Abstract
Objective
This study aimed to estimate the probability of an unfavourable aesthetic outcome (AO) 2 years after breast-conserving therapy (BCT) and evaluate the possible influence of brachytherapy (BT) and external beam radiotherapy (EBRT) boost on patient-reported outcomes (PROs) and AO.
Patients and methods
Patients treated with BCT starting April 2015 were prospectively included. Selection of the boost technique followed an in-house flowchart based on the depth of the tumour bed. An electron boost was performed for a superficial clinical target volume (maximum 28 mm under the epidermis), a BT boost was proposed in all other cases. Patients were followed-up for 2 years. AO was scored by the BCCT.core software and the patient. Further PROs were measured with the EORTC QLQ-C30, QOL-BR23 and the BIBCQ questionnaires.
Results
The analysis included 175 patients, 80 received a BT boost and 95 an EBRT boost. BT patients were significantly older; had a higher breast cup and band size, body mass index and surgical specimen weight of the wide excision; more seroma at baseline and less positive surgical section margins than patients in the EBRT group, and more patients drank alcohol. Cancer- and breast cancer-specific quality of life (QOL) and body image did not differ between the boost techniques over time. Although mean scores for breast symptoms and sexual enjoyment did differ significantly over time (p = 0.05 and < 0.01, respectively), the effect was due to differences before boost administration. Measured with BCCT.core, AO was unfavourable in 28% of patients 2 years after treatment (31% scored by the patient) and results were similar in the BT and EBRT groups.
Conclusion
Using the presented flowchart (See Verhoeven et al. [16]), AO and PROs on QOL or body image up to 2 years after BCT are not influenced by the boost technique.
Zusammenfassung
Zielsetzung
Ziel war es, die Wahrscheinlichkeit eines ungünstigen ästhetischen Ergebnisses (AE) 2 Jahre nach brusterhaltender Therapie (BCT) abzuschätzen sowie den möglichen Einfluss von Brachytherapie (BT) und perkutanem Strahlentherapie(ERBT)-Boost auf patientenberichtete Ergebnisse (PBE) und AE zu beurteilen.
Patienten und Methoden
Prospektiv inkludiert wurden Patientinnen, die ab April 2015 mit BCT behandelt wurden. Die Auswahl der Boost-Technik erfolgte über einen hauseigenen Ablaufplan abhängig von der Tiefe des Tumorbetts. Ein Elektronenboost wurde durchgeführt bei einem oberflächlichen klinischen Zielvolumen (weniger als 29 mm unter der Epidermis), ein BT-Boost bei allen anderen Fällen. Die Patientinnen wurden bis zu 2 Jahre nachbeobachtet. Das AE wurde durch die Patientinnen und die BCCT.core-Software bewertet. Weitere PBE wurden mit dem EORTC QLQ-C30, QOL-BR23 und dem BIBCQ-Fragenbogen gemessen.
Ergebnisse
Die Studie beinhaltete 175 Patientinnen: 80 bekamen einen BT- und 95 einen ERBT-Boost. Patientinnen der BT-Gruppe waren signifikant älter, hatten eine größere Brustgröße, einen größeren Brustumfang, einen größeren Body-Mass-Index, höheres chirurgisches Probengewicht der breiten Exzision, mehr Serom zu Beginn der Analyse, weniger positive Resektionsränder und mehr Alkoholkonsum als die der ERBT-Gruppe. Krebs- oder brustkrebsspezifische Lebensqualität (QOL) und Körperbild unterschieden sich zwischen beiden Boost-Techniken im Zeitverlauf nicht signifikant. Durchschnittswerte für Symptome der Brust und sexuellen Genuss waren im Zeitverlauf zwar signifikant unterschiedlich zwischen beiden Gruppen, waren aber Unterschieden vor Verabreichung des Boosts geschuldet (jeweils p = 0,05 und < 0,01). Gemessen mit BCCT.core war das AE bei 28 % der Patientinnen 2 Jahre nach Behandlung ungünstig (31 % bewertet durch die Patientinnen); die Ergebnisse beider Gruppen waren vergleichbar.
Schlussfolgerung
Unter Verwendung des vorgestellten Ablaufs hat die benutzte Boost-Technik bis zu 2 Jahre nach BCT keinen Einfluss auf AE und PBE im QOL oder Körperbild.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Every year, 1.7 million women are diagnosed with breast cancer, making it the most common malignancy in women worldwide [1]. Breast-conserving therapy (BCT), including breast-conserving surgery followed by radiotherapy (RT), is the cornerstone of locoregional treatment, since randomized trials have shown survival rates equal to mastectomy [2, 3]. Standard adjuvant RT starts with whole-breast irradiation and is followed by a sequential boost to the tumour bed in high-risk patients [4]. The boost can be administered either by external beam radiotherapy (EBRT) or by brachytherapy (BT). The oncological outcome of breast cancer patients has improved during recent decades, resulting in a growing number of long-term survivors and 10-year survival rates exceeding 70% [5, 6]. This high number has imposed a new, growing demand to identify the long-term toxicity of treatment and its impact on quality of life (QOL; [7]).
Prior research on long-term toxicity due to breast cancer RT has created insight into cardiac and pulmonary toxicity and secondary malignancies [8, 9]. However, for other outcomes such as aesthetic outcome (AO), the available data remain unclear, mainly due to heterogeneity between studies. Different studies use a variety of methods to assess AO, such as scoring by the physician, scoring by the patient or automated scoring based on photographs. Moreover, AO is reported using a large set of outcome parameters such as asymmetry, scar appearance or skin colour differences [10]. In 2012, Cardoso et al. published guidelines to homogenize future research on AO [10]. Recently, knowledge on long-term toxicity and the treatment itself has gained attention regarding the effect on QOL. Health-related QOL represents the patient’s physical, psychological and social response to the disease and its treatment. The term “patient-reported outcome measures” (PROMs) was introduced, comprising health-related QOL and also broader concepts such as patient satisfaction with care [11]. In clinical practice, insight into patient-reported outcomes (PROs) could help generate a roadmap of recovery for the patients which predicts functioning in specific areas over time, to help answer the patients’ questions and set appropriate expectations, and which could even be incorporated into the process of shared decision-making [12, 13].
With similar local control between different boost techniques, it seems interesting to further assess the influence on AO and PROs. Firstly, PROs might analyse the burden of the different RT techniques in the short-term. An EBRT and a BT boost have some intrinsic differences, such as the longer treatment duration with EBRT (5–8 daily fractions for EBRT versus 1 fraction for BT), the outpatient procedure with EBRT compared to day-clinic hospitalization with BT, the invasive procedure and anaesthesia in case of BT, and the anxiety related to the BT procedure itself [14]. Secondly, long-term PROs on AO, QOL and body image are of interest. The boost techniques namely also differ from a radiobiological point of view, with a theoretical advantage of BT on AO due to higher doses to small volumes and a skin-sparing effect, and 2–3-times larger irradiated volumes with a sequential EBRT boost [14, 15].
The purpose of the present study was to prospectively follow a group of patients treated with BCT for 2 years and to estimate the probability of an unfavourable AO 2 years after RT. In this study, the influence of the RT boost technique on short- and long-term outcomes was evaluated in the BCT setting. We have therefore investigated the relationship of boost techniques to PROs on QOL and body image as well as AO.
Patients and methods
Patient cohort
All consecutive patients treated for breast cancer with BCT with curative intent who consulted the RT department at the University Hospitals Leuven, Belgium, between April 2015 and April 2016 were invited to participate in this prospective cohort study. Informed consent was obtained from all individual participants. Patient-, tumour- and treatment-related characteristics were prospectively collected. The study was approved by the Clinical Trial Centre and the Ethical Committee of our institution.
BCT included whole-breast irradiation followed by a boost to the tumour bed. Two fractionation schedules were applied for whole-breast irradiation: normofractionation (50 Gy in 25 fractions) and hypofractionation (42.56 Gy in 16 fractions). For the boost, either 16 Gy in 8 fractions (20 Gy in case of positive margins) or 13.3 Gy in 5 fractions (e.g., 18.62 Gy in case of positive surgical section margins) were applied with EBRT. High dose-rate BT doses were 8.5 Gy in 1 fraction (10 Gy in case of positive margins). For selection of the boost technique, an in-house developed flowchart based on the depth of the tumour bed was used. For a clinical target volume lying more than 28 mm beneath the epidermis, BT was chosen over an electron boost because of skin doses. If the patients refused a BT boost or it was technically impossible, a photon boost was suggested [16]. Patients were informed on the proposed boost technique within the first or second week of RT. Treatment regimens were discussed at the multidisciplinary tumour board. Regional nodal irradiation was allowed. Hormonal therapy included tamoxifen and aromatase inhibitors in different regimens and was prescribed in hormone receptor-positive breast cancer patients. Chemotherapy was given according to standard protocol and included epirubicin, cyclophosphamide and taxanes.
Patient-reported outcome measures
Several tools have been used to measure PROs in patients with a history of breast cancer [17]. In 2017, the International Consortium for Health Outcomes Measurement developed a standard set of value-based measures for breast cancer [18]. The working group recommended use of at least a cancer-specific and a breast cancer-specific questionnaire and, optionally, an extra questionnaire to capture extra outcomes. Based on this guideline and the review by Kanatas et al., the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 and QLQ-BR23 were used for cancer-related QOL and the Body Image After Breast Cancer Questionnaire (BIBCQ) for body image [18, 19].
The QLQ-C30 was developed as a cancer-specific QOL questionnaire. It has 30 items that form five functional scales (physical, role, emotional, cognitive and social), a global QOL scale, three symptom scales (fatigue, pain and nausea/vomiting), five single-item symptom measures (dyspnoea, insomnia, appetite loss, constipation and diarrhoea), and one financial impact question. These are coded with the same response categories (scored 1 to 4): “not at all”, “a little”, “quite a bit” and “very much”; except for the global QOL scale, which is scored as a visual analogue scale ranging from 1 (very bad) to 7 (excellent). All the scales and single-item scores are transformed to range from 0 to 100. A high score for a functional scale or global QOL indicates better levels of functioning and/or global QOL, with a score above 80 considered to be satisfactory. A high score for a symptom scale or item represents a high level of symptoms or problems, with a score below 20 considered to be satisfactory [20]. Clinical significance between groups is interpreted according to Osaba et al.: the mean differences from 5 to 10 points can be considered minimal, from 10 to 20 points intermediate and mean differences of more than 20 points are considered large [21].
The QLQ-BR23 module consist of 23 items assessing disease symptoms, side effects related to different treatment modalities (surgery, chemotherapy, radiotherapy and hormonal therapy), body image, sexual functioning and future perspective. It incorporates five domains: body image, systemic therapy side effects, breast symptoms, arm symptoms and sexual functioning. In addition, single items assess sexual enjoyment, hair loss and future perspective. The scoring approach is identical to that for the functioning and symptom scales and for the single items in QLQ-C30 [22].
The BIBCQ was specifically designed to measure the long-term impact of breast cancer on body image in a multidimensional fashion. It is a measure with a 53-item questionnaire comprising six optional items specific to women with two breasts [23]. The BIBCQ is suggested to evaluate various treatment forms for breast cancer [19].
Besides the three questionnaires, PROs were also assessed for AO: patients were asked to score their AO according to Harris et al. as “excellent”, “good”, “fair” or “poor” at baseline and 2 years after RT [24].
Objective aesthetic outcome analysis
An anterior view picture of the patient was taken with the patients’ hands on the hips. The BCCT.core software was used to evaluate AO [10, 25]. Based on the semi-automatic localization of fiducial points (nipple complex, breast contour, sternal jugular notch, mark 25 cm beneath the jugular notch), the software first measures asymmetry, skin colour changes and surgical scar appearance. Secondly, the set of measures is automatically converted into an overall objective AO: excellent, good, fair or poor [25].
Follow-up
Patients were evaluated at pre-set timepoints: at baseline; after surgery and before the start of RT; before the start of the boost; at the end of RT; between 3 and 6 months after RT; 1 year after RT and 2 years after RT. No photographs were taken at the end of RT because of the acute breast changes immediately after the removal of interstitial needles in case of a brachytherapy boost.
Statistical analysis
AO was dichotomized into excellent/good (“favourable”) versus fair/poor (“unfavourable”). The sample size was calculated to independently estimate AO for the BT and the EBRT group assuming a 25% unfavourable AO measured with BCCT.core after 2 years. Calculations were performed for estimating with 80% power the 95% confidence interval (CI) with a halfwidth of 10%. For each group—EBRT (i.e. electrons or photons) and BT—76 patients were needed [26].
Proportions of 2-year AO classifications were estimated with the Wilson 95% CI, excluding patients with unfavourable AO at baseline. Differences in proportions between the BT and the EBRT group were tested using Fisher’s exact test. The Mann–Whitney U test was used to compare the groups in terms of continuous or ordinal variables. Linear models were used for the analysis of continuous or ordinal scale scores. A random intercept for patients was modelled to account for the longitudinal structure of the data. Logistic regression was used for analysis of the binary AO score, with estimations based on generalized estimating equations to deal with longitudinal data. To assess whether the difference in mean outcomes between the BT and EBRT groups differed over time, we tested the interaction between the boost technique and time. A significant interaction would imply that the differences between patients with a BT and an EBRT boost would vary over time. Multivariable models were used to correct for variables that significantly differed between treatment groups.
Analyses were performed using SAS software (version 9.4 of the SAS System for Windows, SAS Institute, Heidelberg).
Results
Patient cohort
The analysis included 175 patients, 4 patients refused participation. A BT boost was received by 80 patients and 95 were treated with an EBRT boost (91 with electrons and 4 with photons). Patients in the BT group were significantly older; had higher breast cup, breast band size, body mass index (BMI) and surgical specimen weight of the wide excision; more seroma at baseline and less positive surgical section margins for the invasive and/or in situ component compared to patients in the EBRT group, and more patients drank alcohol (any vs. none; Table 1). Furthermore, patients in the EBRT group were more frequently treated with normofractionation compared to the BT group.
All patients filled in the QLQ-C30 and QLQ-BR23, scored their AO and had a photographic evaluation at baseline. Missing data during follow-up can be seen in Table 2.
No toxicity evaluation by means of photographs was available for 5 patients 2 years after RT (2 in the EBRT group and 3 in the BT group). One patient from the EBRT group underwent a contralateral mastectomy between 1 and 2 years after RT and was excluded from further follow-up. The baseline scores of the functional and symptom scales of the QLQ-C30, QLQ-BR23 and BIBCQ are reported in Table 3.
Short-term patient-reported outcomes
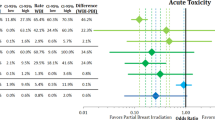
Mean scores for breast symptoms and sexual enjoyment differed significantly between the two patient groups in the short-term. Patients in the EBRT group showed an increase in breast symptoms (QLQ-BR23) in the short-term compared to the BT group, with a p-value of 0.050 for the interaction with time (Fig. 1). With mean difference −6.0 and p-value 0.060, the only minimally clinically relevant and trend towards a statistically significant difference was observed before the boost was administered [21]. The boost can thus not explain the difference between the groups. Sexual enjoyment (QLQ-BR23) significantly decreased in the EBRT group compared to the BT group (p < 0.01 for interaction). Similar to the breast symptoms, the only marginally significant difference was observed before the boost was administered; thus, the boost cannot explain the difference (p = 0.053). However, mean differences also seemed minimally clinically relevant after the boost and 2 years after RT (mean differences −9.5 and 8.1, respectively; [21]). Given this ambiguous result, the analysis was repeated with sexual enjoyment as a binary score (“no”, score 1 versus “yes”, scores 2–4). No difference could be observed between the two treatment groups over time (p = 0.56 for interaction).
Long-term patient-reported outcomes
There was no significant difference in mean scale PRO scores upon comparing the two boost techniques over time after correction for group differences (age, BMI, alcohol, breast cup and band size, surgical specimen weight, positive surgical margins and seroma) for any of the EORTC QLQ-C30 global health status, physical, role, emotional, cognitive or social functioning, fatigue, nausea and vomiting, pain, dyspnoea, insomnia, appetite loss, constipation, diarrhoea or financial difficulties scores; nor for the EORTC QLQ-BR23 body image, sexual functioning, future perspective or arm symptoms or for the BIBCQ vulnerability, body stigma, limitations, transparency and arm concerns scale.
There was a trend towards increased body concerns (BIBCQ) in the BT group compared to the EBRT group from 1 year after RT onwards (p = 0.08 for interaction; Fig. 2). The only marginally significant difference was observed 2 years after RT (p = 0.047) and the mean difference then was 1.7.
The BCCT.core scores for AO were unfavourable for 3 patients (1.71%) at baseline, for 37 (21.39%) before the boost, for 33 (19.41%) 3 to 6 months after RT, for 43 (20.61%) 1 year after RT and for 47 (27.81%) patients 2 years after RT. In univariable analysis, the proportion of patients with an unfavourable AO 2 years after treatment was greater in the BT group than in the EBRT group (p = 0.02). This statistically significant difference disappeared after correction for baseline group differences (age, BMI, breast cup and band size, surgical specimen weight, seroma, positive surgical margins, use of alcohol and fractionation scheme of the whole-breast irradiation; p = 0.77; Fig. 3).
At baseline, 6 patients (3.43%) scored their AO as unfavourable, whereas this rose to 53 patients (31.36%) 2 years after RT. The proportion of patients with an unfavourable AO 2 years after treatment was significantly higher in the BT group (p < 0.01). However, after correcting for baseline group differences, the p-value was 0.19.
Discussion
Local control and survival are the key objectives in breast cancer treatment and long-term survival rates are high [6]. With different techniques and treatment schedules resulting in similar oncological outcomes, the current challenge is to define factors that could guide treatment decisions. The purpose of the present study was to assess the possible effect of boost technique on PROs and AO. Therefore, we prospectively evaluated a cohort of 175 patients treated with BCT over 2 years.
First of all, it is important to stress that in the present cohort, a flowchart was used basing the choice of the tumour bed boost technique on the depth of the clinical target volume [16]. Using this flowchart has proven to result in equal local control rates for the two techniques.
This study found that there is no difference between EBRT and BT patient groups regarding short-term PROs on QOL and body image. Differences between the boost techniques such as treatment duration, the in- or outpatient procedure, the invasiveness of the procedure or the anxiety associated with the treatment do not influence short-term PROs on QOL and body image. To the best of our knowledge, we are the first to examine PROs in terms of the boost technique.
However, two PROs did differ significantly over time when comparing the two boost technique groups: breast symptoms and sexual enjoyment. First of all, with mean scores under 80, both PROs indicated unsatisfactory levels at baseline. Secondly, the p-value for both outcomes only just trended towards significance before the administration of the boost, so the results are independent of the boost technique.
Interpretation of the mean differences for sexual enjoyment after administration of the boost and 2 years after RT according to Osaba et al. did, however, indicate minimal clinical relevance (values between 5 and 10), but the difference was not statistically significant [21]. Given this ambiguous result, the analysis was repeated with sexual enjoyment as a binary score, also resulting in no difference between the two treatment groups over time (p = 0.56). Our data also show that factors which could potentially lead to impaired sexual enjoyment linked to the RT boost, such as an unfavourable AO or an impaired body image, were not observed in our study cohort. This also fits with literature data from population-based studies showing that breast cancer survivors report more frequent physical and menopausal symptoms than healthy women, yet report QOL and sexual functioning comparable to that of healthy age-matched women [27].
This study revealed no difference between the patient groups in terms of long-term PROs on QOL, body image and AO. There was no significant difference in cancer-specific or breast cancer-specific QOL and body image upon comparing EBRT and BT boost techniques over time.
Two years after treatment, AO was unfavourable in 28% of the patients as measured by BCCT.core and in 31% when scored by patients themselves. The BCCT.core has been shown to be a reliable instrument to measure cosmesis, and in contrast to what has been reported previously, patients in the present cohort judge their AO similarly to the BCCT.core software and not more positively [10, 28]. There is no evidence for a difference between the boost techniques with respect to AO 2 years after RT in the present cohort.
RT mainly influences AO by causing fibrosis and asymmetry, differences in colour such as hyper- or hypopigmentation, and skin telangiectasia [29,30,31,32,33]. Increasing the administered RT dose, for example in case of positive surgical margins or in the case of a boost, has been shown to worsen AO [29, 34]. However, results are conflicting with regard to the influence of the boost technique on AO [35, 36]. The reason for the inconsistency in the literature might be found in the selection criteria for the boost technique. Women with inadequate breast tissue or tumours in inaccessible locations were, for example, preferably treated with electrons; however, these factors may in themselves be associated with unfavourable AO [36]. Patients in the present cohort were treated with an electron boost or a BT boost based on the depth of the clinical target volume. The practical convenience of the electron boost is chosen for clinical target volumes up to 28 mm below the epidermis. The risk for telangiectasia increases with high doses in the first 5 mm of the skin and doses increase with increasing electron energy [37]. A BT boost is then the first choice for a deeper lying clinical target volume, also because boost volumes are smaller with BT than with a sequential photon boost [38]. It is thereby known that to optimize cosmetic results after BCT, both the total dose and the irradiated volume should be kept as low as possible [15]. Kelemen et al. have shown that the risks of breast oedema and breast fibrosis increase by 21 and 12%, respectively, for every 10 cm3 increase in boost volume [39]. If a BT was refused or technically impossible, a photon boost was suggested [16]. More information on the possible influence of dose–volume metrics is needed, but beyond the scope of this article. Regardless of the boost, several other patient-, tumour- and treatment-related factors play an important role [30, 36, 40]. We have therefore conducted multivariable analyses taking into account the relevant factors that differed between the two treatment groups: age, breast cup and band size, BMI, surgical specimen weight, the presence of a seroma at baseline, surgical section margins, alcohol and whole-breast irradiation fractionation schedule.
Over the past years, efforts have been made to integrate the sequential boost simultaneously into the whole-breast irradiation. With this technique, the whole breast and the boost are irradiated with EBRT, regardless of the depth of the clinical target volume. These changes have led to shorter treatment times and smaller boost volumes compared to the sequential EBRT boost, without compromising effectiveness or safety. Firstly, concerning toxicity, a large amount of acute toxicity data and a smaller amount of long-term sequelae are reassuring, with some studies even concluding improved AO [41,42,43,44]. Furthermore, cosmetic results were mostly reported to be independent of the timing of the boost. Secondly, to the best of our knowledge, a sequential BT boost and a simultaneous EBRT boost have not been evaluated with regard to PROs. We can assume that short-term PROs on QOL are comparable for a sequential or a simultaneous EBRT boost. Our study comparing PROs between EBRT and BT is thus not only of historical interest, but also currently pertinent in the evaluation of contemporary techniques such as a sequential BT boost or a simultaneous boost with EBRT.
Standard practice at our hospital was to treat all patients undergoing BCT with a sequential boost to the tumour bed. An important remark thereby is that according to current guidelines, the boost should be considered only in high-risk patients [4, 45]. The risk profile of a patient should, however, not alter the obtained results. A possible limitation of this study is that follow-up is currently at 2 years and it is therefore possible that not all patients will have expressed their final level of toxicity. The study is ongoing, and a re-analysis is planned at 5 years. However, there is evidence to suggest that the results at 2 years are predictive for late normal tissue outcomes 5 years after RT [46, 47]. Also, the effect of the boost on fibrosis presents in the first 3 years after RT [48]. A strength of this study is the very low number of losses to follow-up; only 5 patients lacked photographic evaluation at 2 years.
Conclusion
We have shown that PROs on QOL or body image and AO are not influenced by the boost technique if an EBRT boost is given for a clinical target volume lying in the upper 28 mm beneath the epidermis and if a BT boost is administered for deeper lying volumes. These data can be used when informing the patient on different boost techniques.
References
Ferlay J, Soerjomataram I, Ervik M et al (2014) GLOBOCAN 2012 v1.1, Cancer incidence and. [Online] Lyon, France: International Agency for Research on Cancer. http://globocan.iarc.fr. Accessed 25 Mar 2018
Fisher B, Anderson S, Bryant J et al (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347:1233–1241
Litière S, Werutsky G, Fentiman IS et al (2012) Breast conserving therapy versus mastectomy for stage I–II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol 13:412–419
Senkus E, Kyriakides S, Ohno S et al (2015) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl5):v8–30 (on behalf of the ESMO Guidelines Committee)
Desantis C, Ma J, Bryan L, Jemal A (2014) Breast Cancer Statistics. Ca Cancer J Clin 64:52–62
Allemani C, Minicozzi P, Berrino F et al (2013) Predictions of survival up to 10 years after diagnosis for European women with breast cancer in 2000–2002. Int J Cancer 132:2404–2412
Ewertz M, Jensen A (2011) Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol 50:187–193
Darby SC, Ewertz M, McGale P et al (2013) Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 368:387–398
Taylor C, Correa C, Duane F et al (2017) Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol 35:1641–1649
Cardoso MJ, Cardoso JS, Vrieling C et al (2012) Recommendations for the aesthetic evaluation of breast cancer conservative treatment. Breast Cancer Res Treat 135:629–637
Department of Health (2007) Guidance on the routine colletion of patient reported outcome measures (PROMS). http://www.gov.uk
Friese C, Harrison J, Janz N et al (2017) Treatment-associated toxicities reported by patients with early-stage invasive breast cancer. Cancer 123:1925–1934
Baumhauer J (2017) Patient-reported outcomes—are they living up to their potential? N Engl J Med 377:6–9
Jalali R, Singh S, Budrukkar A (2007) Techniques of tumour bed boost irradiation in breast conserving therapy: current evidence and suggested guidelines. Acta Oncol 46:879–892
Borger JH, Kemperman H, Smitt HS et al (1994) Dose and volume effect on fibrosis after breast conservation therapy. Int J Radiat Oncol Biol Phys 30:1073–1081
Verhoeven K, Kindts I, Laenen A et al (2015) A comparison of three different radiotherapy boost techniques after breast conserving therapy for breast cancer. Breast 24:391–396
Chen C, Cano S, Klassen A et al (2010) Measuring quality of life in oncologic breast surgery: a systematic review of patient-reported outcome measures. Breast J 16:587–597
Ong W, Schouwenburg M, van Bommel A et al (2017) A standard set of value-based patient-centered outcomes for breast cancer. The International Consortium for Health Outcomes Measurement (ICHOM) initiative. Jama Oncol 3:677–685
Kanatas A, Velikova G, Roe B et al (2012) Patient-reported outcomes in breast oncology: a review of validated outcome instruments. Tumori 98:678–688
Aaronson N, Ahmedzai S, Bergman B et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Osaba D, Rodrigues G, Pymes J et al (1998) Interpreting the significance of changes in health-related quality of-life scores. J Clin Oncol 16:139–144
Sprangers M, Groenvold M, Arraras J et al (1996) The European Organization for Research and Treatment of Cancer breast cancer-specific quality of life questionnaire module: first results from a three-country field study. J Clin Oncol 14:2756–2768
Baxter NN, Goodwin PJ, Mcleod RS, Dion R, Devins G, Bombardier C (2006) Reliability and validity of the body image after breast cancer questionnaire. Breast J 12:221–232
Harris J, Levene M, Svensson G, Hellman S (1979) Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys 5:257–261
Cardoso JS, Cardoso MJ (2007) Towards an intelligent medical system for the aesthetic evaluation of breast cancer conservative treatment. Artif Intell Med 40:115–126
Chow S, Shao J, Wang H (2008) Sample size calculations in clinical research, 2nd Ed. s.l. edn. Chapman & Hall/CRC (Biostatistics Series)
Ganz P, Rowland J, Desmond K, Meyerowitz B, Wyatt G (1998) Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. J Clin Oncol 16:501–514
Heil J, Dahlkamp J, Golatta M et al (2010) Aesthetics in breast conserving therapy: do objectively measured results match patients’ evaluations? Ann Surg Oncol 18:134–138
Vrieling C, Collette L, Fourquet A et al (2000) The influence of patient, tumour and treatment factors on the cosmetic results after breast-conserving therapy in the EORTC ‘boost vs. no boost’ trial. Radiother Oncol 55:219–232 (EORTC Radiotherapy and Breast Cancer Cooperative Groups)
Van Limbergen E, van der Schueren E, Van Tongelen K (1989) Cosmetic evaluation of breast conserving treatment for mammary cancer. 1. Proposal of a quantitative scoring system. Radiother Oncol 16:159–167
Pezner R, Patterson M, Hill L et al (1985) Breast retraction assessment: an objective evaluation of cosmetic results of patients treated conservatively for breast cancer. Int J Radiat Oncol Biol Phys 11:575–578
Triedman S, Osteen R, Harris J (1990) Factors influencing cosmetic outcome of conservative surgery and radiotherapy for breast cancer. Surg Clin North Am 70:901–916
Kurtz J (1995) Impact of radiotherapy on breast cosmesis. Breast 4:163–169
Poortmans P, Collette L, Horiot J et al (2009) Impact of the boost dose of 10 Gy versus 26 Gy in patients with early stage breast cancer after a microscopically incomplete lumpectomy: 10-year results of the randomised EORTC boost trial. Radiother Oncol 90:80–85
Fourquet A, Campana F, Mosseri V et al (1995) Iridium-192 versus cobalt-60 boost in 3–7 cm breast cancer treated by irradiation alone: final results of a randomized trial. Radiother Oncol 34:114–120
Budrukkar AN, Sarin R, Shrivastava SK, Deshpande DD, Dinshaw KA (2007) Cosmesis, late sequelae and local control after breast-conserving therapy: influence of type of tumour bed boost and adjuvant chemotherapy. J Clin Oncol 19:596–603
van Limbergen E, Briot E, Drijkoningen M (1990) The source-skin distance measuring bridge: a method to avoid radiation teleangiectasia in the skin after intersitital therapy for breast cancer. Int J Radiat Oncol Biol Phys 18:1239–1244
Poortmans P, Bartelink H, Horiot JC et al (2004) The influence of the boost technique on local control in breast conserving treatment in the EORTC ‘boost versus no boost’ randomised trial. Radiother Oncol 72:25–33
Kelemen G, Varga Z, Lázár G et al (2012) Cosmetic outcome 1–5 years after breast conservative surgery, irradiation and systemic therapy. Pathol Oncol Res 18:421–427
Vrieling C, Collette L, Fourquet A et al (2000) The influence of patient, tumour and treatment factors on the cosmetic results after breast-conserving therapy in the EORTC ‘boost vs. no boost’ trial. Radiother Oncol 55:219–232 (EORTC Radiotherapy and Breast Cancer Cooperative Groups)
van der Laan HP, Dosma WV, Maduro JH et al (2007) Three-dimensional conformal simultaneously integrated boost technique for breast-conserving radiotherapy. Int J Radiat Oncol Biol Phys 68:1018–1023
Bantema-Joppe EJ, Schilstra C, de Bock GH et al (2012) Simultaneous integrated boost irradiation after breast-conserving surgery: physician-rated toxicity and cosmetic outcome at 30 months’ follow-up. Int J Radiat Oncol Biol Phys 83:e471–e477
Dellas K, Vonthein R, Zimmer J et al (2014) Hypofractionation with simultaneaous integrated boost for early breast cancer. Strahlenther Onkol 190:646–653 (ARO Study Group)
Lansu J, Essers M, Voogd A et al (2015) The influence of simultaneous integrated boost, hypofractionation and oncoplastic surgery on cosmetic outcome and PROMs after breast conserving therapy. Eur J Surg Oncol 41:1411–1416
Gradishan WJ, Anderson BO (2017) National comprehense cancer network guidelines breast cancer. Version 2. www.nccn.org. Accessed 25 Mar 2018 (on behalf of the NCCN Breast Cancer Panel Members)
Turesson I, Nyman J, Holmberg E, Odén A (1996) Prognostic factors for acute and late skin reactions in radiotherapy patients. Int J Radiat Oncol Biol Phys 36:1065–1075
Hennigs A, Hartmann B, Rauch G et al (2015) Long-term objective cosmetic outcome after breast-conserving therapy. Breast Cancer Res Treat 153:345–351
Immink JM, Putter H, Bartelink H et al (2012) Long-term cosmetic changes after breast-conserving treatment of patients with stage I–II breast cancer and included in the EORTC ‘boost versus no boost’ trial. Ann Oncol 23:2591–2598
Funding
This project is realized with the support of Kom op tegen Kanker.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I. Kindts, A. Laenen, M. Christiaens, H. Janssen, E. VanLimbergen and C. Weltens declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kindts, I., Laenen, A., Christiaens, M. et al. Comparison of brachytherapy and external beam radiotherapy boost in breast-conserving therapy: Patient-reported outcome measures and aesthetic outcome. Strahlenther Onkol 195, 21–31 (2019). https://doi.org/10.1007/s00066-018-1346-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1346-7