 21). Eight patients received additional local treatments to different lesions. Liver function was predominantly moderately restricted (Child A: 29, Child B: 6, Child C: 1). Treatment planning was based on 4D-computed tomography, dose/fractionation varied depending on location and size, most commonly 3 fractions of 12.5 Gy (65% isodose) and 5 fractions of 8 Gy (80% isodose).
Results
Median follow-up was 15 months. Local recurrence was observed in 3 lesions (7%), resulting in 1‑and 2‑year local control rates of 93%. The only significantly predicting factor was the use of abdominal compression. New hepatic lesions occurred in 19 patients (52%), 1‑ and 2‑year freedom-from-hepatic-failure (FFHF) was 39% and 32%, respectively. Only the number of treated lesions was predictive for FFHF. Sixteen patients have died, resulting in 1‑ and 2‑year overall survival (OS) of 64% and 41%, respectively, significantly impacted by the number of treated lesions and Child–Pugh class. Severe acute and late toxicity (≥grade 3) was observed in 3% and 8%, respectively. 6 patients (17%) received liver transplantation (OLT) after SBRT, of whom 5 showed pathological complete remission.
Conclusion
SBRT (±TACE) in highly pretreated HCC is effective and associated with excellent LC and low toxicity. SBRT may be used as definitive or bridging treatment prior to OLT. Patients with multifocal lesions have significantly decreased 1‑ and 2‑year FFHF and OS.
Zusammenfassung
Ziel
Ziel war die retrospektive Evaluation der Bestrahlungstherapie mittels Körperstereotaxie (SBRT) beim hepatozellulären Karzinom (HCC).
Methoden
Die retrospektive Analyse umfasst 36 Patienten (45 Läsionen), welche zwischen 2011 und 2017 behandelt wurden. Lokale Vorbehandlungen erhielten 27 Patienten. Aktuell wurden 15 Patienten mittels alleiniger SBRT und 21 mittels SBRT nach selektiver transarterieller Chemoembolisation (TACE) der gleichen Läsion behandelt. Bei 8 Patienten erfolgten zusätzlich lokale Behandlungen anderer Läsionen. Die Leberfunktion war überwiegend gering eingeschränkt (Child A: 29, Child B: 6, Child C: 1). Die Therapieplanung erfolgte mittels 4‑D-Computertomographie, die Dosis/Fraktionierung war abhängig von Lokalisation und Größe, üblicherweise 3 × 12,5 Gy (65%-Isodose) oder 5 × 8 Gy (80%-Isodose).
Ergebnisse
Die mediane Nachbeobachtungszeit betrug 15 Monate. Es traten 3 Lokalrezidive auf (7%) auf, die Ein-und 2‑Jahres-Lokalkontrollrate betrug 93%. Der einzige signifikante prädiktive Faktor war die Verwendung einer Bauchpresse. Bei insgesamt 19 Patienten (52%) traten neue hepatische Läsionen auf, das leberspezifische krankheitsfreie Ein- und 2‑Jahres-Intervall (FFHF) betrug 39% bzw. 32%. Lediglich die Zahl der behandelten Läsionen zeigte sich als prädiktiv für das FFHF. Es starben 16 Patienten, das Ein-und 2‑Jahres-Gesamtüberleben (OS) betrug 64% bzw. 41%. Die Anzahl der behandelten Läsionen und die Child-Pugh-Klasse waren signifikant mit dem OS assoziiert. Schwere akute oder späte Nebenwirkungen (≥Grad 3) traten bei 3% bzw. 8% der Patienten auf. Bei 6 Patienten (17%) wurde eine Lebertransplantation nach SBRT durchgeführt, hiervon zeigten 5 eine komplette pathologische Remission.
Schlussfolgerung
Die SBRT (±TACE) bei multipel vorbehandeltem HCC ist effektiv, toxizitätsarm und erzielt eine exzellente Lokalkontrolle. Sie eignet sich als definitive Therapiealternative oder als Überbrückung vor Lebertransplantation. Patienten mit multifokalen Herden zeigten ein signifikant reduziertes Ein- und 2‑Jahres-FFHF und -OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Primary liver tumors are among the most common malignancies and tumor-related causes of death worldwide [1]. Resection or orthotopic liver transplantation (OLT) are the main curative treatment options, with 5‑year survival rates of up to 60% after resection and 50–70% after transplantation [2]. Unfortunately, many patients are not suitable for resection due to comorbidities, poor liver function, major vascular involvement or multifocal spread of disease [1, 3]. According to common guidelines, locoregional therapies should be considered in these patients as definitive treatment or as bridging in patients awaiting liver transplantation [4]. Locoregional treatments are broadly categorized into arterially directed therapies (transarterial chemoembolization [TACE], transarterial chemoembolization with drug-eluting beds [DEB-TACE], or selective internal radiotherapy [SIRT]) and into local ablative techniques like radiofrequency ablation (RFA), percutaneous alcohol injection, microwave ablation, or minimally invasive stereotactic body radiotherapy (SBRT). Each of these treatments has its limitations and must be weighed in particular against the anticipated remaining liver function, the extent and location of the disease, and the existing co-morbidities. For example, lesions directly adjacent to major vessels or bile ducts are not well suited for RFA [5, 6] and patients with portal vein thrombosis are not eligible for TACE [2, 6]. Radiotherapeutic treatment paradigms have changed in the last decades in favor of highly conformal and precise treatment techniques such as intensity-modulated radiotherapy (IMRT) and stereotactic body radiation therapy (SBRT). The latter is a highly conformal technique delivering large doses in a small number of fractions [5]. It sufficiently spares dose to adjacent organs at risk due to its sharp dose fall-off outside the target, resulting in low toxicity rates, especially regarding radiation-induced liver disease (RILD), compared to the historical reports using conventional radiation therapy techniques [7,8,9,10]. Due to the enhanced biological effectivity of large single doses, adequate tumor control is maintained, as shown by the experience from other body sites [11,12,13]. For example, in stage I lung cancer, SBRT has shown to result in at least equal local control and overall survival rates compared to surgery [14]. After its first description in HCC patients by Bloomgren et al. in 1995 [15], liver SBRT has gained increasing attraction in the last decade mainly due to its low toxicity profile. Several retrospective studies have shown encouraging outcomes, with low to moderate toxicity and high local control rates (1 year LC: 90–100%) [1], but no randomized trials comparing SBRT to other local treatment options have been conducted so far and only scarce prospective data on the employment of SBRT in the treatment of HCC are available. Moreover, no generally accepted criteria for patient selection or a generally accepted dose and fractionation concept exists. We therefore report our experience with SBRT with or without prior selective TACE to the same lesion in patients with HCC.
Methods
SBRT has been implemented for HCC treatment at our center in 2011. Patient numbers steadily increased in the following years, with most of the included patients treated since 2015. For the current analysis, we retrospectively analyzed all HCC patients who underwent SBRT to 1–2 liver lesions.
Patient evaluation
Pretreatment investigations included at least MRI and/or contrast-enhanced multiphasic liver CT, CT staging to exclude distant metastases, and liver function tests. The indication for SBRT was seen in patients ineligible for other local treatment options (except TACE) or in case of multifocal HCC in combination with other local treatments. SBRT was scheduled based on multidisciplinary evaluation as a definitive treatment or bridging prior to planned orthotopic liver transplantation (OLT). The decision for listing was made by an interdisciplinary transplant board (without the presence of a radiation oncologist) based on internationally accepted criteria (Milan criteria, Model for End-Stage Liver Disease = MELD score) and legal considerations regarding transplant surgery. Patients suitable for OLT according to the transplant board decision and able to tolerate major surgery were scheduled for OLT. Afterwards, the decision about the adequate bridging strategy was made by another interdisciplinary board including surgeons, gastroenterologists, medical oncologists, interventional radiologists, and radiation oncologists. The strategy for bridging was chosen based on the size, localization, and number of lesions as well as on patient-related factors such as tolerability of surgery, comorbidities, and performance status, taking into account all possible advantages and disadvantages of the available techniques on an individual basis.
Treatment
Treatment of the irradiated lesions consisted of SBRT alone in 15 patients, while 21 patients received selective TACE to the same lesions upfront of SBRT (within 6 weeks). Additional local treatments to different lesions within 6 weeks of SBRT were performed in 8 patients. Of these patients, 6 received RFA, one received selective TACE, and one a combination of RFA and TACE. 27/36 patients had a median of 2 (range 1–8) previous local treatments (surgery, RFA, TACE, or SIRT), only 9 patients were treatment naïve. In 9 patients (25%), two lesions were treated at the same time, while 27 patients (75%) received SBRT to one lesion. OLT was performed in 6 patients after SBRT with a median interval of 6 months (range 1–8), including the only patient with Child-Pugh class C (CP C) cirrhosis.
Prior to SBRT, patients usually received CT-guided implantation of 1–3 fiducials (Visicoil™, IBA dosimetry GmbH, Schwarzenbruck, Germany or Gold-Anchor™, MBP Scherer Medizinprodukte, Krustetten, Austria) per lesion (n = 25), unless enhancement of lipiodol in patients with prior TACE (n = 11) was deemed sufficient to guide the procedure.
Patients were immobilized using a vacuum pillow in combination with an alpha-cradle. Abdominal compression was implemented in 2014, and since then, only discarded in patients not able to tolerate compression (for example, patients with large herniations after previous surgery). Patients treated prior to 2014 received SBRT using an ITV concept without abdominal compression. In total, 22 patients (66%) were treated with abdominal compression.
Treatment planning was based on contrast-enhanced 4D-CT. Gross tumor volume (GTV) was contoured as the visible tumor on the free-breathing CT and on all respiratory phases of the 4D-CT supplemented by information from MRI, if available. An internal target volume (ITV) was constructed and enlarged by an isotropic margin of 6 mm to obtain the planning target volume (PTV). Dose was generally prescribed to the PTV-surrounding isodose. Dose and fractionation varied dependent on localization, size, motion, and liver function. The most common schemes were 3 fractions of 12.5 Gy prescribed to the 65% isodose (64%) and 5 fractions of 8 Gy to the 80% isodose (22%) delivered every other day. No general difference in dose prescription was made between definitive and bridging treatments. Implanted fiducials or lipiodol enhancement were contoured to receive a fiducial or lipiodol ITV, which was used for daily patient set-up. Treatment was performed using daily CBCT image guidance.
Follow-up examinations (including physical examination, laboratory tests and MRI/CT of the liver) regularly took place at our department or the departments of gastroenterology/oncology every 3 months for the first year, every 6 months for the second year, and annually thereafter.
Statistical and legal considerations
Acute and late toxicity was scored according to CTCAE v4.03. Acute toxicity included events from the time of SBRT until 90 days after; late toxicity was defined as events occurring after more than 90 days from SBRT until disease progression. Severe toxicity was defined as ≥grade 3. Marked deterioration of liver function was defined as an increase in CP class or in case of CP C, as acute liver failure from SBRT until disease progression. Biologically effective dose (BED) at isocenter (maximum dose) was calculated according to the formula BED = nd[1 + d/alphabeta] with n being the number of fractions, d the single dose, and alphabeta = 10 for tumor control. Local control (LC) was defined as absence of tumor progression in the region of the treated lesion. Freedom-from-hepatic-failure (FFHF) was defined as absence of tumor progression in the liver. All time-to-event data were calculated from the first day of SBRT using the Kaplan–Meier method. All endpoints and subgroup analyses are reported referring to patients (not lesions) unless otherwise specified. Differences in subgroups were assessed by the log-rank test for univariate analysis. Due to the low number of events, multivariate analysis was not performed. P-values < 0.05 were considered significant. Patients who received OLT after SBRT were censored at the time of transplantation, except for in the comparison of OLT and non-OLT patients. The analysis was in accordance with the declaration of Helsinki in its latest version and was approved by our independent Ethics Committee.
Results
The majority of included patients had been treated since 2015 (Fig. 1), resulting in a median follow-up of 15 months for the entire cohort (3–73 months).
Patient characteristics
We analyzed 36 patients with 45 treated lesions. Median age was 63 years (31–83), 72% were male and the median Karnofsky performance score (KPS) was 90% (60–100%). HCC was based on liver cirrhosis in all patients, with a moderately restricted liver function in the majority (Child A: 29 [81%], Child B: 6 [17%], Child C: 1 [3%]). BCLC score was often advanced (BCLC A: 9 [25%], BCLC B: 12 [33%], BCLC C: 14 [39%], BCLC D: 1 [3%]). The main causes of the underlying liver cirrhosis were toxic (28%) and infectious (hepatitis B/C) liver damage (31%), 2 patients had autoimmune hepatitis (6%) and the remaining 13 (36%) had cryptogenic liver cirrhosis. Median GTV volume on free-breathing CT was 6.7 ccm (0.8–249) per lesion and median PTV volume per lesion was 60.3 ccm (14.9–512). For detailed patient characteristics see Table 1.
Local control
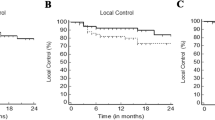
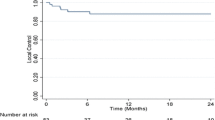
Local recurrence (LR) was observed in 2/36 patients (6%) and in 3/45 lesions (7%), translating into estimated 1‑ and 2‑year LC rates of 93% (per lesion, Fig. 2). Both patients with local recurrences also showed hepatic progression outside the irradiated region at the same time and died shortly afterwards of progressive liver disease. The use of abdominal compression was the only factor with a significant impact on LC (100% vs. 82% with vs. without abdominal compression, p = 0.027; Fig. 2). Pretreatment alpha-fetoprotein values (AFP) ≥ 10 showed a trend for poorer 1‑ and 2‑year local control (p = 0.067; 100% vs. 83% after 1 and 2 years) and larger GTV volumes tended to have worse LC (100 vs. 86% after 1 and 2 years; p = 0.088). No significant association between BED and local control was observed (Table 2).
Freedom-from-hepatic-failure
A total of 19 patients (52%) showed hepatic failure, resulting in estimated 1‑ and 2‑year FFHF rates of 39% and 32%, respectively (Fig. 3a). Median time to hepatic progression in those patients was 5.5 months (range 1–37). Only the number of lesions treated with SBRT (1-year FFHF single 46% vs. 0% multiple, p = 0.006) was significantly predictive for FFHF (Fig. 3b). The same result was seen in patients treated with any local ablative therapy in multiple lesions (two lesions with SBRT ± TACE or one with SBRT ± TACE and one with RFA/TACE) compared to those treated with SBRT ± TACE only in one lesion (1- and 2‑year FFHF single lesion 48% [1-and 2‑year] vs. 25% and 0% for multiple lesions, p = 0.004 [Fig. 3c]). Regarding the number of local liver-directed pretreatments, there was a trend toward improved FFHF in patients without any pretreatment (1-year FFHF and 2‑year FFHF without pretreatment 77% vs. 28% with pretreatments; p = 0.064). No significant association between BED and FFHF was observed, see Table 2.
Overall survival
So far, 16 patients (44%) have died, translating into an estimated median survival of 22 months with 1‑ and 2‑year OS rates of 64% and 41%, respectively (Fig. 4a) regarding all patients. The number of irradiated lesions was significantly predictive for OS (1-year OS single 81% vs. 22% multiple, p < 0.001; 2‑year OS 63% vs. 0%, p < 0.001 [Fig. 4d]). Patients with multiple lesions—regardless of the type of current treatment (two lesions with SBRT ± TACE or one with SBRT ± TACE and one with RFA/TACE)—also showed a significantly decreased OS (1-year OS single 78% vs. 47% multiple, 2‑year OS 59% vs. 18%, p = 0.021 [Fig. 4e]). Child–Pugh class (CP class) prior to SBRT was also predictive for OS (1-year OS CP A 70% vs. 33% CP B, 2‑year OS 45% vs. 0%, p = 0.043 [Fig. 4b]). BCLC score was also predictive for OS after 1 and 2 years (p = 0.041 [Fig. 4c]). No significant association between BED and overall survival was observed (Table 2). Similar results were obtained if the 6 patients with OLT were excluded from analysis (data not shown).
Toxicity
CT-guided fiducial placement was feasible without any severe acute complications. In two patients, one of the fiducials was dislocated into the vascular system, both remained asymptomatic. SBRT was well tolerated in general. Aside from mild side effects of grade 1 in 14% and grade 2 in 8% (mainly fatigue and gastrointestinal symptoms like nausea or abdominal pain), only one patient (3%) developed a severe acute toxicity. This patient already suffered from CP C cirrhosis prior to SBRT and developed acute hepatic failure (grade 4) after SBRT which was ultimately salvaged by OLT. This patient was still alive at last follow-up. No grade 5 toxicities attributable to SBRT were observed.
Late toxicity possibly related to SBRT was also generally mild. Only three patients (8%) developed grade 3 late toxicities. Two showed marked deterioration of liver function, with a decline in CP class from A (6 points) to class B (8 points) scored as grade 3; one of them also showed symptoms of fatigue, ascites, and abdominal pain clinically consistent with RILD symptoms, scored as grade 3. Mean liver dose was 12 and 15 Gy, although the second patient had the largest GTV (249 ccm) of all patients. However, our predefined dose constraints (defined as mean liver dose minus GTV < 18 Gy and >700 ml of liver receiving less than 15 Gy) were met in both patients. The third patient suffering from a centrally located large HCC developed cholangitis and a stricture of the proximal common hepatic bile duct. He was treated by antibiotics and repeated stent placement, but this side effect finally resolved (toxicity scored as grade 3). Maximum dose to the central biliary tract was 37.5 Gy in 3 fractions in this patient. No grade 4/5 late toxicities attributable to SBRT were observed.
SBRT ± TACE
Additional TACE to the same lesion within 6 weeks before SBRT was performed in 21 patients. In 11 of these patients, no fiducial markers were necessary because of sufficient lipiodol enhancement.
No significant differences were found for the addition of TACE compared to SBRT alone in any of the endpoints, see Table 2.
SBRT as bridging prior to OLT
SBRT was performed as bridging to transplant in 6 patients with 7 lesions. Three of them had liver cirrhosis CP A, 2 of them CP B, and one had CP C. Causes of the underlying liver cirrhosis were alcohol related in 2 patients, infectious (hepatitis B/C) in 2 patients, and the remaining 2 patients had autoimmune hepatitis. All patients fulfilled EASL criteria for diagnosis and Milan criteria for OLT eligibility. OLT was performed after a median interval of 6 months (range 1–8 months) from SBRT. Median follow-up from SBRT was 18 months (range 7–73) in those patients. All patients remained locally and distantly controlled until OLT. In 5/6 patients and 6/7 lesions, no residual HCC (pathologic complete response) was found in the explanted liver. The only patient with residual disease had been treated with SBRT only and received OLT early (1 month) after SBRT. No severe acute toxicity from SBRT was observed, except deterioration of liver function in the CP C patient (grade 4) salvaged by OLT, who is still alive. One patient died shortly after OLT due to postoperative complications (septic shock) and one due to distant failure 9 months after OLT, resulting in 1‑ and 2‑year OS rates of 66%. Comparing the OLT group with the non-OLT patients, the OLT group showed similar OS after 1 year (both 66%) but an increased OS rate after 2 years (66 vs. 43% in patients without OLT), but this difference did not reach statistical significance (p = 0.49).
Discussion
SBRT outcome
We observed encouraging 1‑ and 2‑year LC rates of 93% in our cohort of patients treated with SBRT for HCC. These results are in line with other series reporting 1‑year LC rates of 65–100% using similar SBRT approaches [9, 16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. For example, Bujold et al. [24] observed a 1-year LC control rate of 87% in the largest published prospective (phase I/II) trial including 102 HCC patients, which showed similar patient characteristics to our cohort with regard to lesion size and number. Nabavizadeh et al. [30] and Jeong et al. [31] recently published large retrospective series and found even higher 1‑year LC rates of 97% and 99%. Based on those and our results, SBRT seems to be very effective in controlling disease at least locally. The high (so-called ablative) doses used within this approach seem to effectively destroy all malignant cells in the vast majority of the treated lesions, as shown by the high pathological complete response rate of 86% achieved in our patients who received OLT later on. Interestingly, we identified the use of an abdominal compression device as the only significant predictive factor for LC. Abdominal compression has been shown to reduce respiratory motion of the liver [33], thus resulting in smaller ITV and PTV volumes, which might have prompted a more adequate target volume coverage. However, this finding should be regarded very cautiously given the low number of events in our study. In contrast, we could not confirm the prognostic value of several factors described by others like dose, fractionation, and lesion size [9, 21, 23], probably again due to the small sample size and the low number of events.
Regarding OS, we observed 1‑ and 2‑year rates of 64% and 41%. These findings are in the range of published series describing 1‑year survival rates of 32–94% [9, 16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31], although the wide range clearly indicates the inclusion of inhomogeneous patient groups in the published reports. We identified the number of treated lesions as well as CP class and BCLC stage prior to SBRT as predictive factors for OS in our cohort. Surprisingly, the number of lesions has been evaluated with regard to OS in only very few of the studies including patients with multiple lesions. Bujold et al. [24] reported on 102 patients enrolled in two prospective phase II trials and did not find a correlation of OS with the number of lesions. Su et al. [27] analyzed 132 Chinese patients retrospectively and reported a non-significant but remarkable decrease in 2‑year OS of 85%, 77%, and 63% in patients with 1, 2, or 3 lesions. Moreover, the number of lesions was predictive for progression-free survival (PFS) in their study. Scorsetti et al. [9] did not directly evaluate the number of lesions as a prognostic factor. However, they included only patients with up to three lesions with a single diameter <6 cm and reported a significant decrease in OS for patients with a cumulative GTV diameter of >5 cm, indicating that patients with more than one lesion may be at a higher risk for death. In contrast, many groups have consistently reported CP class as a prognostic factor for OS [9, 17, 20, 23, 31]. For example, Lasley et al. [17] described a median OS of 45 months in CP A versus 17 months in CP B patients in their prospective trial. Huang et al. [23] retrospectively analyzed 119 patients and reported a significantly decreased 3‑year OS of 62% in CP B patients compared to 86% in CP A patients. CP class was also confirmed as an independent prognostic factor in their multivariate analysis. Moreover, CP B patients seem to be at a higher risk for severe toxicities [17, 20] and thus might be not ideal candidates for SBRT or should at least be selected very carefully. A similar or even higher prognostic value has been reported for BCLC stage. For example, Marrero et al. [34] analyzed 239 patients and found that BCLC stage had the best prognostic stratification compared to several other commonly used staging systems.
In contrast to the encouraging results with regard to LC and OS, we observed a high number of liver outfield failures translating into rather poor 1‑ and 2‑year FFHF rates of 39% and 32%, respectively. The only factor with a significant impact on FFHF was the number of treated lesions. Similar results have been described by others in comparable patient cohorts. For example, Takeda et al. [28] enrolled 101 patients in a phase II trial combining TACE and SBRT and found a 3-year intrahepatic-failure-free rate of only 34%, although only patients with a single lesion were eligible. Bujold et al. [24] described 11 infield but 61 outfield intrahepatic failures in their series of 102 patients and Huang et al. [23] also identified outfield intrahepatic failure as the main pattern of recurrence. This may implicate that patients with multiple lesions detected on imaging have a higher risk for further subclinical disease in general and might not be ideal candidates for SBRT or other local treatments. However, most of our patients had already been treated with other local treatments and received SBRT only because they were deemed ineligible for other treatments except TACE or systemic agents. In this heavily pretreated cohort, the risk for developing further recurrences might be simply increased compared to treatment-naïve patients due to selection of an unfavorable biology.
Toxicity
With the possibility of outfield failures and the need for salvage treatments after locally ablative therapies, preservation of liver function and toxicity issues gain importance in the decision process for specific treatments. With SBRT, we observed a high treatment compliance with generally mild side effects in the majority of patients. Severe acute and late toxicities were only observed in 3% and 8% of our patients, respectively. These results compare favorably with other series, which reported acute grade 3+ side effects in 5–37% including up to 7% deaths [1], mainly in CP B patients. The main severe side effect regardless of its onset in our series was deterioration of liver function in 8% of our patients. Distinctly higher rates (13–29%) of a decline in CP class after SBRT have been reported by others [18, 24, 35], although some authors described a marked recovery over time [24]. However, some of these series included a higher percentage of CP B patients and treated larger lesions resulting in more dose to normal liver tissue. Both factors have been shown to be associated with toxicity [17, 35]. For example, Lasley et al. [17] observed severe liver toxicity of 38% in CP B compared to 11% in CP A patients and described several dose–volume factors associated with increased liver toxicity, especially in CP B patients. Culleton et al. [25] evaluated specifically patients with CP B/C and observed a decline of ≥2 points in 63% after 3 months. Therefore, SBRT should be used with caution in patients with impaired pretreatment liver function (CP B), while it seems to be generally well tolerated in CP A patients. Moreover, a recent systematic review demonstrated well-preserved quality of life after SBRT at least similar or even favorable compared to other approaches [36].
SBRT as bridging to transplant
OLT is the most effective treatment for HCC with underlying cirrhosis [37]. However, the availability of OLT may be limited by donor organ shortages resulting in prolonged waiting times [37]. Therefore, effective treatments have to be offered in between to maintain disease control until availability of a donor organ (so-called bridging) [37]. Similarly to definitive treatment, different methods—including RFA, TACE, SIRT, and SBRT—are currently in use, depending on the location and size of the lesion and patientsʼ comorbidities [37]. In our cohort, we observed a pathological complete response (pCR) in the explanted liver in 5/6 (83%) patients treated with OLT after SBRT at a median interval of 6 months. These results compare favorably with the (rare) evidence published in the literature. For example, Uemura et al. [38] reported 28% pCR and 22% extensive pathological partial responses (pPR) after SBRT with 40–50 Gy in 4–6 fractions followed by OLT in 11 patients. O’Connor et al. [39] found a pCR rate of 27% in 10 patients treated with SBRT (51 Gy in 3 fractions) followed by OLT and Moore et al. [40] observed 27% pCR and 55% pPR in a series of 11 patients treated by SBRT with 30–54 Gy in 3–5 fractions. This difference cannot be easily attributed to different doses, as BEDs in all studies were at least similar and considered ablative. A longer time interval from SBRT to OLT may theoretically also result in higher pCR rates; however, our median interval (6 months) was in the range of the mentioned studies (4–8 months) [38,39,40]. However, in contrast to the mentioned studies, SBRT was preceded by TACE within 6 weeks in 4 of 5 patients with pCR in our cohort. TACE alone has been reported to result in 30–44% complete tumor necrosis in explanted livers [37]. Therefore, one may speculate that the combination of TACE and SBRT in the majority of transplanted patients has contributed to the higher pCR rate. However, given the small number of patients, it cannot be fully ruled out that the difference occurred simply by chance or was triggered by other factors. Nevertheless, based on the data from our and other studies, SBRT seems to be a suitable method for bridging patients waiting for OLT.
Limitations
Our study has some limitations, especially its retrospective nature, the rather short follow-up, and the limited number of patients. However, in the absence of randomized trials and with limited data from prospective studies, retrospective evaluation seems a suitable way to gain more information in a heterogenous patient group treated with a variety of slightly different approaches.
Conclusion
SBRT in highly pretreated patients with HCC resulted in excellent LC and acceptable OS with low toxicity. OS was predicted by pretreatment number of lesions and pretreatment CP class. However, especially patients with multiple lesions were at a high risk for outfield intrahepatic failures indicating a possible need for improved patient selection and/or additional therapies. SBRT can be used as definitive treatment or as bridging to OLT.
Abbreviations
- 4D-CT:
-
four-dimensional computed tomography
- AFP:
-
alpha-fetoprotein
- BCLC:
-
Barcelona Clinical Liver Cancer
- BED:
-
biologically effective dose
- CBCT:
-
cone beam computed tomography
- ccm:
-
cubic centimeters
- CP class:
-
Child–Pugh class
- CT:
-
computed tomography
- CTCAE:
-
Common Toxicity Criteria for Adverse Events
- FFHF:
-
freedom from hepatic failure
- GTV:
-
gross tumor volume
- HCC:
-
hepatocellular carcinoma
- IF:
-
in-field
- IMRT:
-
intensity-modulated radiation therapy
- ITV:
-
internal target volume
- KPS:
-
Karnofsky performance score
- LC:
-
local control
- LR:
-
local recurrence
- MRI:
-
magnetic resonance imaging
- OLT:
-
orthotopic liver transplantation
- OS:
-
overall survival
- pCR:
-
pathologic complete response
- PFS:
-
progression-free survival
- pPR:
-
pathologic partial response
- PTV:
-
planning target volume
- RFA:
-
radiofrequency ablation
- RILD:
-
radiation-induced liver disease
- SBRT:
-
stereotactic body radiation therapy
- SIRT:
-
selective internal radiotherapy
- TACE:
-
transarterial chemoembolization
References
Murray LJ, Dawson LA (2017) Advances in stereotactic body radiation therapy for hepatocellular carcinoma. Semin Radiat Oncol 27:247–255
Waller LP, Deshpande V, Pyrsopoulos N et al (2015) Hepatocellular carcinoma: a comprehensive review. World J Hepatol 7:2648–2663
Delis SG, Dervenis C (2008) Selection criteria for liver resection in patients with hepatocellular carcinoma and chronic liver disease. World J Gastroenterol 14:3452–3460
NCCN Clinical Practice Guidelines in Oncology—hepatobiliary cancers version 2/2019. https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
Kalogeridi MA, Zygogianni A, Kyrgias G et al (2015) Role of radiotherapy in the management of hepatocellular carcinoma: a systematic review. World J Hepatol 7:101–112
Gerum S, Heinz C, Belka C et al (2018) Stereotactic body radiation therapy (SBRT) in patients with hepatocellular carcinoma and oligometastatic disease. Radiat Oncol 13:100
Lawrence TS, Robertson JM, Anscher MS et al (1995) Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 31:1237–1248
Dawson LA, Normolle D, Balter JM et al (2002) Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 53:810–821
Scorsetti M, Comito T, Cozzi L et al (2015) The challenge of inoperable hepatocellular carcinoma (HCC): results of a single-institutional experience on stereotactic body radiation therapy (SBRT). J Cancer Res Clin Oncol 141:1301–1309
Boda-Heggemann J, Jahnke A, Chan MK et al (2018) Direct dose correlation of MRI morphological alterations of healthy liver tissue after robotic liver surgery. Strahlenther Onkol 194:414–424
Fleckenstein J, Boda-Heggemann J, Siebelist K et al (2018) Non-coplanar VMAT combined with non-uniform dose prescription markedly reduces lung dose in breath-hold lung SBRT. Strahlenther Onkol 194:815–823
Moustakis C, Blanck O, Ebrahimi Tazehmahalleh F et al (2017) Planning benchmark study for SBRT of early stage NSCLC—results of the DERO working group stereotactic radiotherapy. Strahlenther Onkol 193:780–790
Gkika E, Adebahr S, Kirste S et al (2017) Stereotactic body radiotherapy (SBRT) in recurrent or oligometastatic pancreatic cancer. Strahlenther Onkol 193:433–443
Chang JY, Senan S, Paul MA et al (2015) Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 16:630–637
Blomgren H, Lax I, Näslund I et al (1995) Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 34:861–870
Gerum S, Roeder F (2019) Stereotactic body radiation therapy (SBRT) in patients with hepatocellular carcinoma (HCC): a mini-review. World J Gastrointest Oncol 11:367–376
Lasley FD, Maninna EM, Johnson CS et al (2015) Treatment variables related to liver toxicity in patients with hepatocallular carcinoma, Child-Pugh class A and B enrolled in a phase 1–2 trial of stereotactic body radiation therapy. Pract Radiat Oncol 5:e443–449
Mendez-Romero A, Wunderink W, Hussain SM et al (2006) Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase I–II study. Acta Oncol 45:831–837
Tse RV, Hawkins M, Lockwood G et al (2008) Phase I Study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 26:657–664
Cardenes HR, Price TR, Perkins SM et al (2010) Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol 12:218–225
Kang JK, Kim MS, Cho CK et al (2012) Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer 118:5424–5431
Price TR, Perkins SM, Sandrasegaran K et al (2012) Evaluation of response after stereotactic body radiotherapy for hepatocellular carcinoma. Cancer 118:3191–3198
Huang WY, Jen YM, Lee MS et al (2012) Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 84:355–361
Bujold A, Massey CA, Kim JJ et al (2013) Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 31:1631–1639
Culleton S, Jiang H, Haddad CR et al (2014) Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol 111:412–417
Sanuki N, Takeda A, Oku Y et al (2014) Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol 53:399–404
Su TS, Liang P, Lu HZ et al (2016) Stereotactic body radiation therapy for small primary or recurrent hepatocellular carcinoma in 132 chinese patients. J Surg Oncol 113:181–187
Takeda A, Sanuku N, Tsurugai Y et al (2016) Phase 2 Study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer 122:2041–2049
Moon DH, Wang AZ, Tepper JE (2018) A prospective study of the safey and efficacy of liver stereotactic body radiotherapy in patients with and without prior liver-directed therapy. Radiother Oncol 126:527–533
Nabavizadeh N, Waller JG, Fain R et al (2018) Safety and efficacy of accelerated hypofractionation and stereotactic body radiation theraoy for hepatocellular carcinoma patients with varying degrees of hepatic impairment. Int J Radiat Oncol Biol Phys 100:577–585
Jeong Y, Jung J, Cho B et al (2018) Stereotactic body radiation therapy using a respiratory-gated volumetric-modulated arc therapy technique for small hepatocelluar carcinoma. BMC Cancer 18:416
Chiang CL, Chan MKH, Yeung CSY et al (2019) Combined stereotactic body radio-therapy and trans-arterial chemoembolization as initial treatment in BCLC stage B‑C hepatocellular carcinoma. Strahlenther Onkol 195:254–264
Heinzerling JH, Anderson JF, Papiez L et al (2008) Four-dimensional computed tomography scan analysis of tumor and organ motion at varying levels of abdominal compression during stereotactic treatment of lung and liver. Int J Radiat Oncol Biol Phys 70:1571–1578
Marrero JA, Fontana RJ, Barrat A et al (2005) Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an american cohort. Hepatology 41:707–716
Andolino DL, Johnson CS, Maluccio M et al (2011) Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 81:e447–e453
Mutsaers A, Greenspoon J, Walker-Dilks C et al (2017) Systematic review of patients reported quality of life following stereotactic ablative radiotherapy for primary and metastatic liver cancer. Radiat Oncol 12:110
Rubinstein MM, Kaubisch A, Kinkhabwala M, Reinus J, Liu Q, Chuyy JW (2017) Bridging therapy effectiveness in the treatment of hepatocellular carcinoma prior to orthotopic liver transplantation. J Gastrointest Oncol 8:1051–1055
Uemura T, Kirichenko A, Bunker M, Vincent M, Machado L, Thai N (2019) Stereotactic body radiation therapy: a new strategy for locoregional treatment for hepatocellular carcinoma while awaiting liver transplantation. World J Surg 43:886–893
O’Connor JK, Trotter J, Davis GL, Dempster J, Klintmalm GB, Goldstein RM (2012) Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl 18:949–954
Moore A, Cohen-Naftaly M, Tobar A, Kundel Y, Benjaminov O, Braun M, Issachar A, Mor E, Sarfaty M, Bragilovski D, Hur BR, Gordon N, Stemmer SM, Allen AM (2017) Stereotactic body radiation therapy (SBRT) for definitive treatment and as a bridge to liver transplantation in early stage inoperable hepatocellular carcinoma. Radiat Oncol 12:163
Author information
Authors and Affiliations
Contributions
SG performed data acquisition and participated in patient treatment, statistical analysis, and in drafting the manuscript. CH participated in treatment of the patients and statistical analysis. FW, PP, ED treated the patients and participated in data acquisition. CB revised the manuscript critically. FR participated in data acquisition, statistical analysis, treatment of the patients, drafting the manuscript and critically reviewed the data and the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
S. Gerum, C. Heinz, C. Belka, F. Walter, P.M. Paprottka, E.N. De Toni, and F. Röder declare that they have no competing interests.
Ethical standards
The study was approved by the Ethics committee of the University of Munich (LMU), reference number 617-16. Informed consent was obtained from all individual participants included in the study.
Additional information
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Rights and permissions
About this article
Cite this article
Gerum, S., Heinz, C., Belka, C. et al. Stereotactic body radiotherapy in patients with hepatocellular carcinoma in a multimodal treatment setting. Strahlenther Onkol 196, 334–348 (2020). https://doi.org/10.1007/s00066-019-01540-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-019-01540-8
Keywords
- Liver
- Hepatocellular carcinoma
- Stereotactic body radiation therapy
- Orthotopic liver transplantation
- Multimodal treatment